Use of nifuratel to treat infections caused by clostridium species
A technology of nifuratel and uses, applied in the field of Clostridium difficile-associated diarrhea (CDAD), can solve problems such as unmet medical needs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
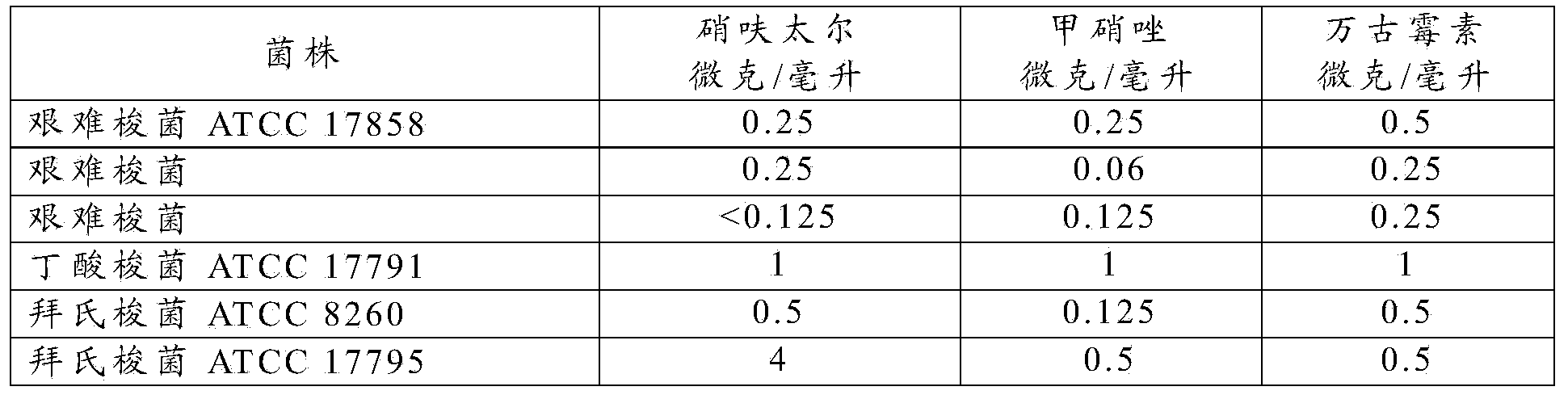
[0018] In vitro studies were performed to investigate 3 C. difficile strains (1 reference strain ATCC17858 and 2 clinical isolates), 1 reference strain of Clostridium butyricum (ATCC17791) and 2 reference strains of C. ) susceptibility to nifuratel and compared with metronidazole and vancomycin. According to CLSI M11-A7, by broth dilution method, supplemented with hemin (Hemin) (5 μg / ml), vitamin K1 (1 μg / ml), lysed horse blood (5%) and oxidase (1 :25v / v) Determination of MIC (Minimum Inhibitory Concentration) in Brucella Broth. Nifuratel, vancomycin, and metronidazole (previously dissolved in dimethyl sulfoxide) were added to the medium. All compounds were tested at concentrations ranging from 0.125-128 μg / ml.
[0019] The in vitro activity of nifuratel against tested strains of C. difficile (MIC range: ≤0.125-0.25 μg / ml) was comparable to or superior to that of metronidazole (MIC range: 0.06-0.25 μg / ml) and vancomycin (MIC range: 0.25-0.5 μg / ml).
[0020] Fairly good act...
Embodiment 2
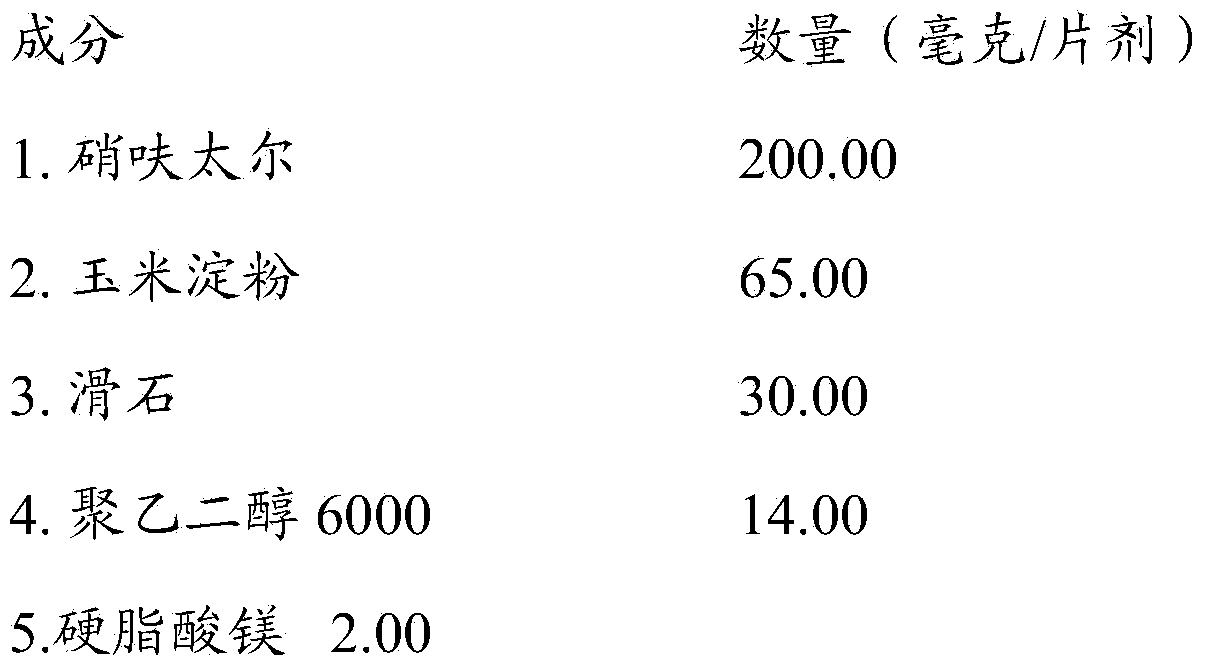
[0024] Oral tablets are manufactured using the following qualitative and quantitative formulations:
[0025]
[0026] The procedure consisted of preparing a (protected from light) binder solution with water and polyethylene glycol heated at 45°C under continuous stirring; then blending nifuratel and starch in a Glatt fluid bed basket until mass Granulation for homogeneity followed by spraying of binder solution followed by drying at an input air temperature of 60°C; followed by addition of talc and magnesium stearate. Compress into tablets in a rotary tablet press with suitable punches.
[0027] The resulting tablets had a yellow smooth surface.
Embodiment 3
[0029] A syrup with the following composition wt. / wt.% was prepared:
[0030]
[0031] preparation
[0032] This recipe is prepared (protected from light) as follows:
[0033] 1) Use deionized water and carboxymethyl cellulose (3.75% in water) to prepare the gel. Let the gel soak overnight.
[0034] 2) Prepare water, sucrose (50%) and sodium chloride (0.5%) solutions separately.
[0035] 3) A mixture of nifuratel (0.4%) and polysorbate 80 (1%) was prepared in water. The mixture is then stirred until homogeneous.
[0036] 4) Add deionized water, sorbitol and glycerin, solution 2) and sucrose in a closed container with a stirrer. The mixture was maintained under continuous stirring. Methylparaben, propylparaben, and silicon dioxide are then added. Heat with stirring at 100°C for 30 minutes. After cooling at 80°C, citric acid was added. After cooling at 40°C, gel 1) and formulation 3) were added with continuous stirring.
[0037] The resulting syrup is a homogeneous ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com