Tetanus toxin-resistant neutralizing antibody as well as preparation and application thereof
A tetanus toxin and anti-tetanus technology, applied in the fields of cellular immunology and genetic engineering, can solve the problems of being in the laboratory research stage, expensive, and foreign virus contamination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
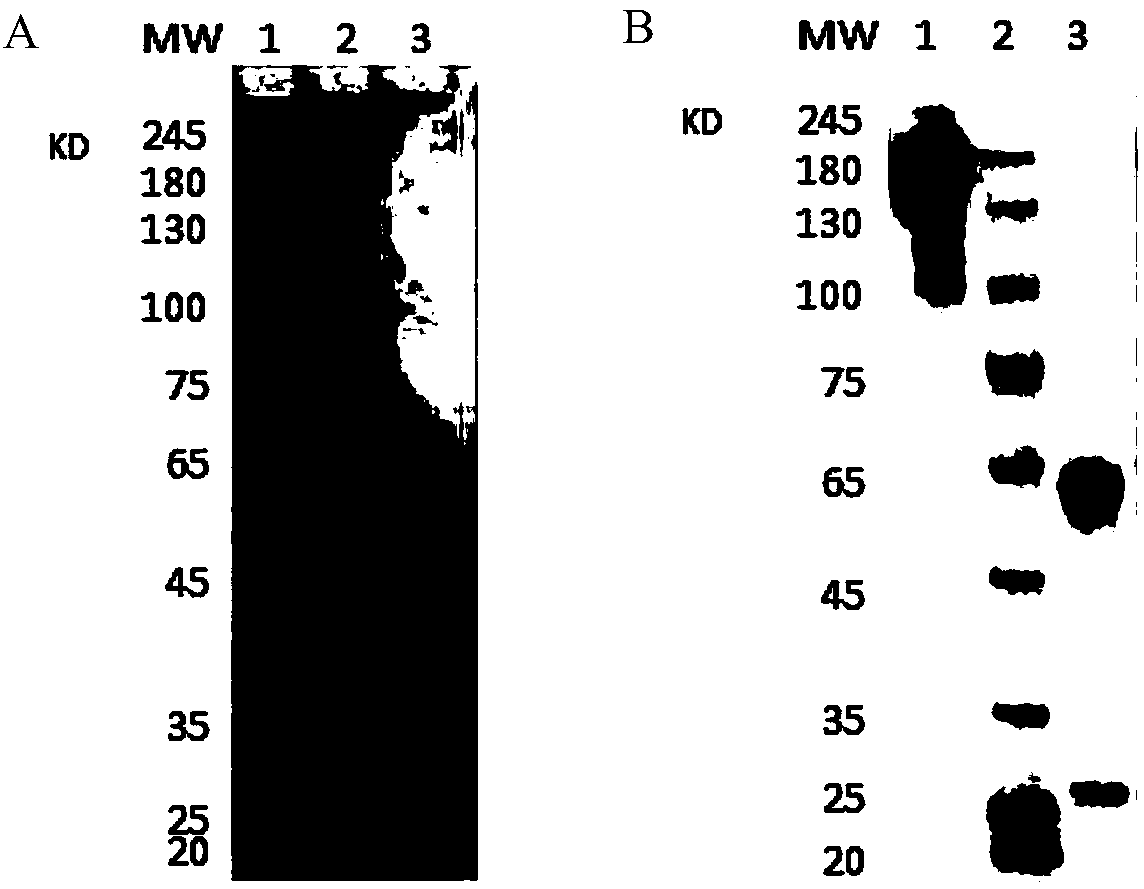
[0067] Example 1 Sorting of B cells by flow cytometry and screening and purification of anti-tetanus toxin antibodies
[0068] 1. PBMC isolation and plasma cell sorting
[0069] 100ml of venous blood was collected from volunteers who had been injected with tetanus vaccine and developed protective antibodies into anticoagulant tubes containing heparin. Use Ficoll to separate 100ml blood samples from mononuclear cells (PBMCs); after cell counting, use BDFACSria flow cytometer to sort from PBMCs, and place single cells with good morphology in a 96-well PCR plate, so that each well contains a memory B cells were stored in a -80°C refrigerator for later use.
[0070] 2. Isolation of antibody variable region genes
[0071] A 96-well plate containing a single B cell was added with 0.5 μM constant region primers of heavy and light chains of each subtype and Superscript III reverse transcriptase, and incubated at 37°C for 1 hour; PCR amplification was carried out under the following ...
Embodiment 2
[0083] Example 2 ELISA detection of neutralizing activity of TRN0012 antibody
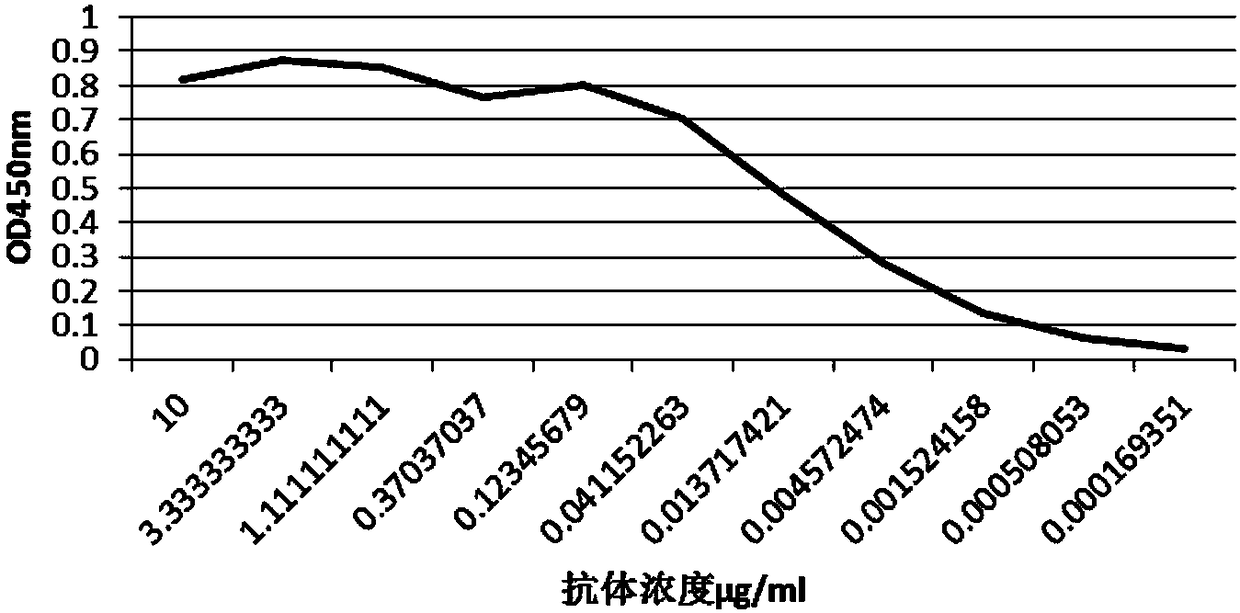
[0084] The standard tetanus toxin was used as the antigen, and the antigen was diluted 10 times with the coating solution to coat the 96-well ELISA plate, 100 μl per well was coated overnight at 4°C, and blocked with the blocking solution for 2 hours at room temperature. After serial dilution of the expressed and purified antibody TRN0012, it was used as the primary antibody and incubated at room temperature for 2 hours, and was incubated with HRP / anti-His-tag (1:2000 dilution) as the secondary antibody at room temperature for 1 hour. After adding the substrate chromogenic solution, place it in the dark at room temperature for 5 minutes. , the reaction was terminated with 2M sodium sulfate, and the detection analysis was performed at a wavelength of 450 nm.
[0085] The result is as figure 2 As shown, after 50,000 dilutions of the expressed and purified antibody (the antibody concentration is app...
Embodiment 3
[0086] Example 3 Kinetic Analysis of Affinity Activity of Antibody TRN0012
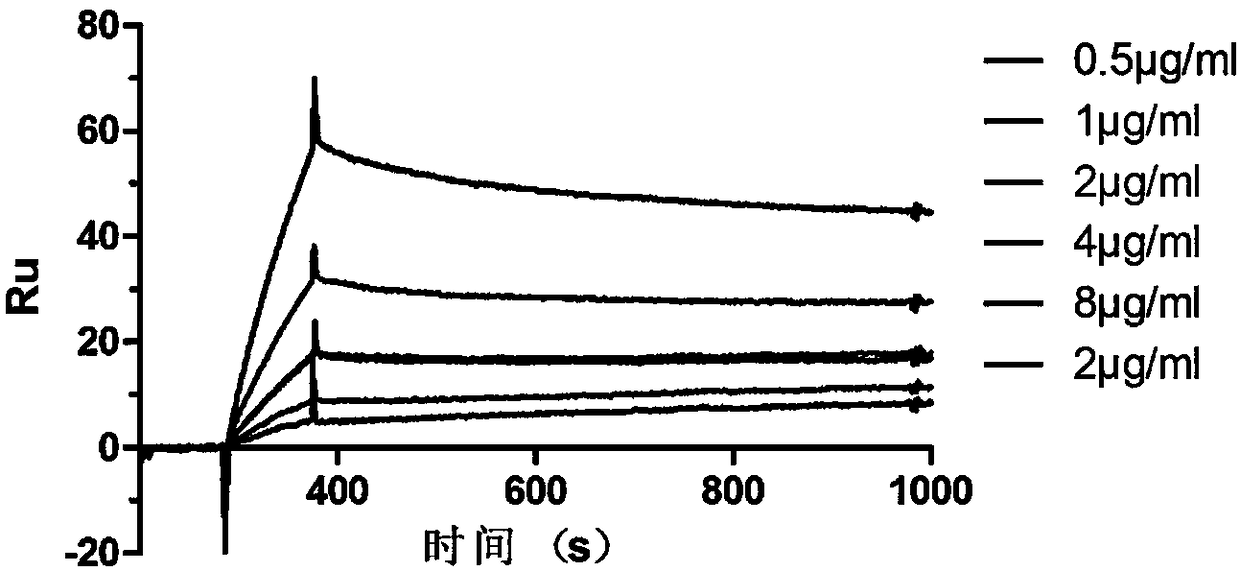
[0087] Using the CM5 chip and human antibody capture kit, the anti-human FC secondary antibody was coupled first, then the anti-tetanus toxin monoclonal antibody was captured, and finally the tetanus toxin protein of different concentrations was used as the analyte. The tetanus standard toxin was diluted with HBS-EP buffer as the analyte, and the analyte flowed through the chip in sequence with gradually increasing concentrations, and the signal curves were respectively obtained. Each concentration was regarded as one cycle, and after completing one cycle, the chip was regenerated with 3 mol / L magnesium chloride to return to the original state of unbound antigen. BiaCore X-100System software was used to analyze the affinity and kinetics of monoclonal antibody binding to tetanus standard toxin (antigen).
[0088] The result is as image 3 As shown, the neutralizing antibody TRN0012 has an affinity of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com