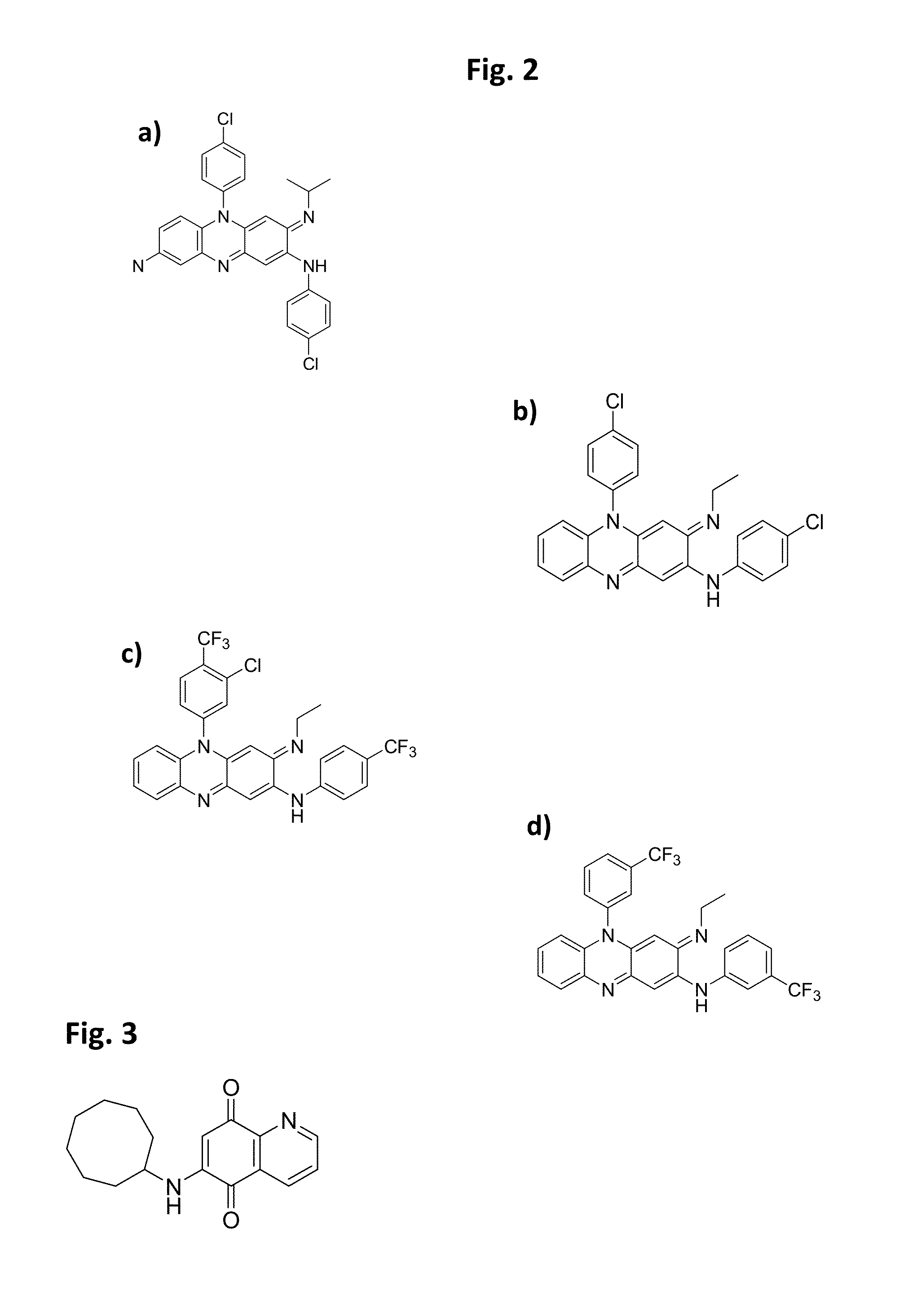
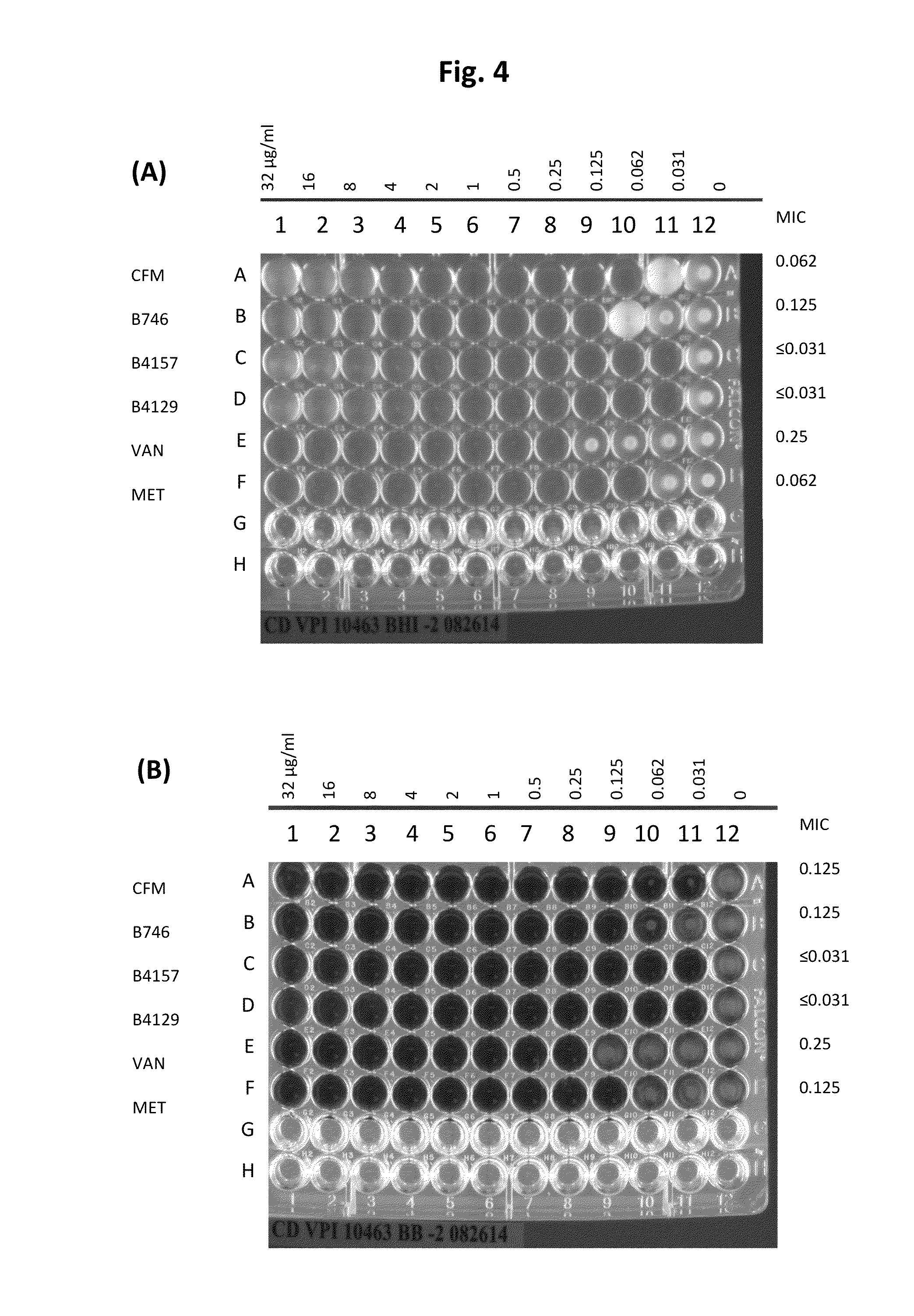
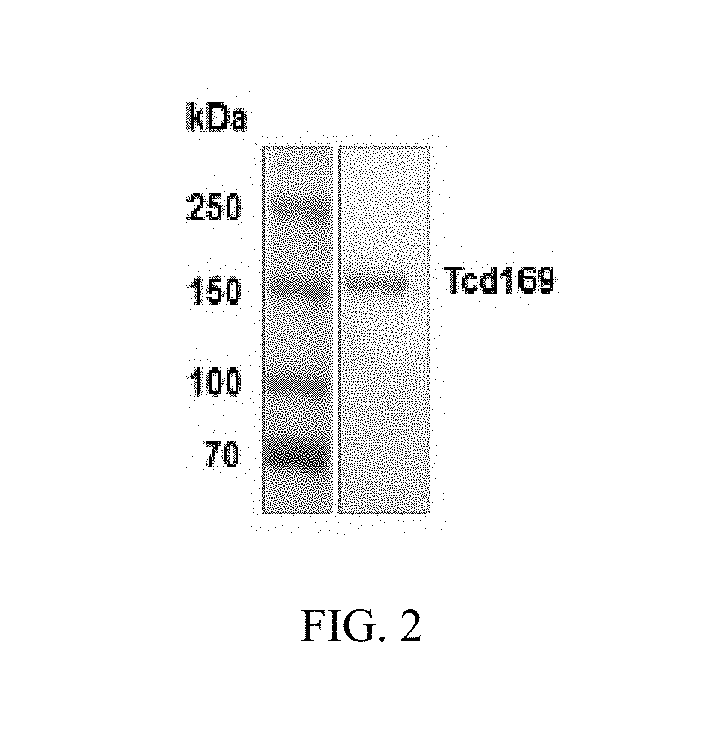
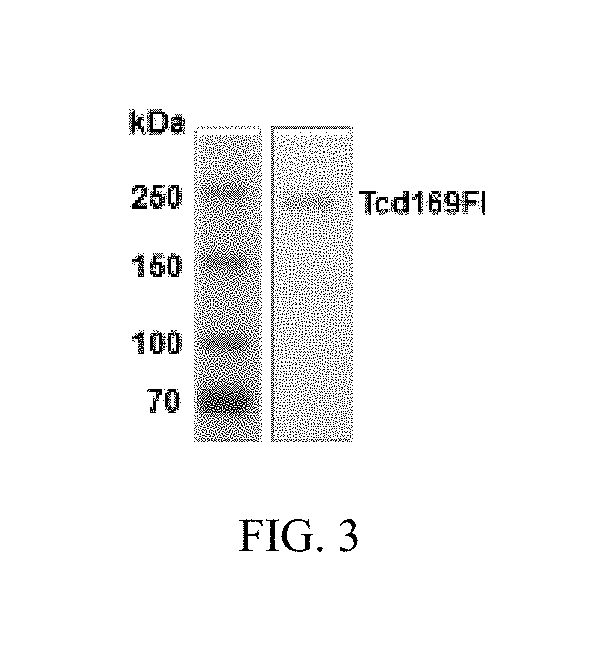
Patents
Literature
35 results about "Clostridial infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
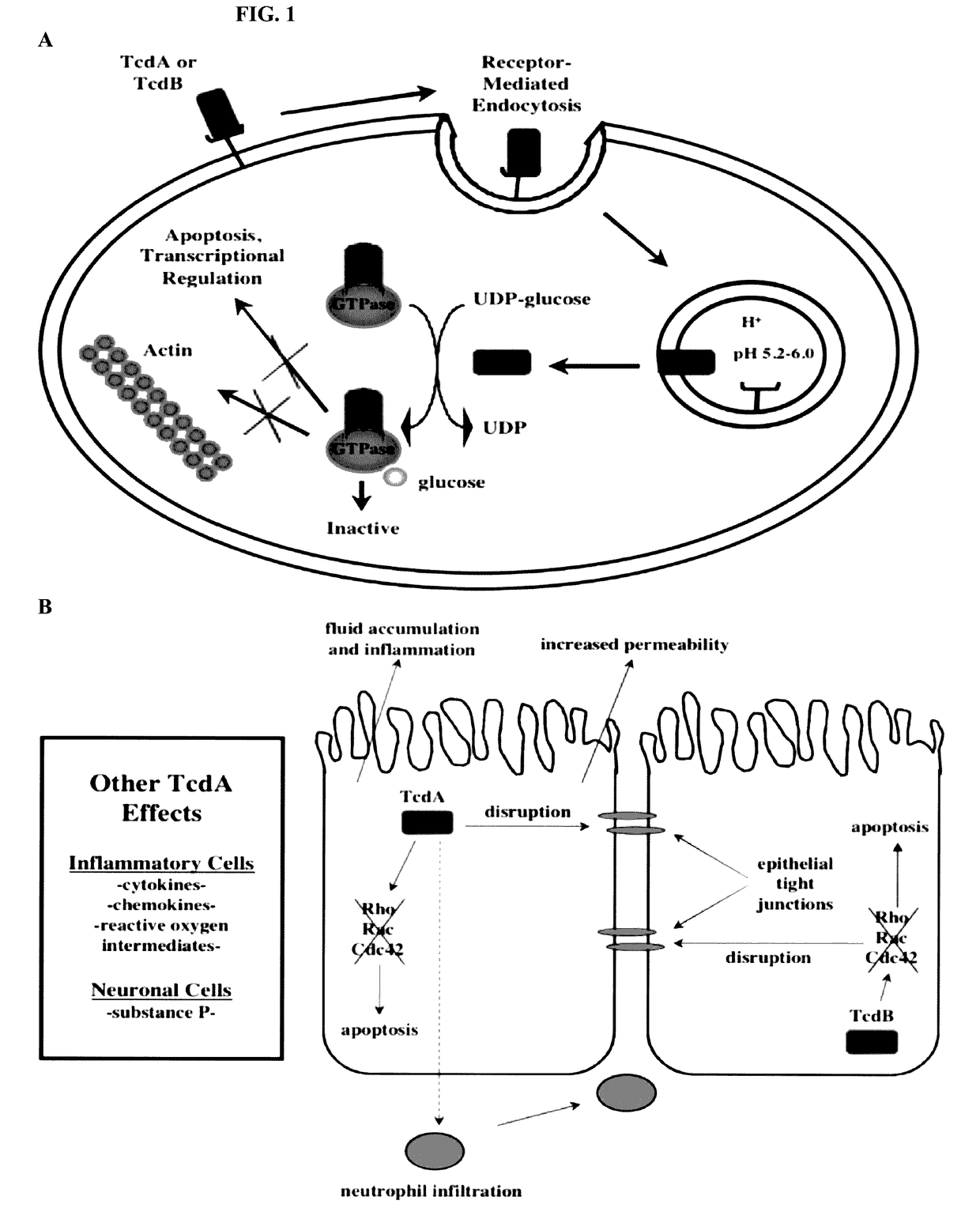
Infections with bacteria of the genus Clostridium.
Method for treatment of disorders of the gastrointestinal system
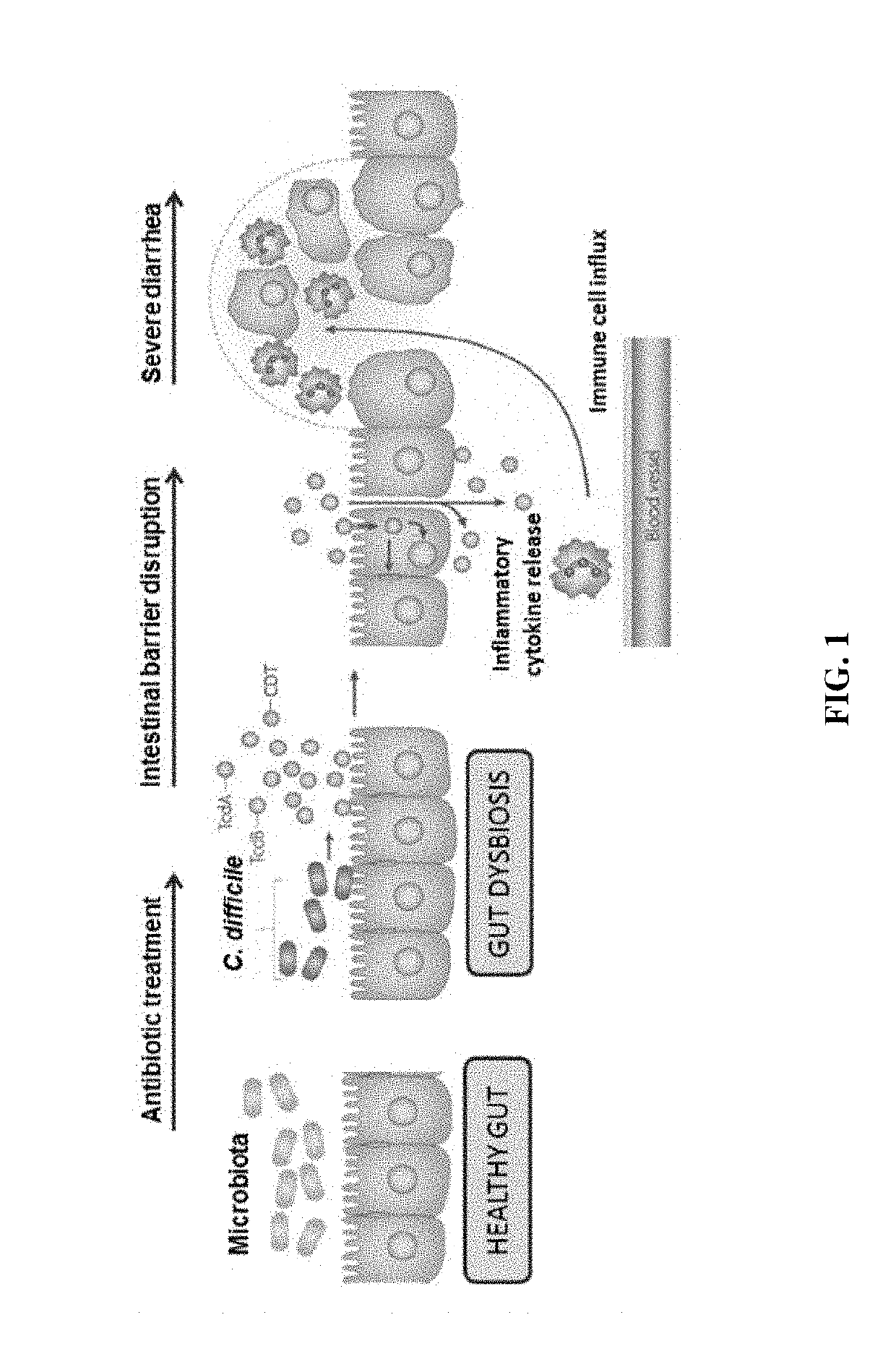
There are provided novel synthetic stool preparations comprising bacteria isolated from a fecal sample from a healthy donor. The synthetic stool preparations are used for treating disorders of the gastrointestinal tract, including dysbiosis, Clostridium difficile infection and recurrent Clostridium difficile infection, prevention of recurrence of Clostridium difficile infection, treatment of Crohn's disease, ulcerative colitis, irritable bowel syndrome, inflammatory bowel disease, and diverticular disease, and treatment of food poisoning such as salmonella. Methods of preparation and methods of use of the synthetic stool preparations are also provided.
Owner:UNIVERSITY OF GUELPH +2
Antibiotic Formulations Providing Reduced Gastrointestinal Side Effects and Clostridium difficile Infection Relapse, and Related Methods
InactiveUS20110240512A1Antibacterial agentsSmall article dispensingClostridial infectionAntibiotic-associated diarrhoea
The invention includes a formulation comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The formulation is prepared in a dosage form for delivery to the gastrointestinal tract. The invention further includes a method of preventing or minimizing the proliferation of Clostridium difficile in the gastrointestinal tract of a mammal undergoing an antibiotic therapy comprising by administration of the formulation of the invention for the duration of the antibiotic therapy; methods of preventing or minimizing the occurrence of antibiotic-associated diarrhea in a mammal undergoing an antibiotic therapy that includes administering the formulation; and methods of preventing or minimizing the occurrence of antibiotic-associated colitis or pseudomembranous colitis in a patient undergoing an antibiotic therapy that includes administering the formulation.Also included are methods of reducing or preventing a failure of treatment for an antibiotic-treatable infection in a patient comprising orally administering the formulation.Described by the invention are formulations for the treatment of a C. difficile infection comprising: (i) a therapeutically effective amount of at least one antibiotic; and (ii) a therapeutically effective amount of at least one probiotic material. The antibiotic includes a non-systemic gram negative antibiotic (such as, for example, fodaximicin) and formulation is prepared in a dosage form for delivery to the gastrointestinal tract.Also included are methods of treatment of a C. difficile infection in mammal that include administering to the digestive tract of the mammal the above described formulation. Such methods provide that the likelihood that the mammal shall experience a reoccurrence of the C. difficile infection is 70% or less.
Owner:TECOPPA BIOPHARMA
Method of prevention and treatment of clostridium difficile infection
InactiveUS20140112985A1Improve developmentGrowth inhibitionAntibacterial agentsSurgical needlesClostridial infectionMicroorganism
This invention relates to prophylactic and / or therapeutic application of microorganism species that are, for example, administered orally as delayed release formulation designed to release its microbial content to the distal small intestine and / or colon in high quantities and density, which is a “normalized” approach to repopulate the colonic flora as a method of prevention and / or treatment of, for example, Clostridium difficile colitis.
Owner:POLONEZ THERAPEUTICS
Antibodies for the treatment of clostridium difficile-associated infection and disease
ActiveUS20130202618A1Improve the quality of lifeConvenient treatmentAntibacterial agentsPeptide/protein ingredientsClostridial infectionBacteroides
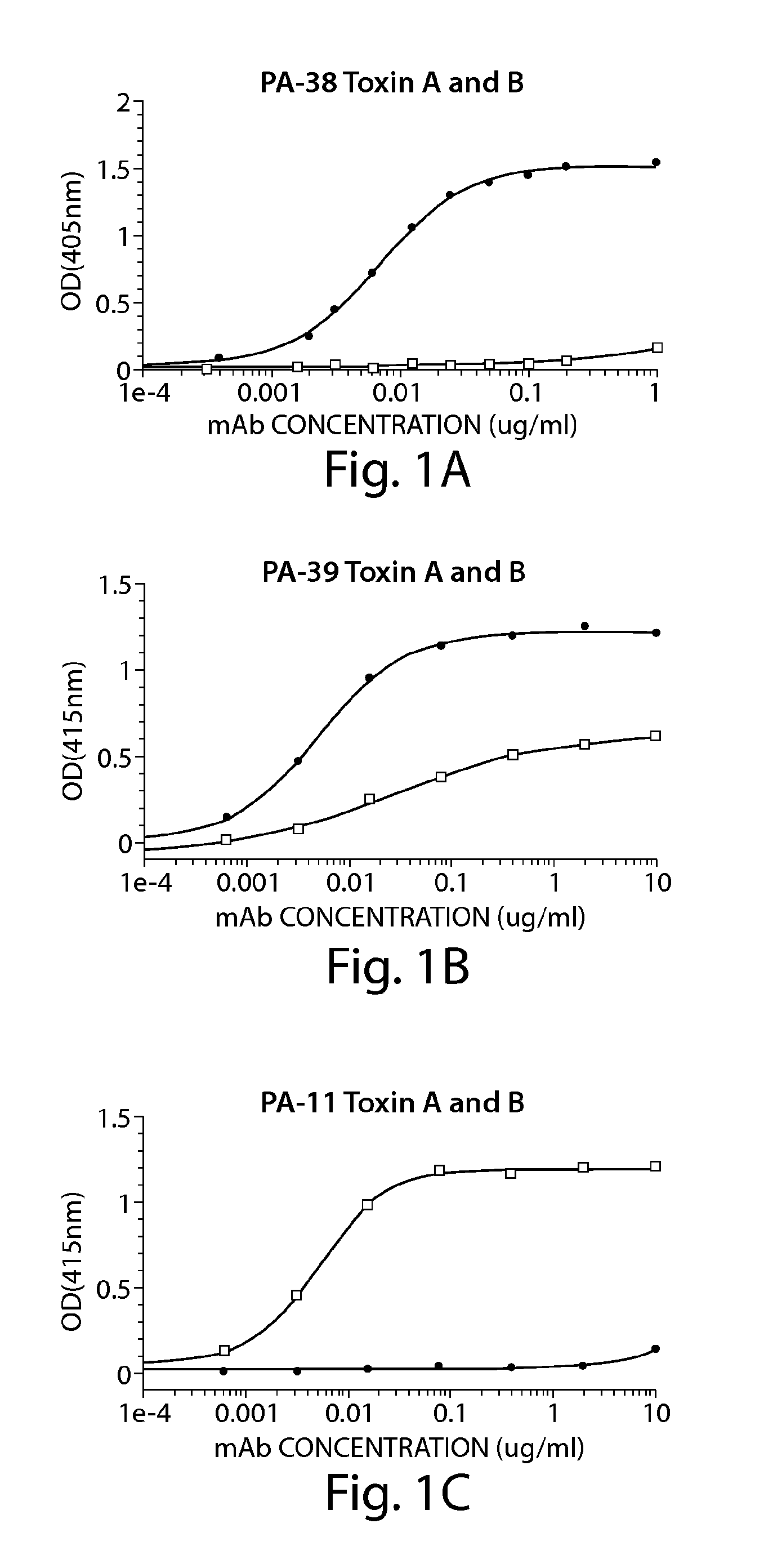
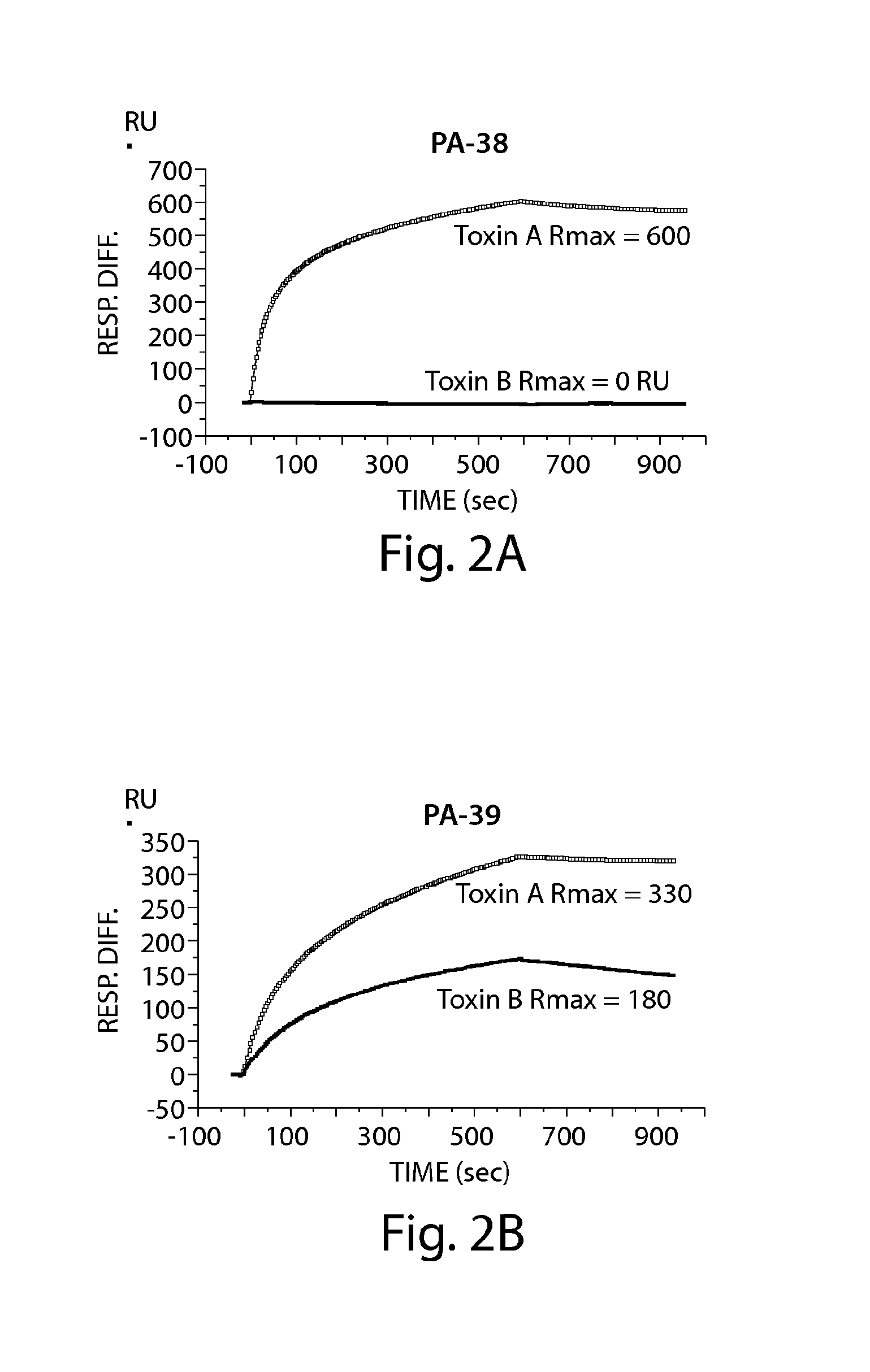
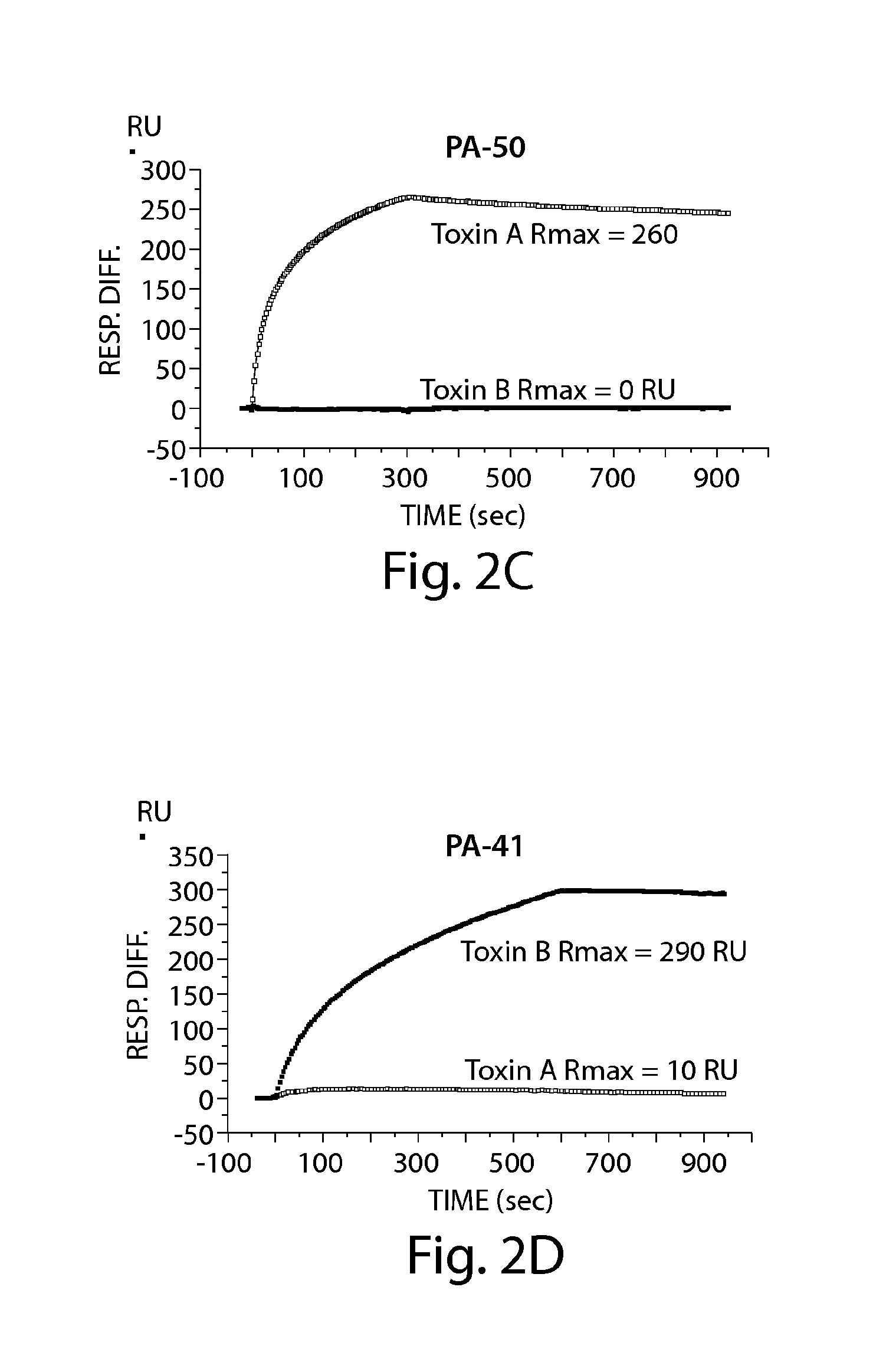
Provided herein are reagents, compositions, and therapies with which to treat Clostridium difficile infection and related disease conditions and pathologies, such as Clostridium difficile-associated diarrhea, resulting from infection by Clostridium difficile bacteria and the enterotoxins produced by these bacteria. In particular, antibodies or antigen-binding fragments thereof that bind specifically to toxin A and / or toxin B of C difficile and neutralize the activities of these toxins; compositions comprising such antibodies; and methods of using the antibodies and the compositions are provided.
Owner:PROGENICS PHARMA INC
Methods and compositions for reducing clostridium difficile infection
InactiveUS20170087196A1Reduce riskReduce severityPeptide/protein ingredientsBacteria material medical ingredientsClostridial infectionClostridium difficile infections
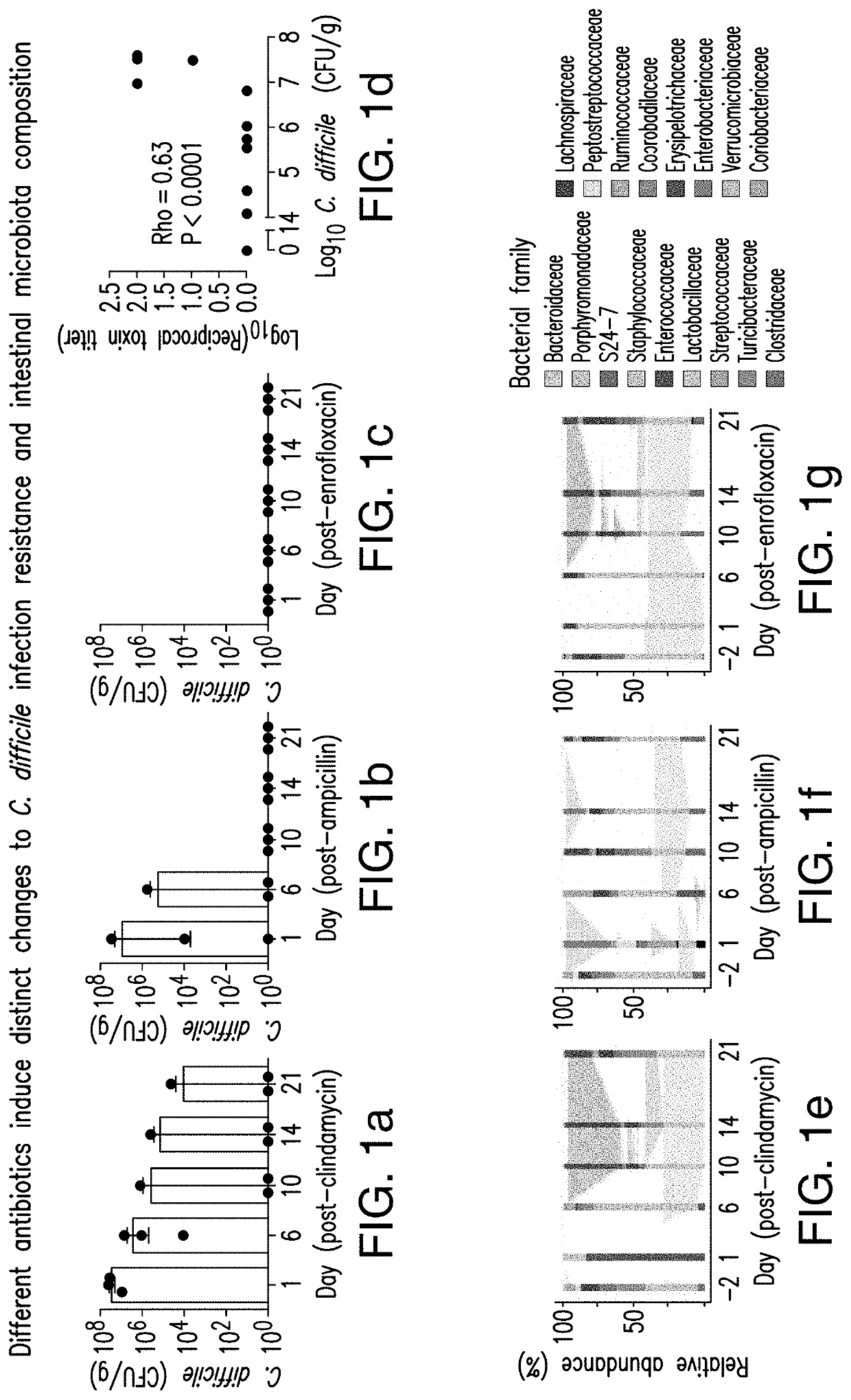
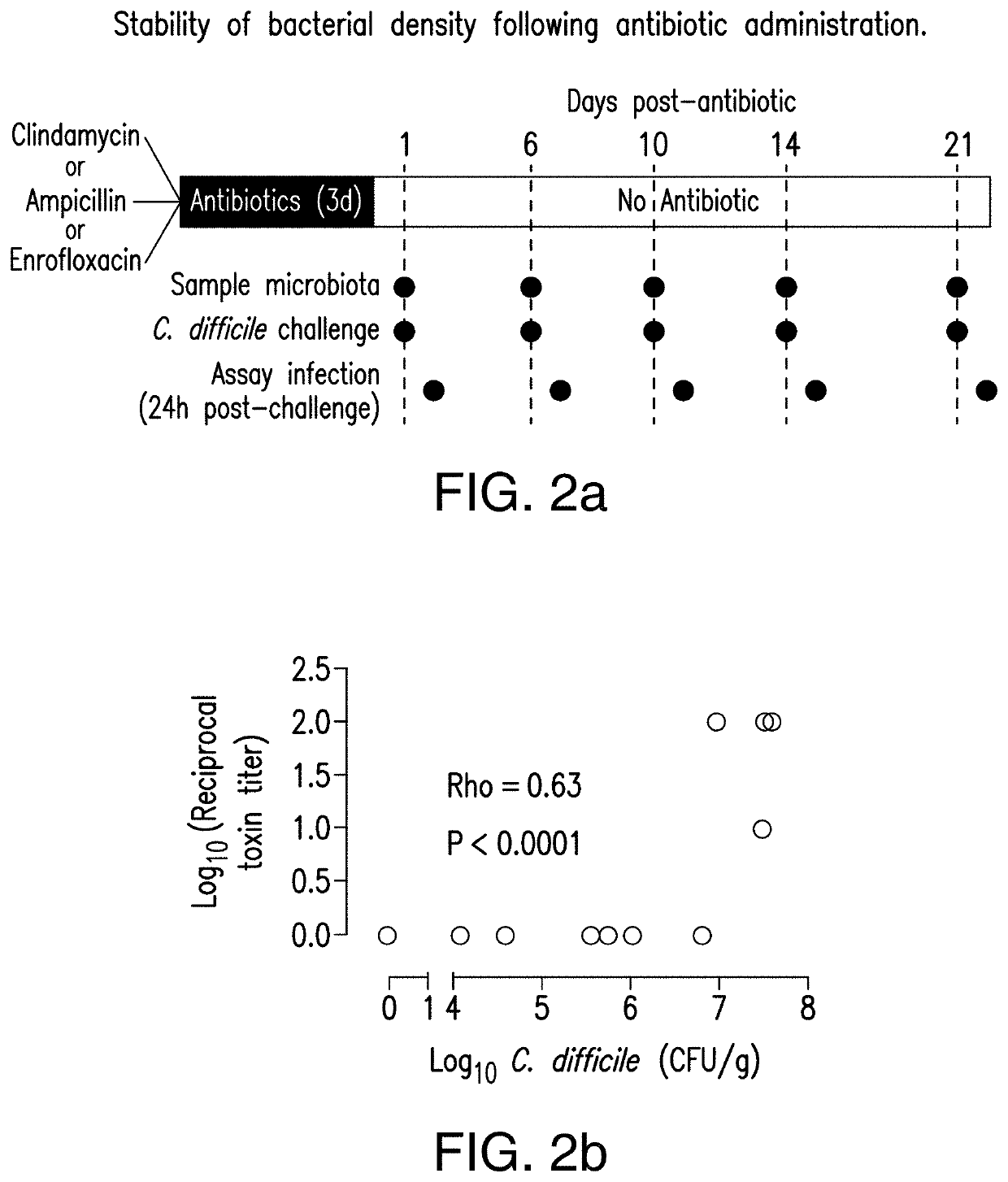
The present invention relates to methods and compositions for reducing the risk and severity of C. difficile infection. It is based, at least in part, on the discovery that a restricted fraction of the gut microbiota, including the bacterium Clostridium scindens, contributes substantially to resistance against C. difficile infection. Without being bound by any particular theory, it is believed that this is achieved through the biosynthesis of secondary bile acids.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Biomarkers of recurrent clostridium difficile infection
ActiveUS20150344940A1Reduce usageBiocideMicrobiological testing/measurementMicroorganismClostridial infection
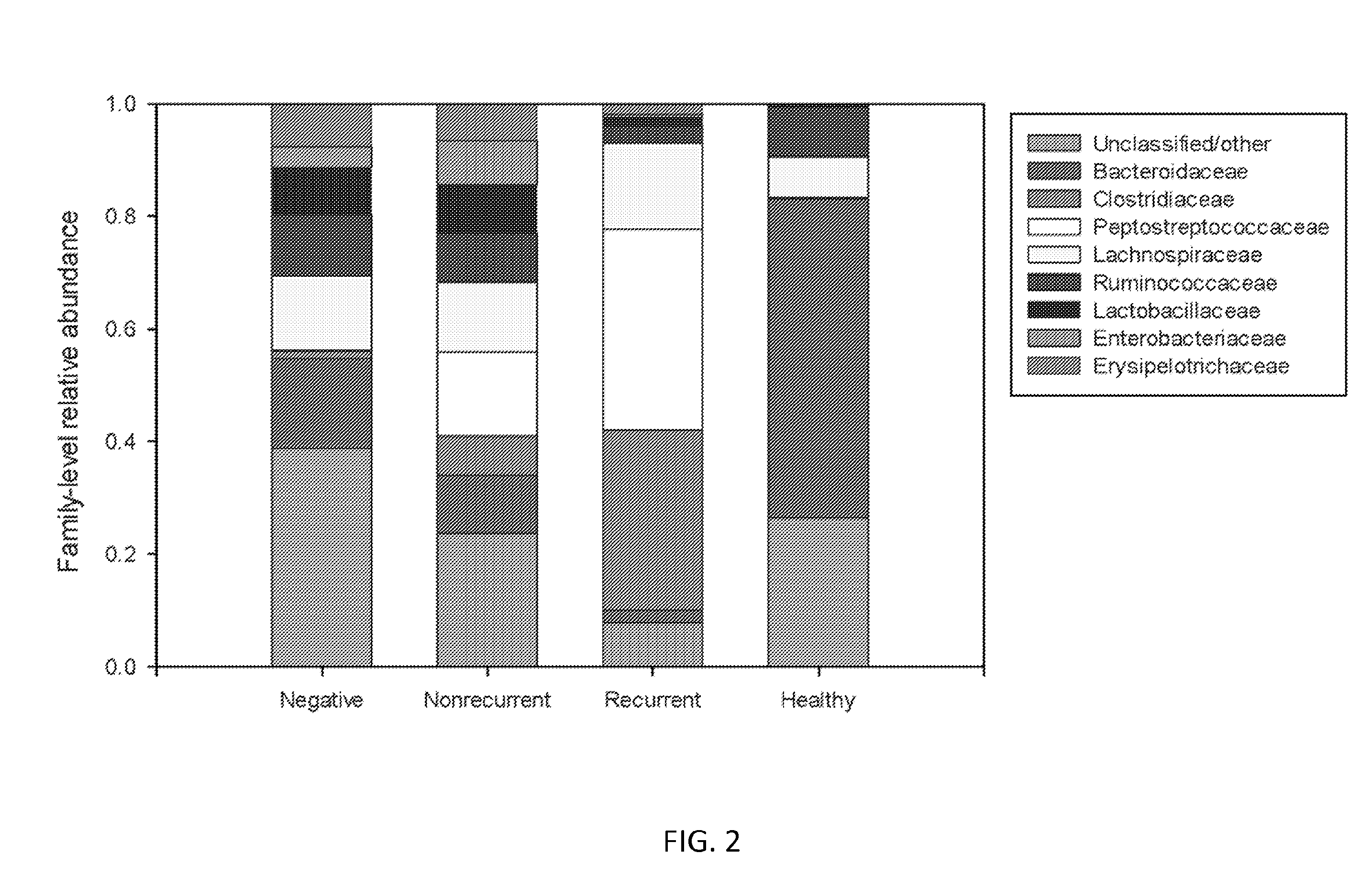
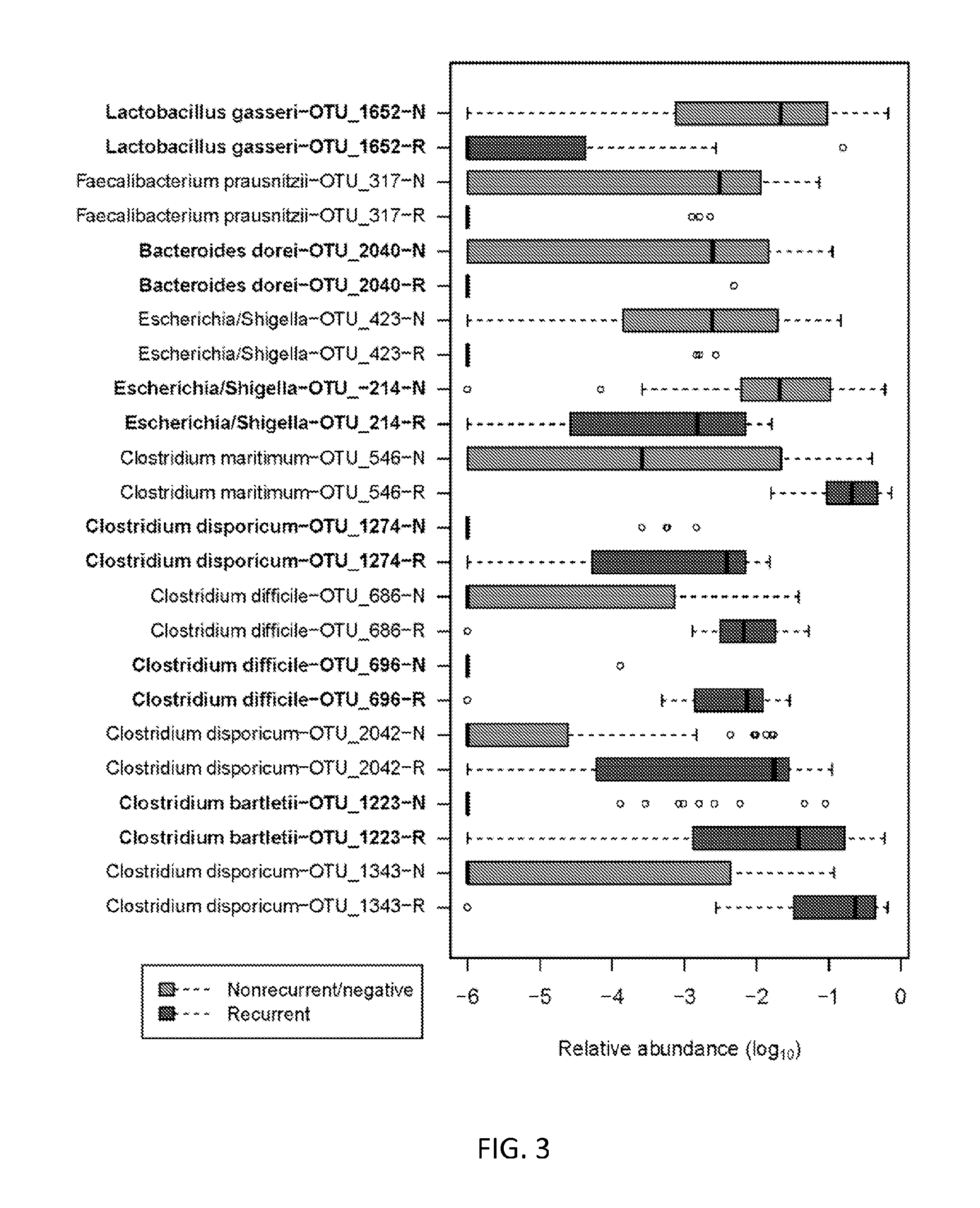
Embodiments of the invention include methods and / or compositions for analysis of samples for C. difficile infection to determine whether or not an individual is at risk for having recurrent C. difficile infection or a CDI misdiagnosis. Methods include characterization of microflora composition from the gut, wherein alterations of the microflora gut composition are indicative of recurrence of infection. Methods include analysis of nucleic acids from the gut, such as 16S rRNA as being identifying of a particular bacteria in the analysis of bacterial populations of the gut.
Owner:BAYLOR COLLEGE OF MEDICINE
Methods and compositions for reducing clostridium difficile infection
ActiveUS20190381113A1Lessen risk of and severityReduce riskPeptide/protein ingredientsBacteria material medical ingredientsClostridial infectionBacteroides
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Compositions for fecal floral transplantation and methods for making and using them and devices for delivering them
This application provides compositions, e.g., formulations, used for gastric, gastrointestinal and / or colonic treatments, and methods for making, storing and using them, including storage, including long term storage, at ambient temperature. Compositions provided herein are useful for treating various diseases or conditions such as autism spectrum disordor, Crohn's Disease, ulcerative colitis, irritable bowel syndrome, and recurrent or primary C.difficile infection.
Owner:CRESTOVO HLDG LLC
Tetanus toxin-resistant neutralizing antibody as well as preparation and application thereof
ActiveCN108314730AAntibacterial agentsImmunoglobulins against bacteriaClostridial infectionClostridium tetani
The invention discloses a tetanus toxin-resistant neutralizing antibody as well as preparation and application thereof. The antibody can be used for preventing, treating and diagnosing tetanus infection and / or treating one or more symptoms mediated by clostridium tetani infection. The invention further provides a method for producing an antibody capable of combining with tetanus toxin immunologically specifically.
Owner:ZHUHAI TRINOMAB BIOTECHNOLOGY CO LTD
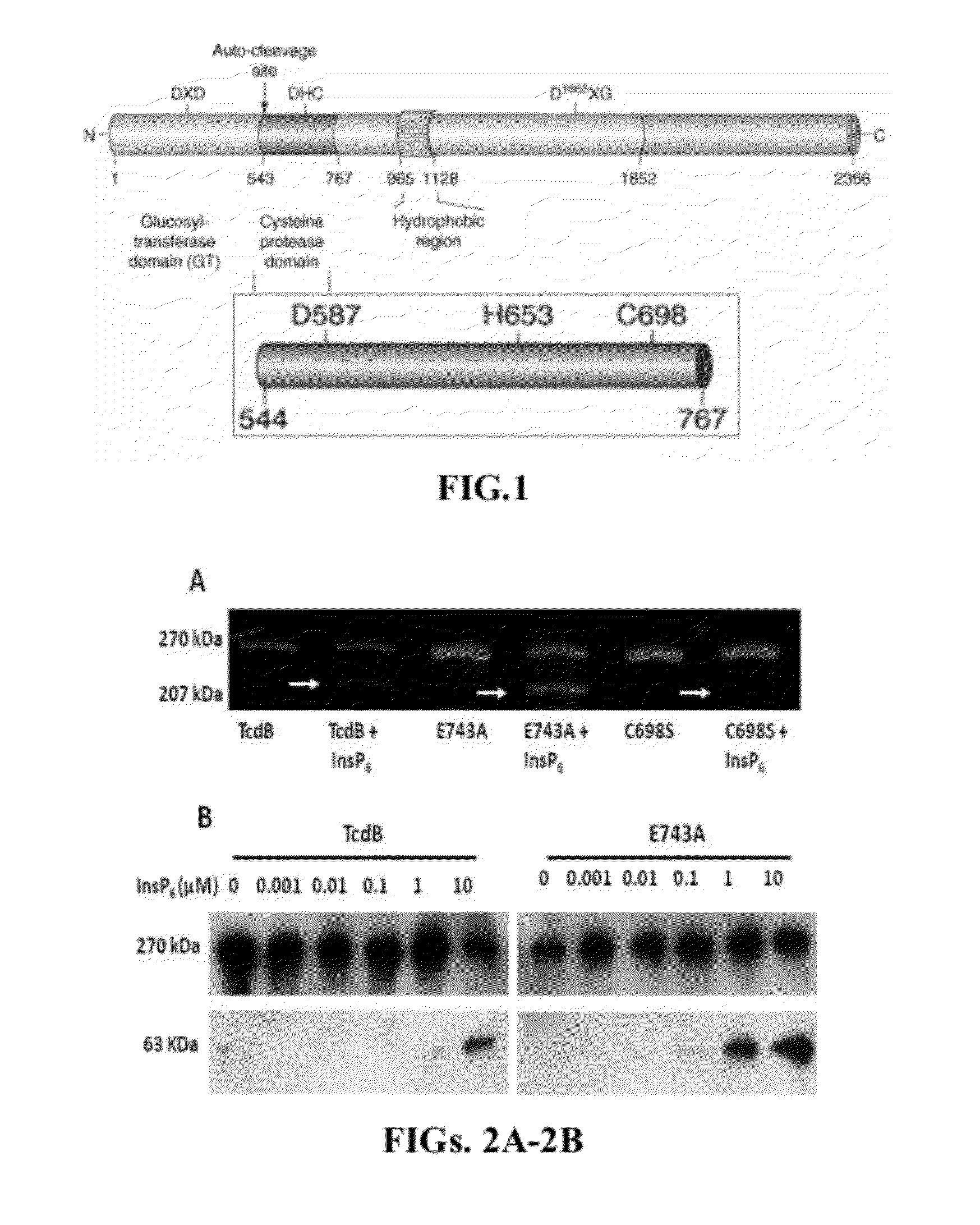
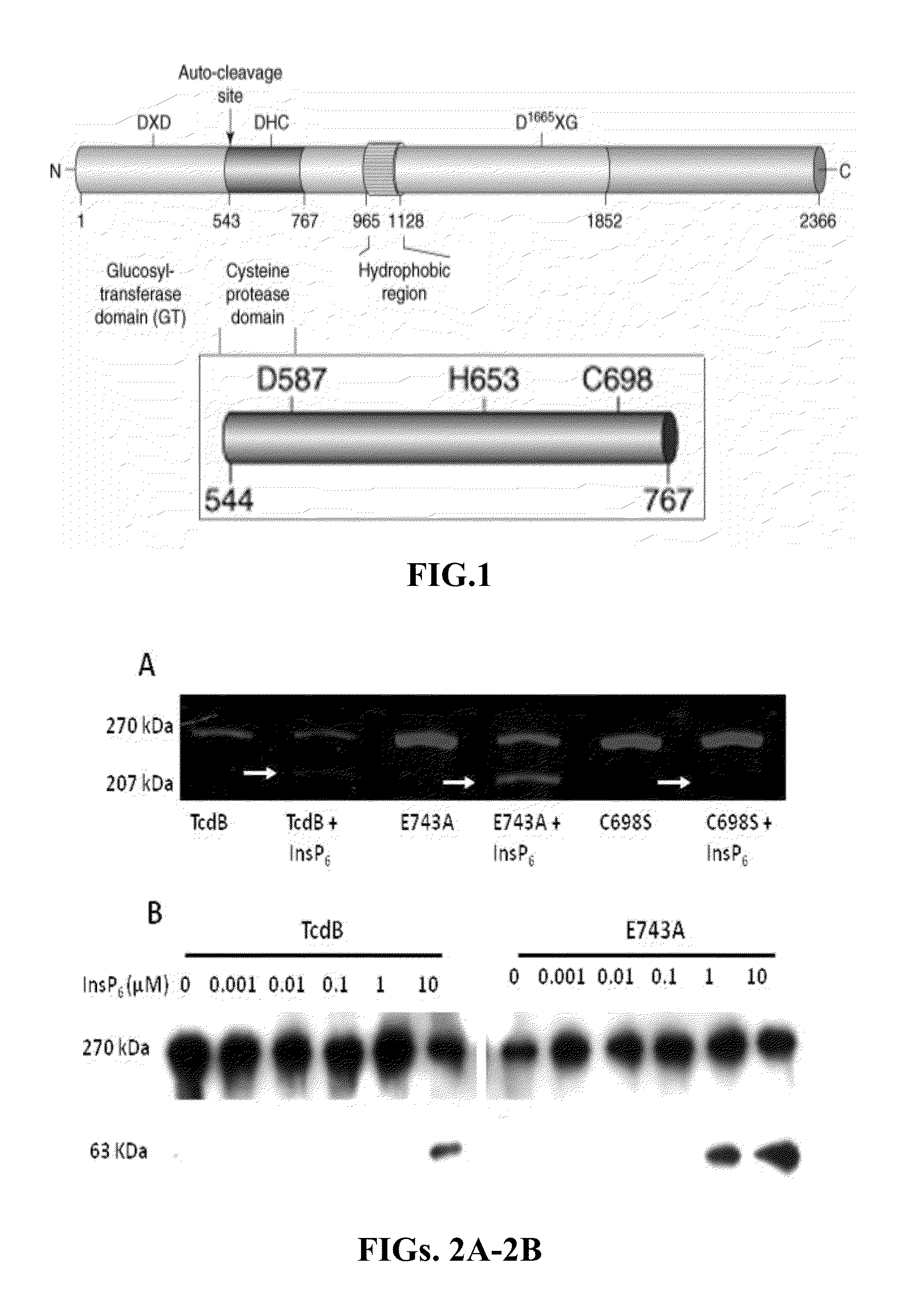
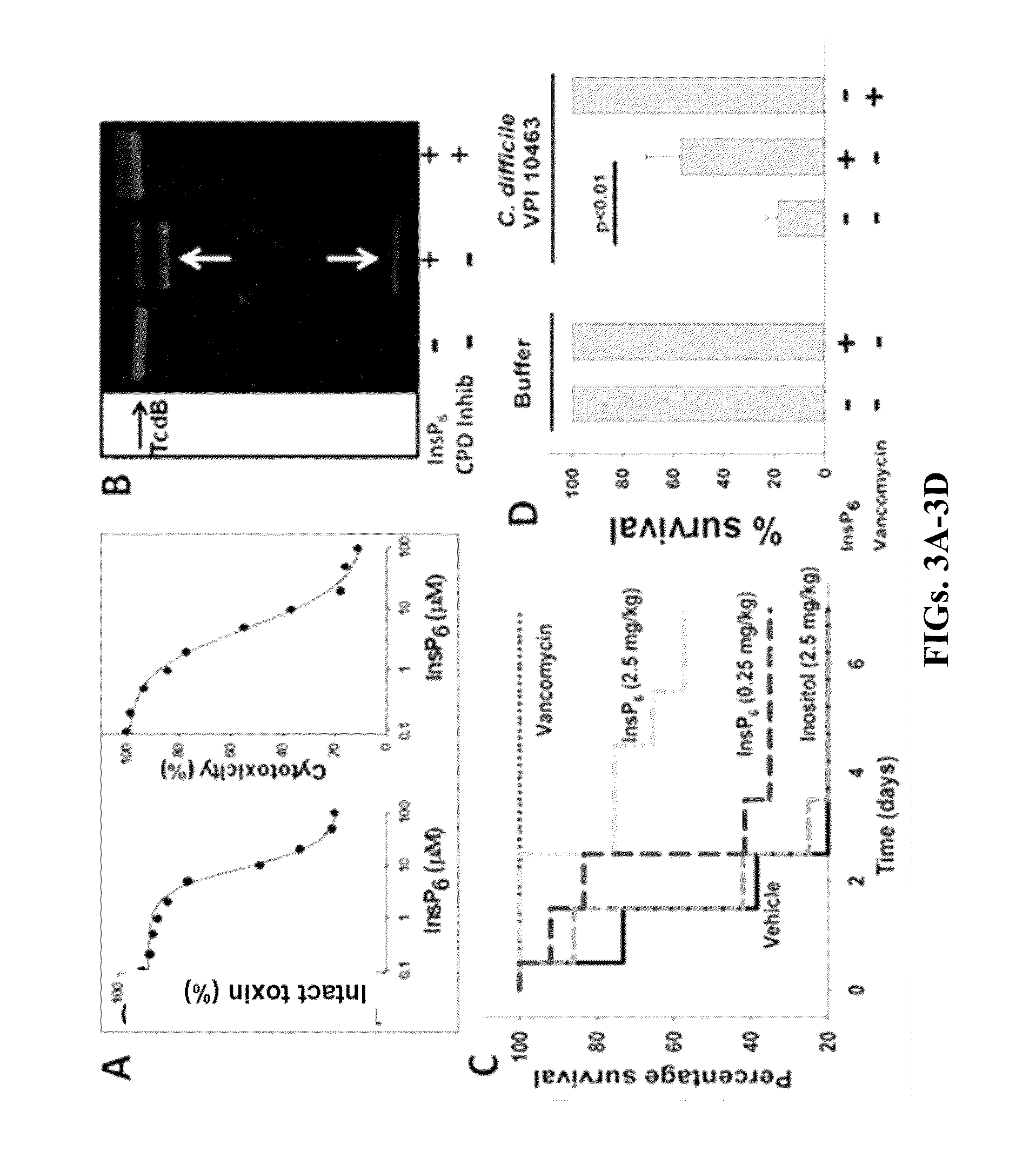
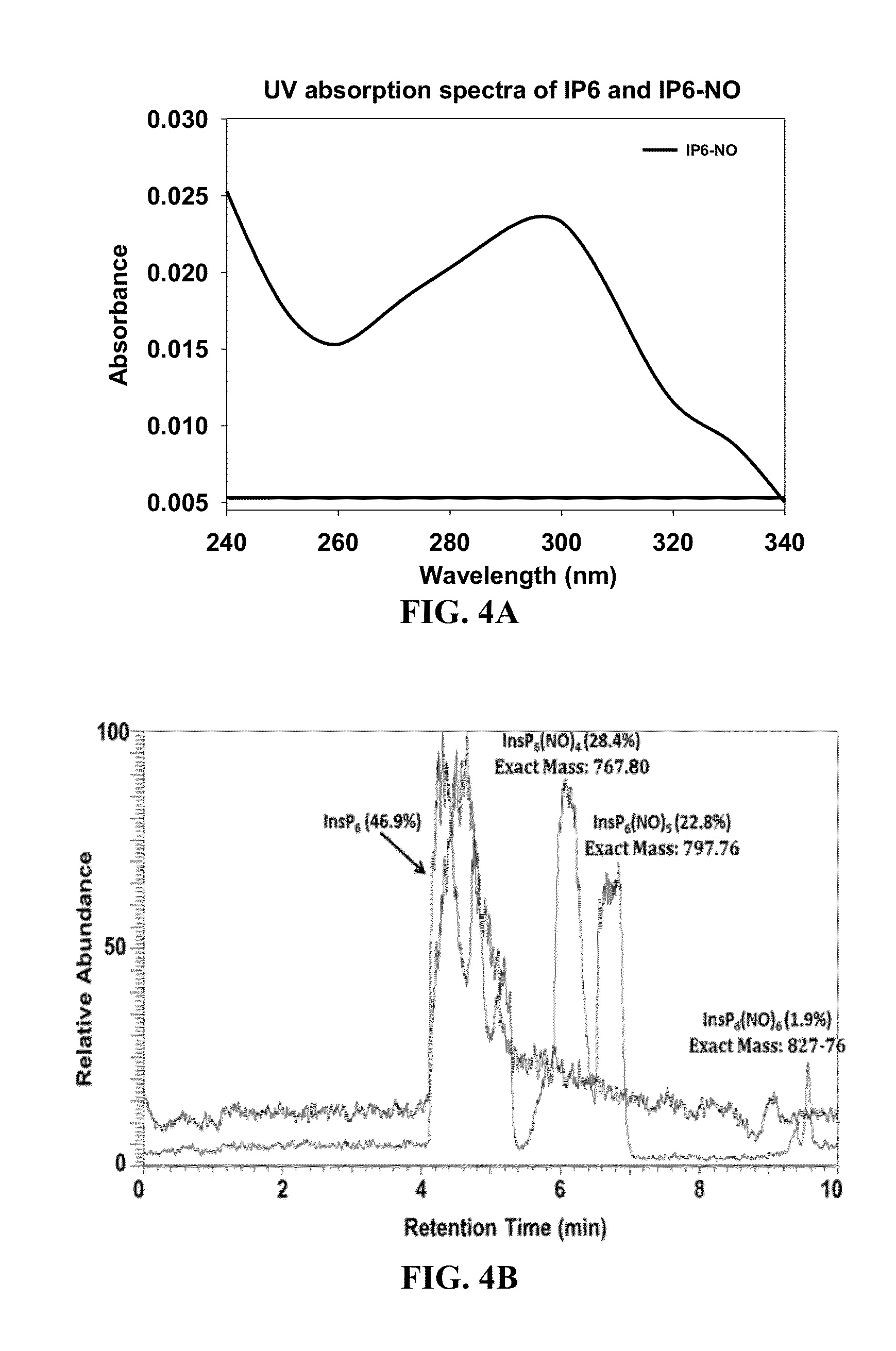
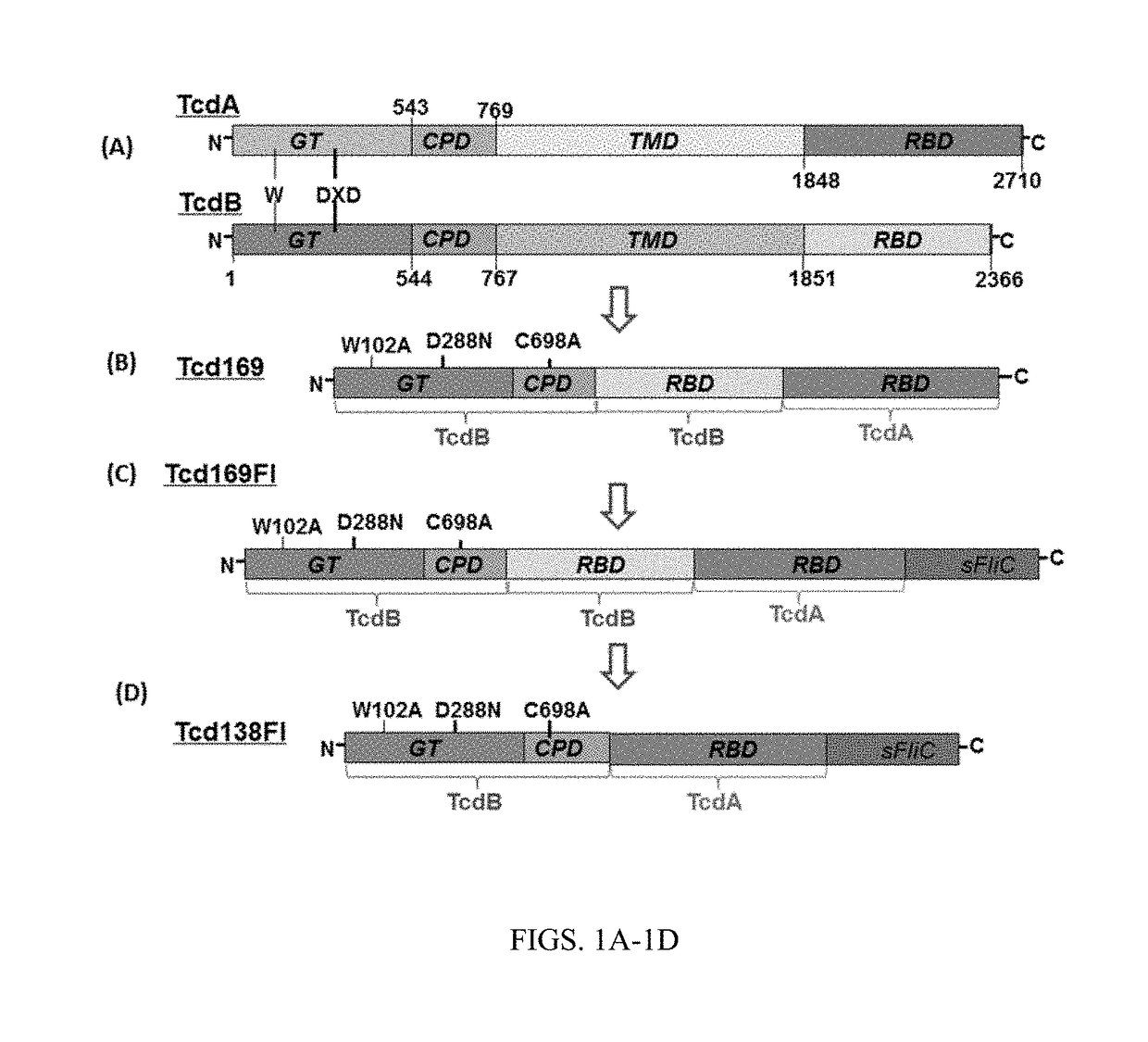
Inositol hexakisphosphate analogs and uses thereof
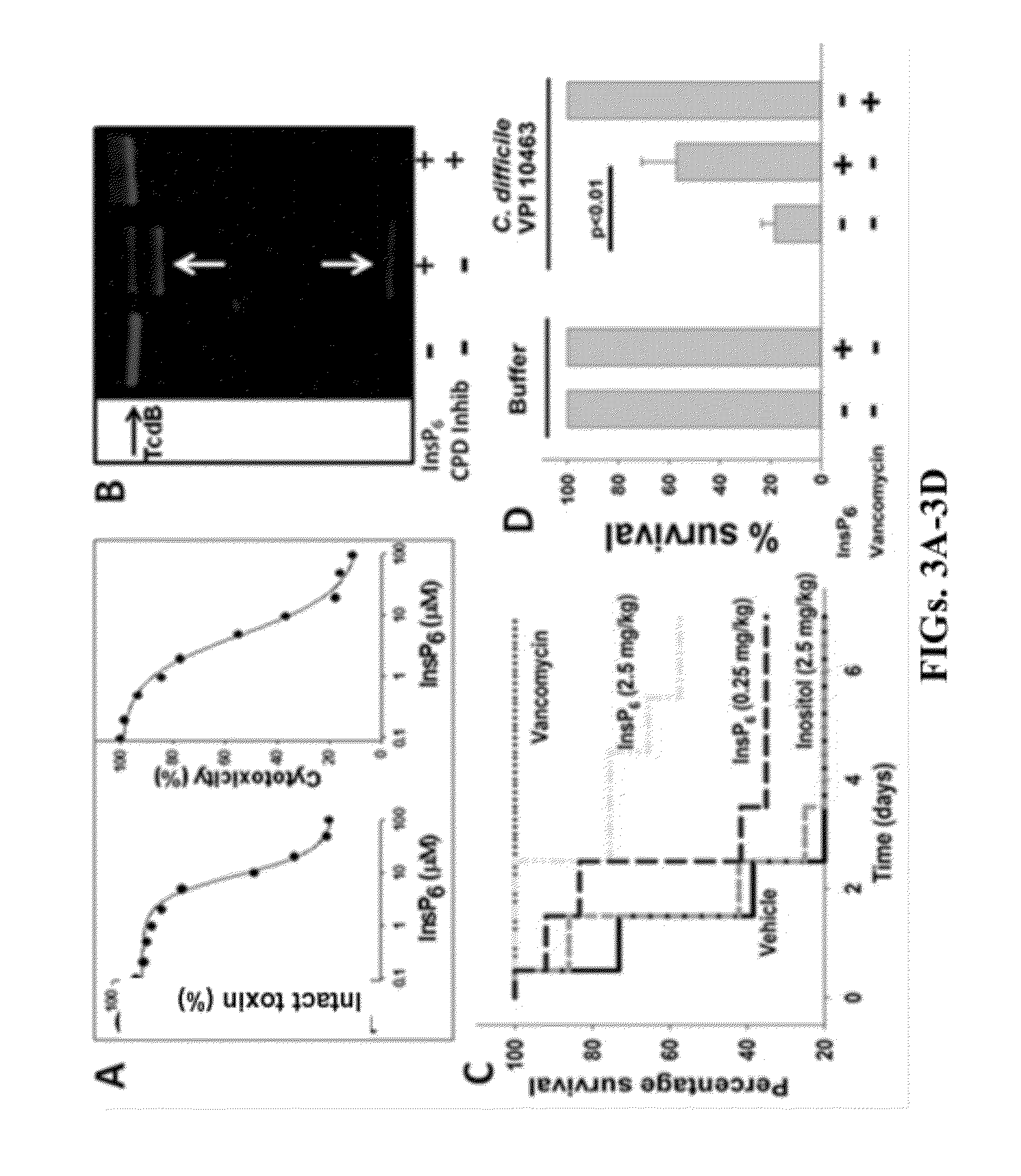
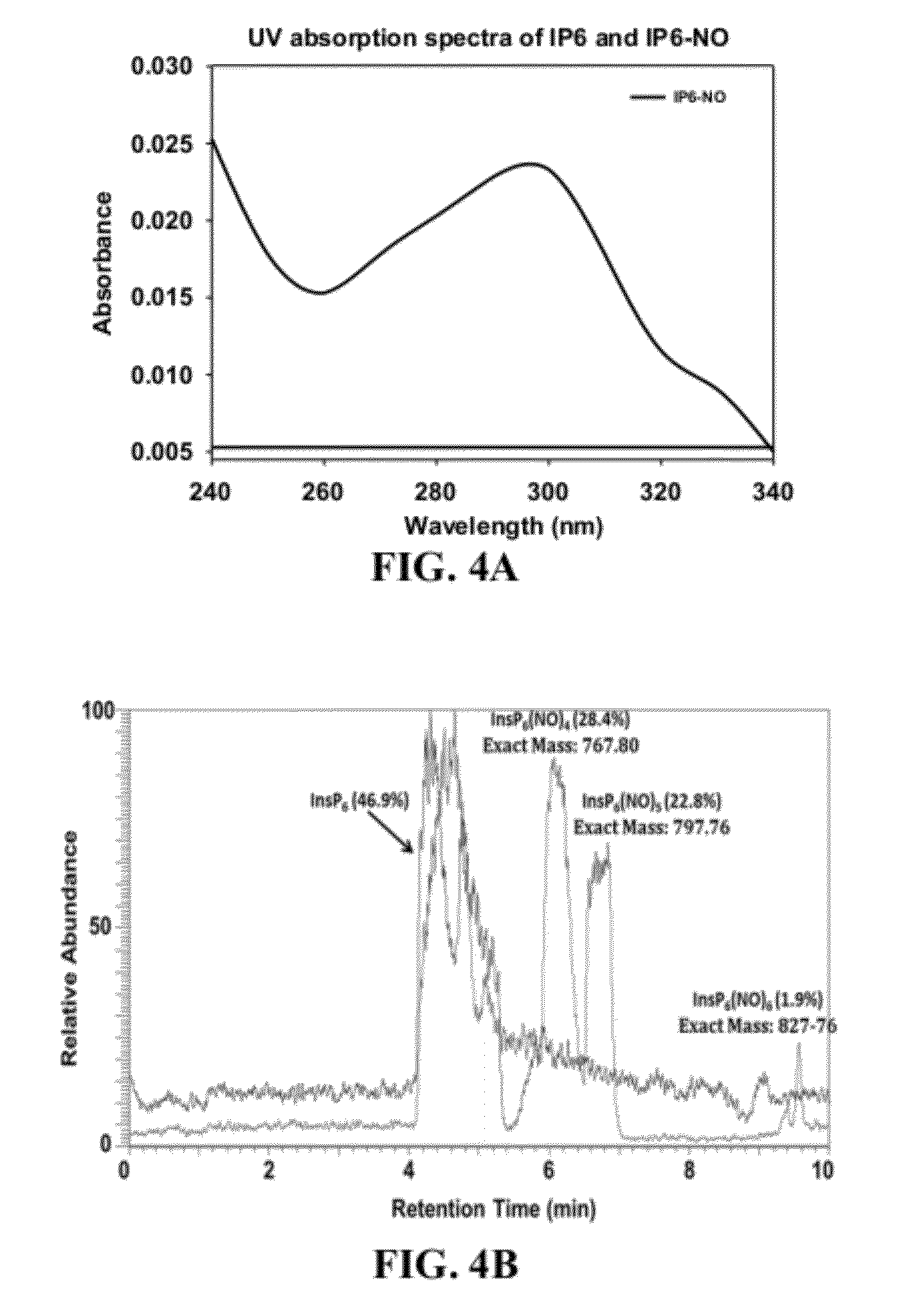
Provided herein are analog and derivative compounds of inositol hexakisphosphate effective to treat a Clostridium difficile infection and to neutralize the bacterial toxins produced by the same. In addition, methods of treating the C. difficile infection and for neutralizing its toxins with the compounds are provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
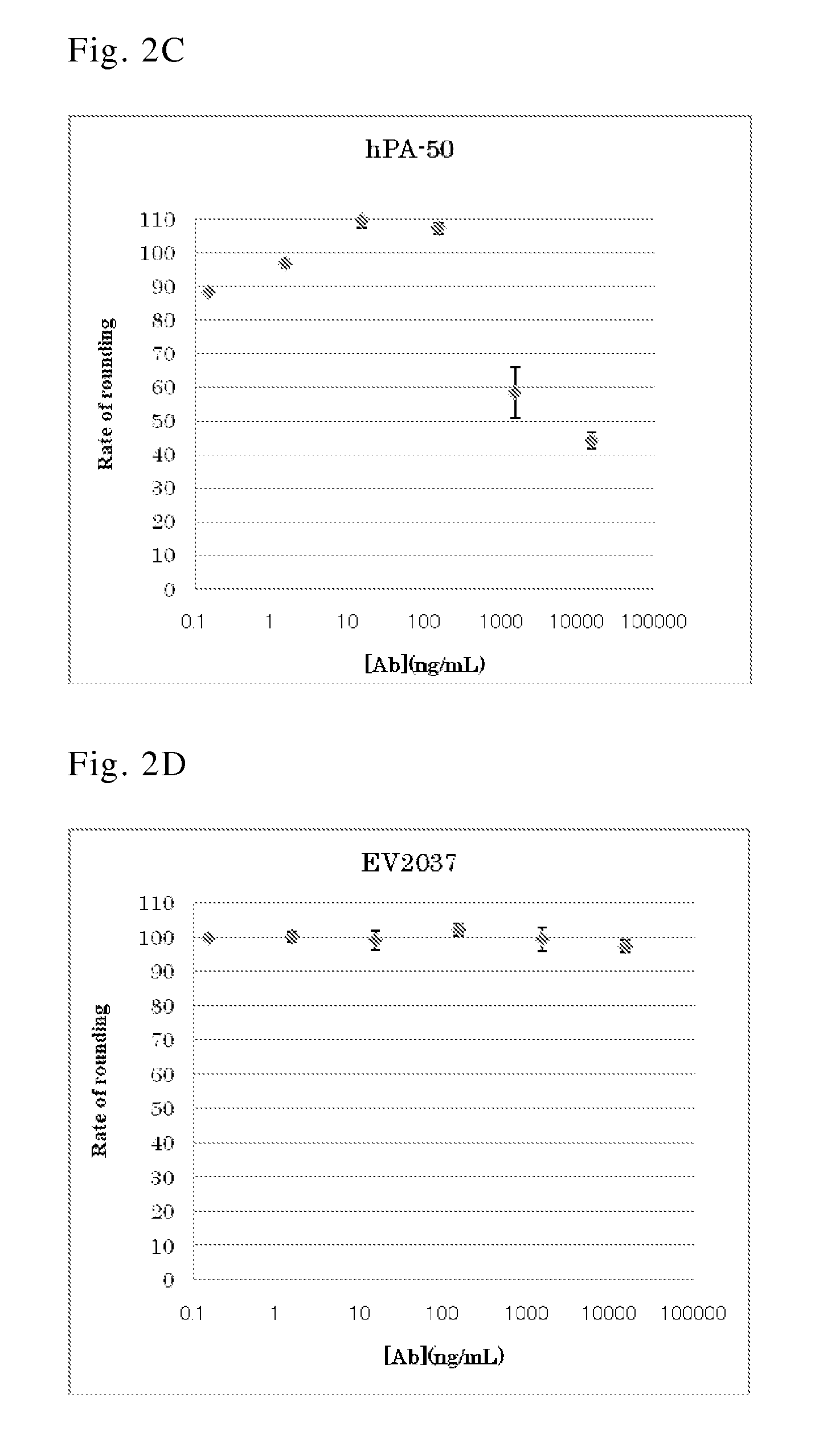
Human antibody specific to toxin produced from clostridium difficile, or antigen-binding fragment thereof
InactiveUS20150259402A1Low immunogenicityAnimal cellsBacterial antigen ingredientsClostridial infectionAntigen Binding Fragment
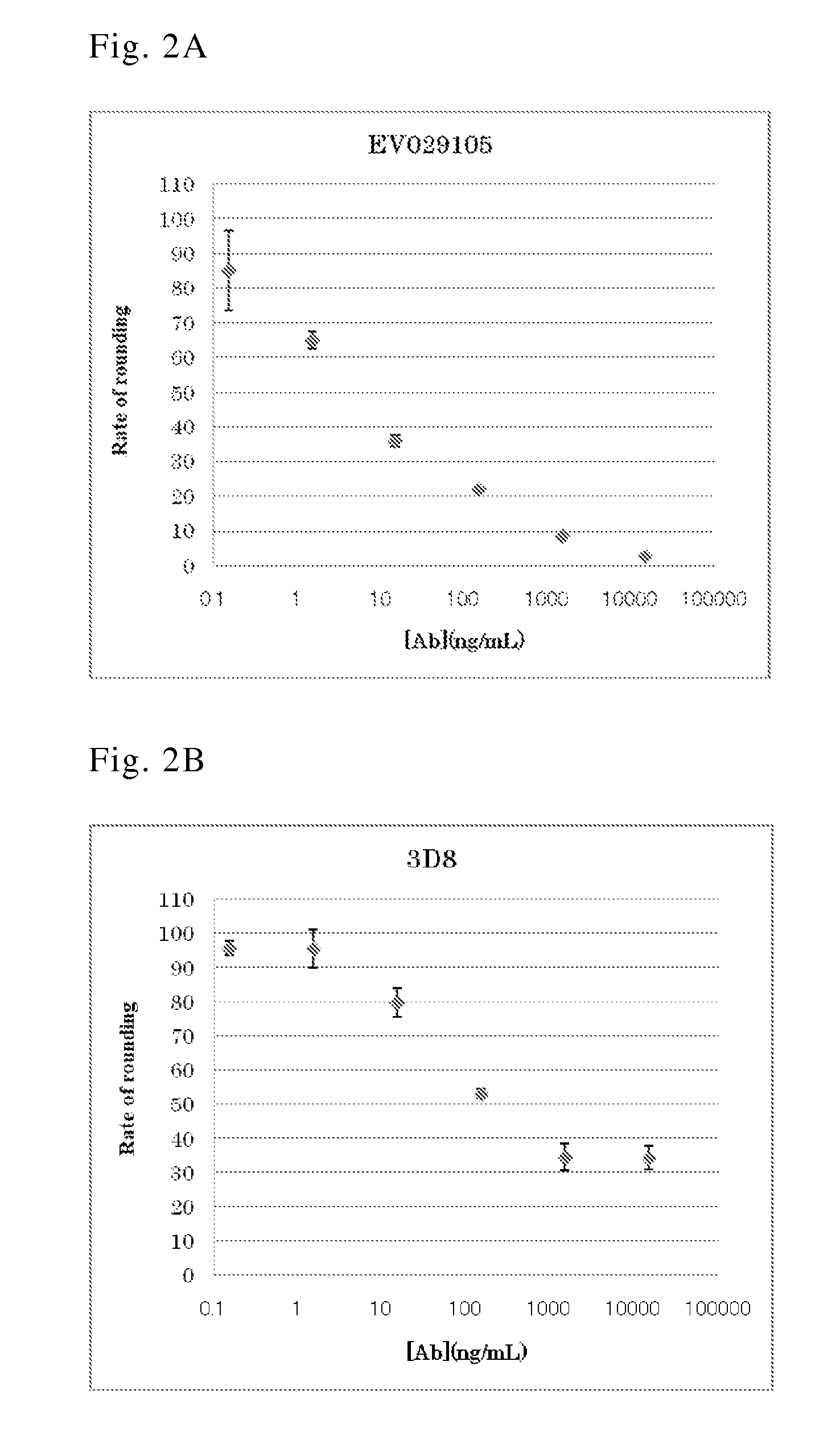
The present invention provides novel human-derived monoclonal antibodies specifically binding to toxins produced by Clostridium difficile (toxin A and toxin B), respectively, and having excellent neutralizing activity, and antigen-binding fragments thereof. The present invention also provides a pharmaceutical composition for the treatment of Clostridium difficile infection comprising any of the antibodies or antigen-binding fragments thereof.
Owner:EVEC
Methods and compositions for the treatment and/or prophylaxis of clostridium difficile associated disease
The present invention relates to methods and compositions for the treatment and / or prophylaxis of Clostridium difficile associated disease (CD AD). In particular, the invention relates to antibodies that bind to C. difficile antigens and are capable of inhibiting C. difficile infection, at least one symptom of C. difficile associated disease, shedding of C. difficile, and C. difficile associated mortality. The compositions of the present application comprise: mammalian or avian antibodies which bind to a C. difficile Toxin B; and mammalian or avian antibodies that bind to a C. difficile vegetative cell antigen and / or a C. difficile endospore antigen.
Owner:IMMURON LIMITED
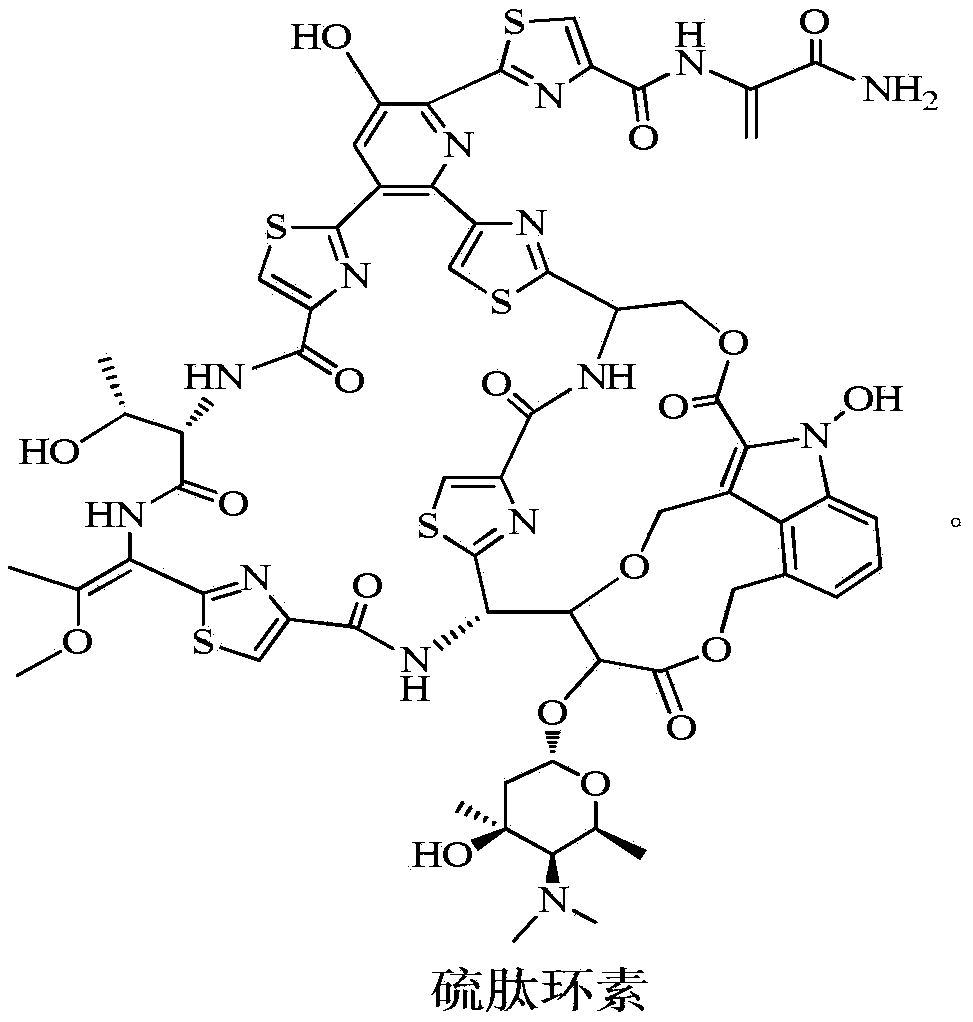
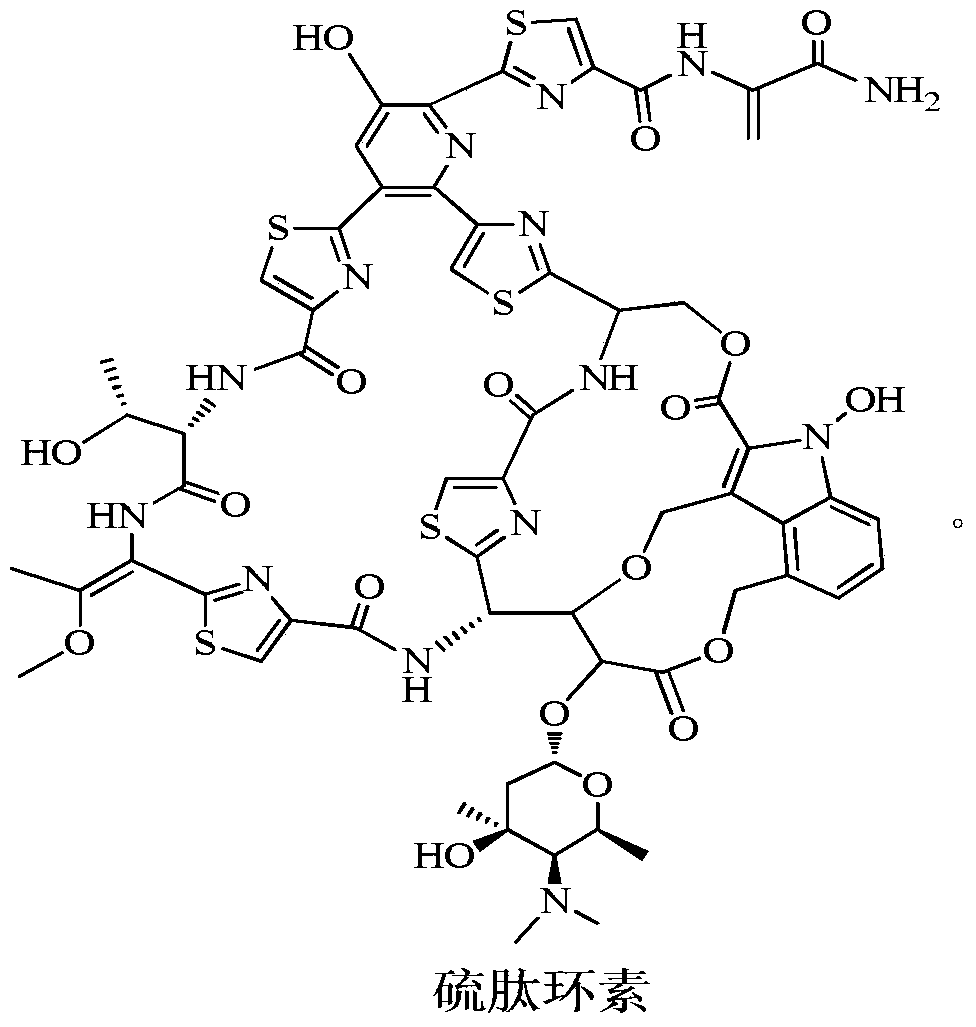
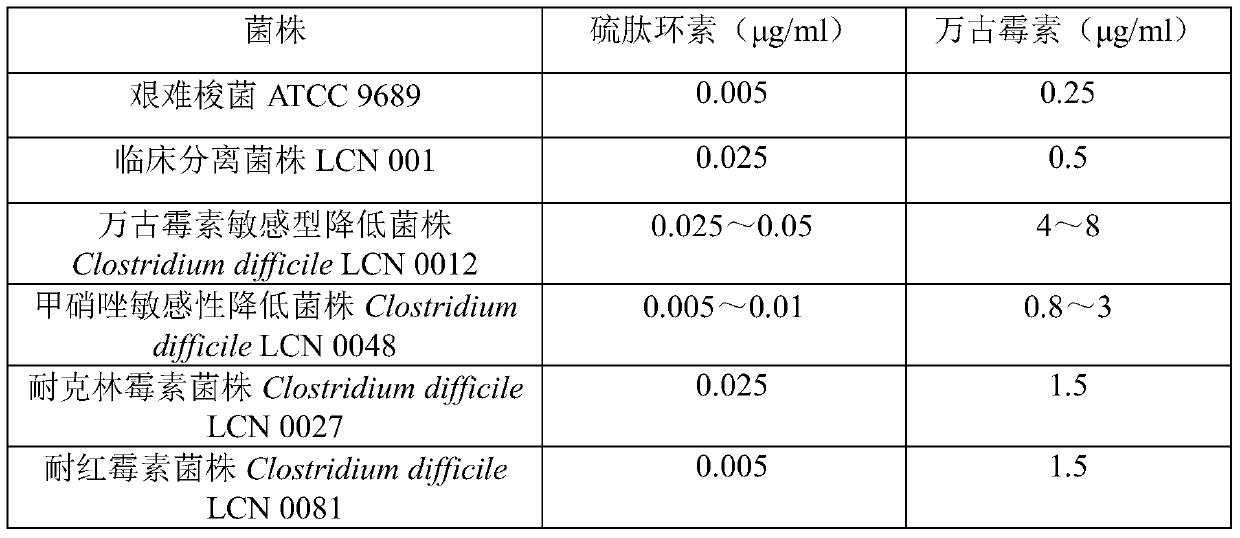
New application of thiopeptcin
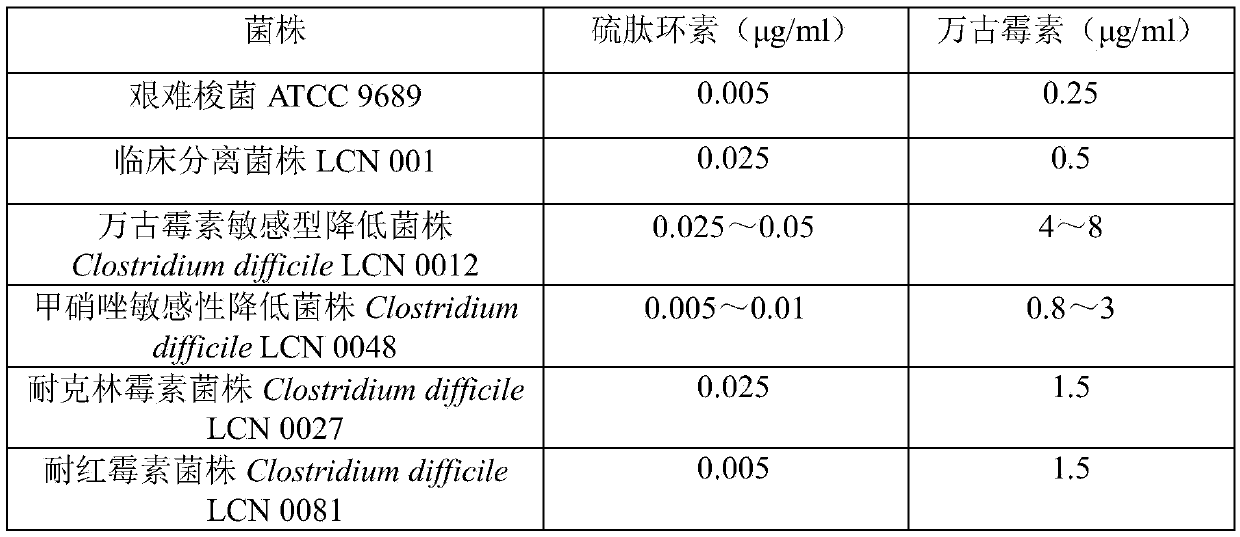
ActiveCN105363018AUnique structureNew mechanism of actionAntibacterial agentsPowder deliveryClostridial infectionDisease
The invention discloses new application of thiopeptcin to preparation of medicine for treating diseases caused by clostridium difficile infection and complications of the diseases. The thiopeptcin has evident activity on the clostridium difficile and the drug-resistant strains of the clostridium difficile and has great clinical application significance. In-vivo tests prove that the thiopeptcin does not enter blood circulation when a patient takes the thiopeptcin orally. According to the features of intestinal administration, the invention further provides a thiopeptcin orally-taken preparation containing a safe and effective dose and a preparing method of the thiopeptcin orally-taken preparation.
Owner:NANJING BIOTICA PHARMA
Methods and Compositions for Treating Clostridium difficile Associated Disease
InactiveUS20160143907A1Reduce additionalBiocidePharmaceutical delivery mechanismDiseaseClostridial infection
Methods for treating a subject infected with Clostridium difficile comprises administering to the subject a pharmaceutical composition comprising a therapeutically effective amount of clofazimine and / or a clofazimine analogue. Pharmaceutical compositions comprising clofazimine and / or an analogue(s) thereof, and optionally one or more additional therapeutic agents, are provided for treating Clostridium difficile infection and diseases or symptoms associated therewith.
Owner:KAMTEK INC
Compositions and methods for c. difficile treatment
InactiveUS20180000872A1CDI clearance rateAntibacterial agentsPowder deliveryClostridial infectionClostridium difficile
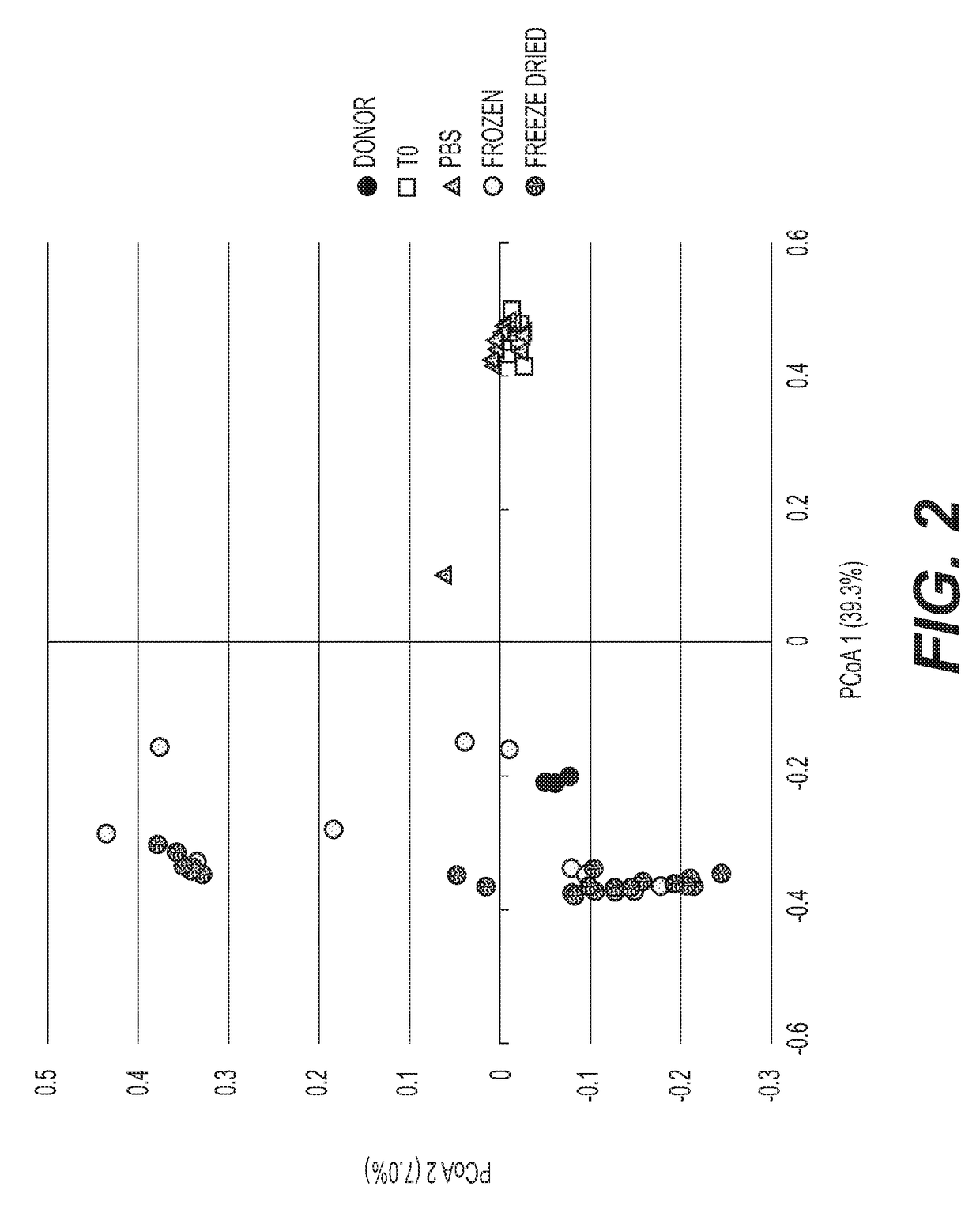
The present disclosure provides compositions and methods for treating Clostridium difficile infection (CDI) including primary and recurrent CDI. In particular, the compositions and methods described herein are capable of achieving a CDI clearance rate of at least 80% through a single oral dose of a pharmaceutical composition comprising a freeze-dried fecal microbiota preparation.
Owner:RGT UNIV OF MINNESOTA
Methods for treating or inhibiting infection by clostridium difficile
InactiveUS20130129695A1Characteristic is differentInhibit expressionBiocideMicrobiological testing/measurementClostridial infectionMedicine
The invention provides methods for treating or inhibiting infection by Clostridium difficile in a subject in need of such treatment, comprising administering an effective amount of a compound binding to a CD3299 riboswitch, as well as assays for identifying compounds useful in such treatment, and the use of particular compounds in such treatment.
Owner:BLOUNT KENNETH
Inositol hexakisphosphate analogs and uses thereof
Provided herein are analog and derivative compounds of inositol hexakisphosphate effective to treat a Clostridium difficile infection and to neutralize the bacterial toxins produced by the same. In addition, methods of treating the C. difficile infection and for neutralizing its toxins with the compounds are provided.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Immunogenic proteins against clostridium difficile
ActiveUS20180344834A1Antibacterial agentsPolypeptide with localisation/targeting motifClostridial infectionClostridium difficile toxin B
Described are immunogenic proteins against Clostridium difficile. Also described are compositions comprising the immunogenic proteins. Further described are methods of preventing or treating a Clostridium difficile infection in a subject in need thereof.
Owner:UNIV OF SOUTH FLORIDA
Application of fresh ginger heart fire-removing decoction in curing diarrhoea caused by clostridium difficile infection
ActiveCN108478757AAlleviate drynessEmbodies multiple componentsDigestive systemSaccharide peptide ingredientsClostridial infectionClinical efficacy
The invention belongs to the technical field of medicines, and particularly relates to application of fresh ginger heart fire-removing decoction in curing diarrhoea caused by clostridium difficile infection. According to the application of the fresh ginger heart fire-removing decoction in curing diarrhoea caused by clostridium difficile infection, based on the existing pharmacological activity ofthe fresh ginger heart fire-removing decoction, through a large number of experiments and in-depth studies, new clinical efficacy of the fresh ginger heart fire-removing decoction is screened, and experimental results show that the fresh ginger heart fire-removing decoction has the application in curing diarrhoea caused by clostridium difficile infection.
Owner:INST OF CHINESE MATERIA MEDICA CHINA ACAD OF CHINESE MEDICAL SCI
Medicine for restraining clostridium difficile thalli and/or restraining germination of clostridium difficile spores
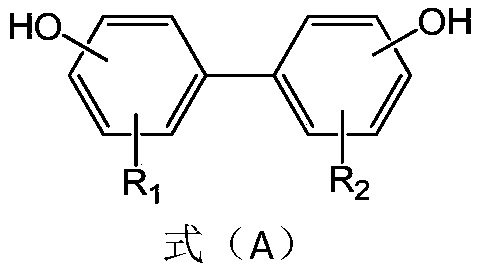
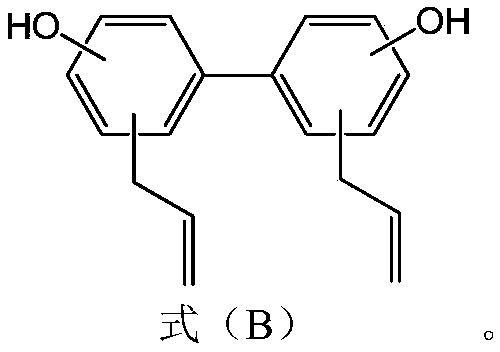
PendingCN110478335APrevention and/or treatment of relapsePrevention and/or treatment of complicationsAntibacterial agentsHydroxy compound active ingredientsSporeClostridial infection
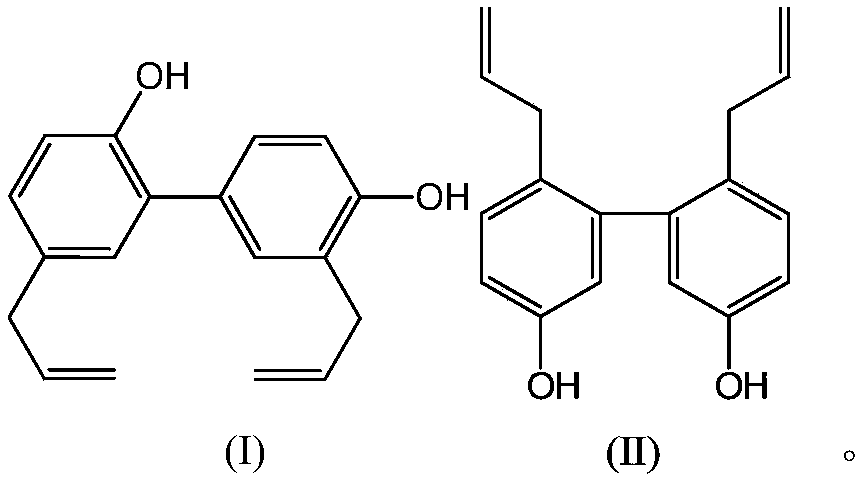
The invention provides an application of a compound as shown in the formula A to preparation of a medicine for restraining clostridium difficile thalli and / or restraining germination of clostridium difficile spores. R1 and R2 are independently selected from C1-3 alkyl group, C2-3 alkenyl and C2-3 alkynyl. Experiment proves that the compound as shown in the formula A, particularly magnolol and honokiol can notably restrain activity of clostridium difficile, can notably restrain germination of clostridium difficile spores, and has good application prospects for preparation of the medicine for preventing and / or treating clostridium difficile infection diseases, recurrence of clostridium difficile infection diseases, and complications of clostridium difficile infection diseases.
Owner:CHENGDU BIOBEL BIOTECHNOLOGY CO LTD
Clostridium difficile antigens
ActiveUS20160319037A1Stimulate immune responseReadily manufactured in large quantitiesAntibacterial agentsBacterial antigen ingredientsClostridial infectionAntigen
Owner:SEC OF STATE FOR HEALTH & SOCIAL CARE
Methods and compositions for the treatment and/or prophylaxis of Clostridium difficile associated disease
The present invention relates to methods and compositions for the treatment and / or prophylaxis of Clostridium difficile associated disease (CD AD). In particular, the invention relates to antibodies that bind to C. difficile antigens and are capable of inhibiting C. difficile infection, at least one symptom of C. difficile associated disease, shedding of C. difficile, and C. difficile associated mortality. The compositions of the present application comprise: mammalian or avian antibodies which bind to a C. difficile Toxin B; and mammalian or avian antibodies that bind to a C. difficile vegetative cell antigen and / or a C. difficile endospore antigen.
Owner:IMMURON LIMITED
Compositions comprising recombinant probiotic bacteria and methods of use thereof
ActiveUS20180000919A1Easy to manageAntibacterial agentsBacterial antigen ingredientsClostridial infectionClostridium difficile infections
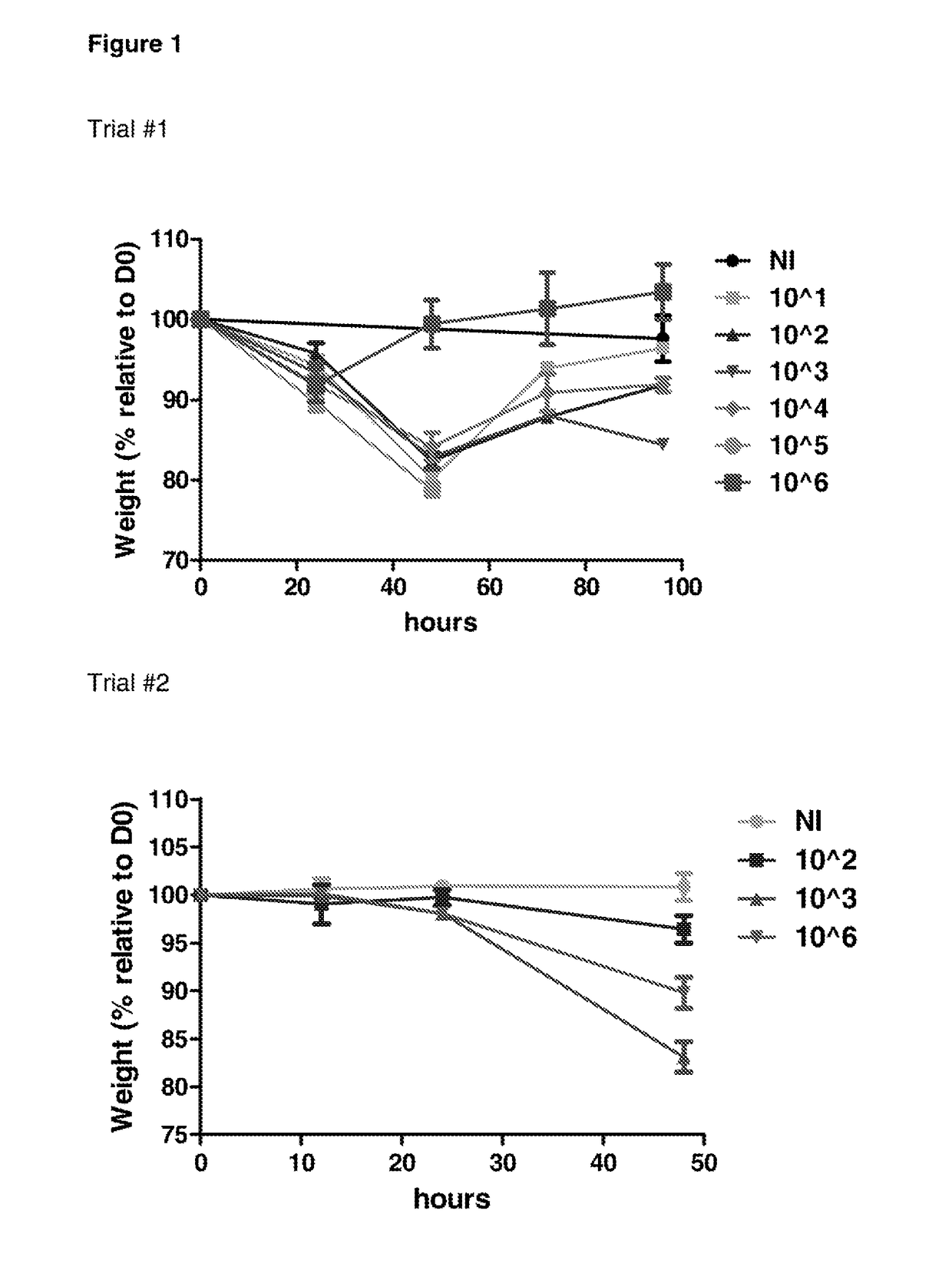
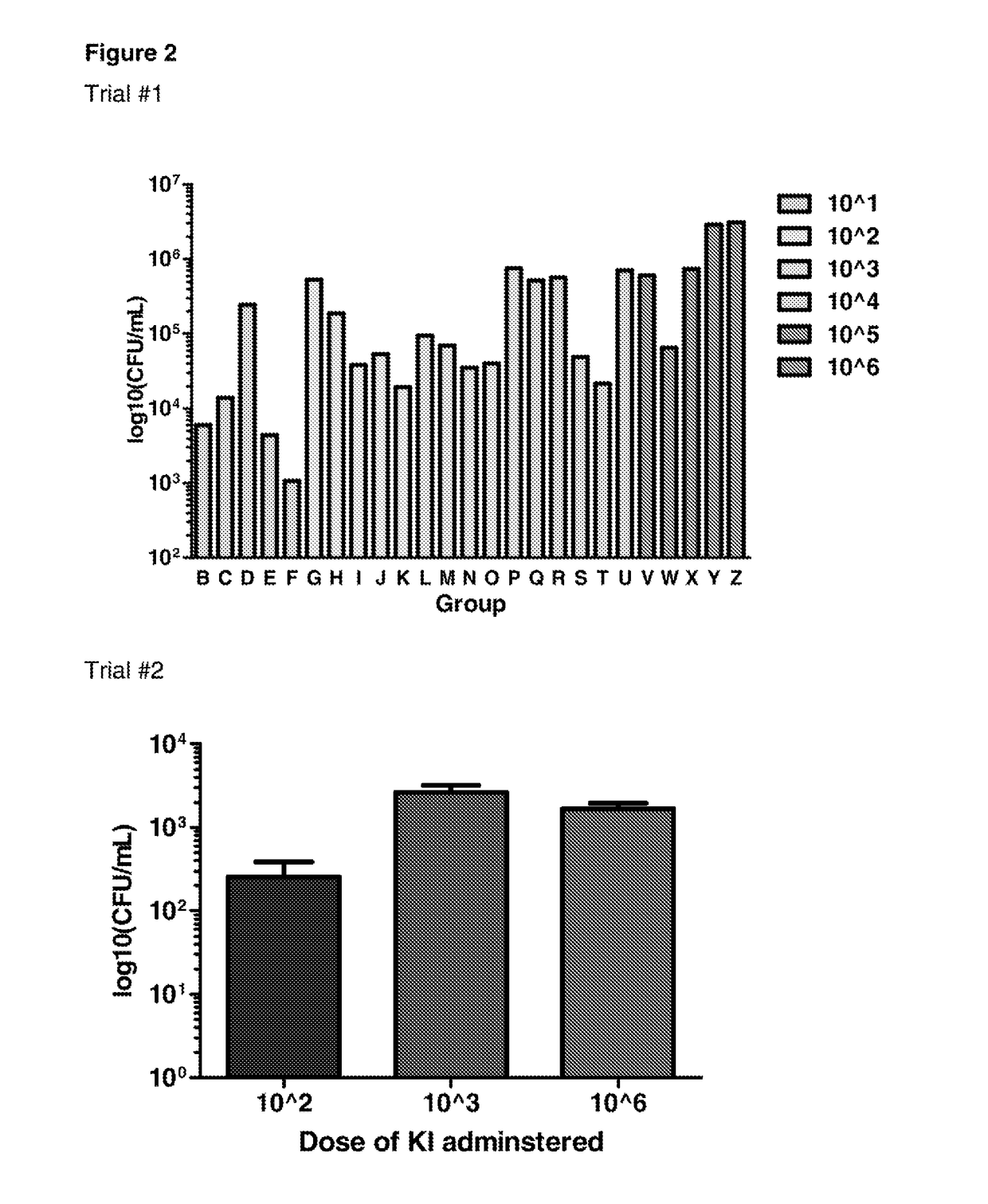
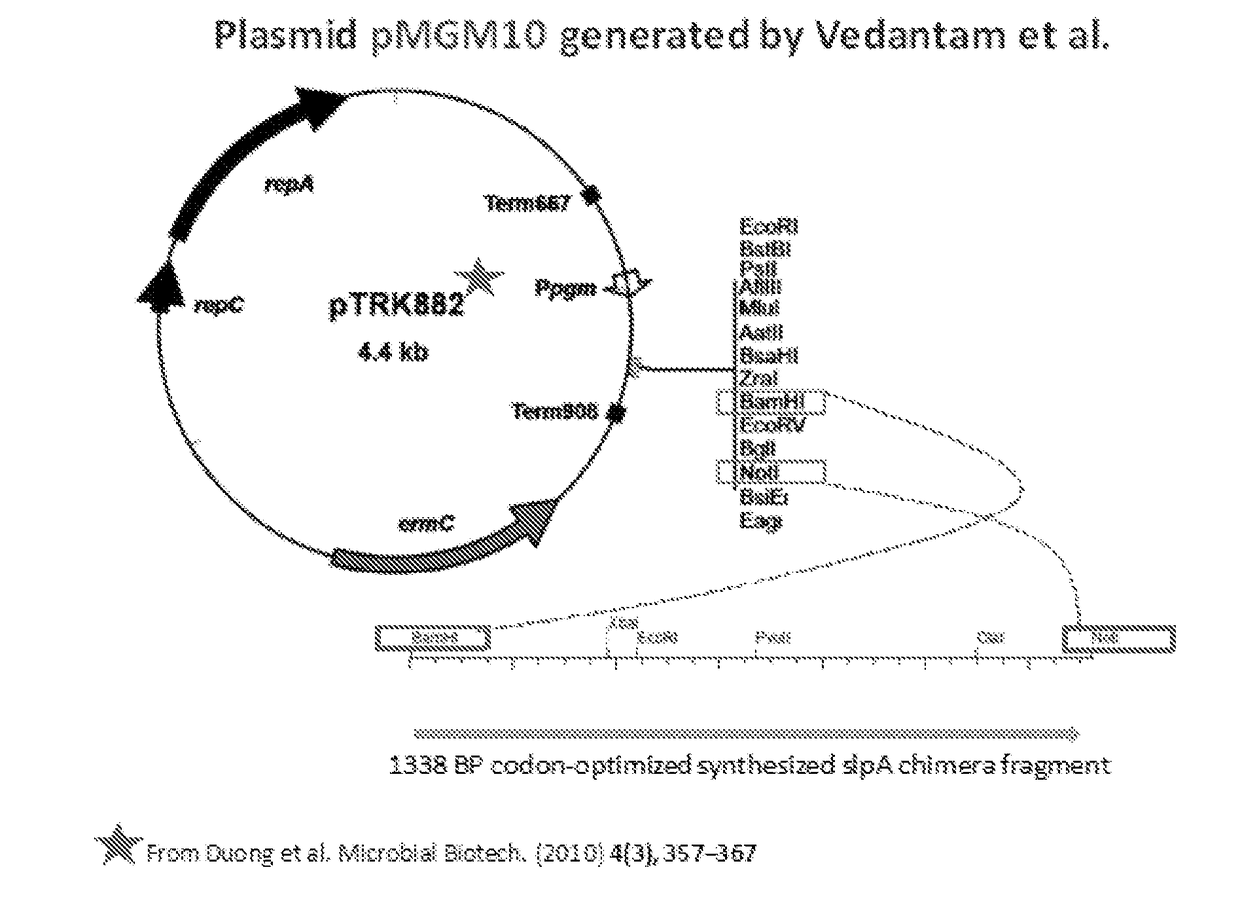
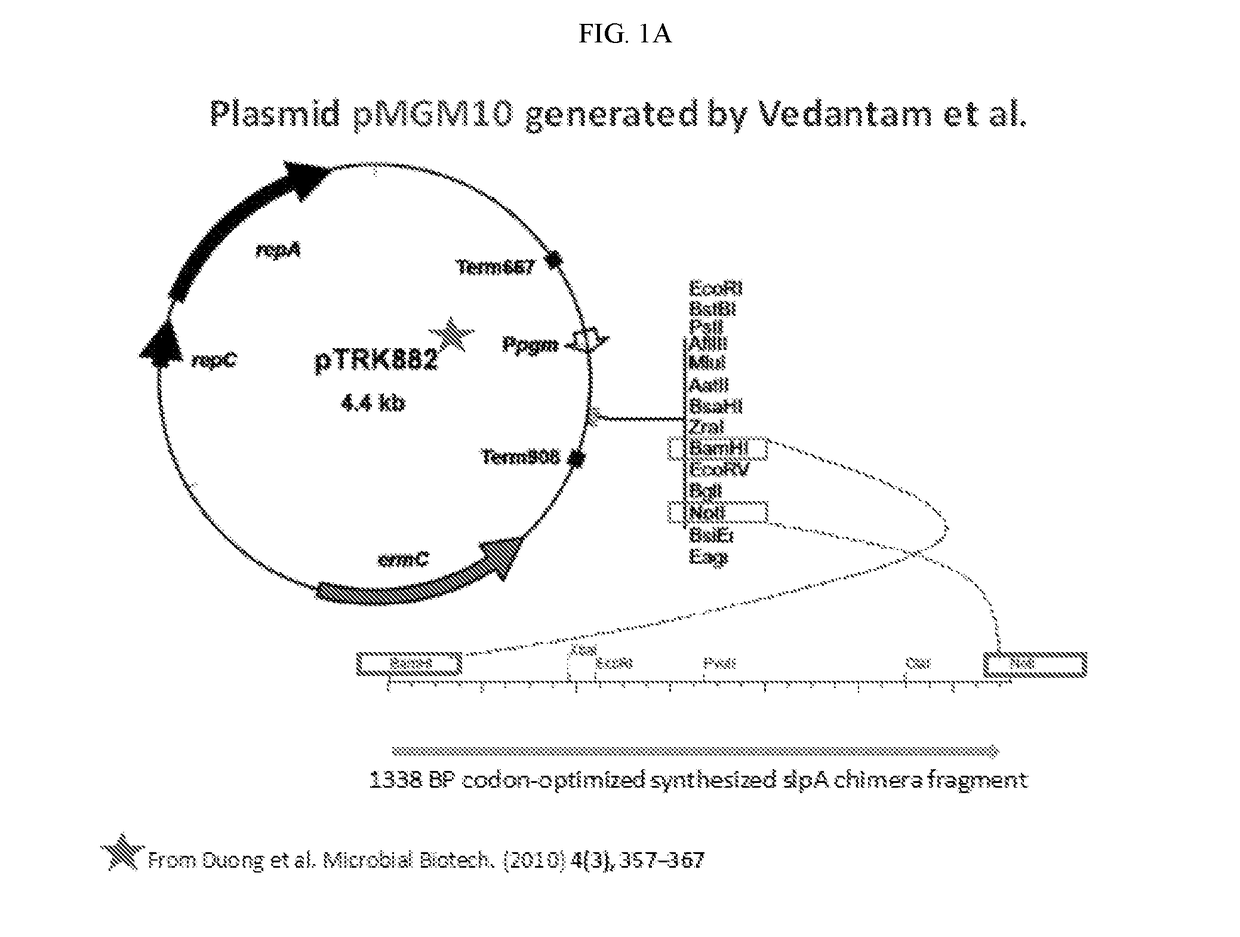
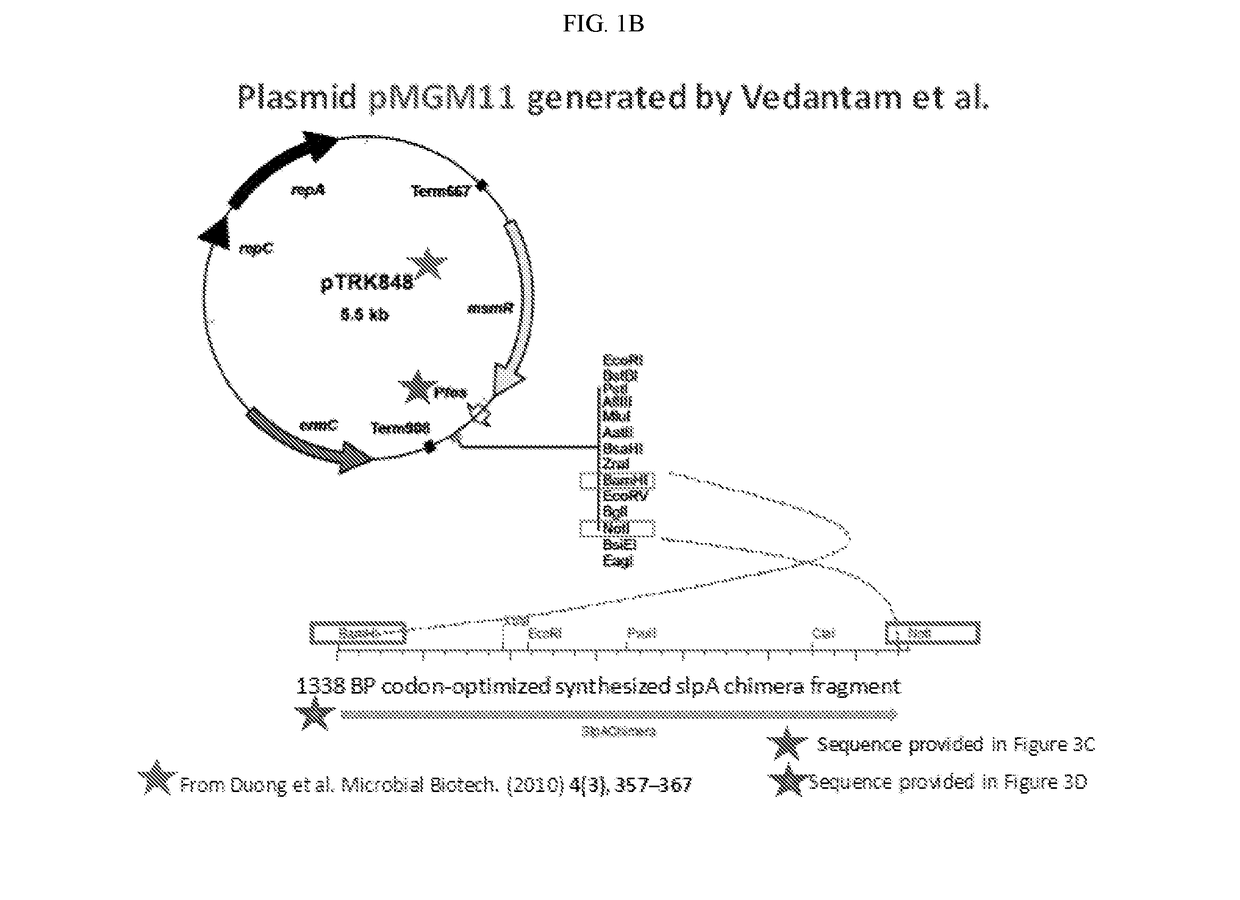
The invention features probiotic bacteria expressing Clostridium difficile SlpA, or fragment thereof, and its use for the treatment or prevention of Clostridium difficile infection and gut colonization.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Compositions and methods for treating clostridium difficile infection
ActiveUS20190290732A1Reduce mortalityReduce severityAntibacterial agentsOrganic active ingredientsClostridial infectionAntibiotic-associated diarrhoea
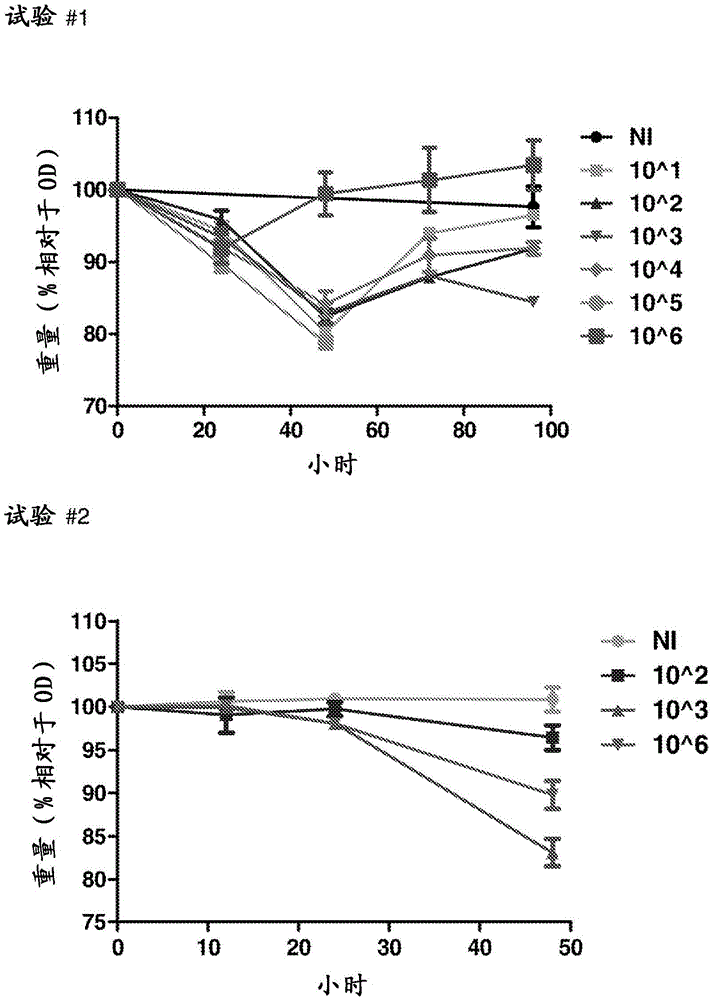
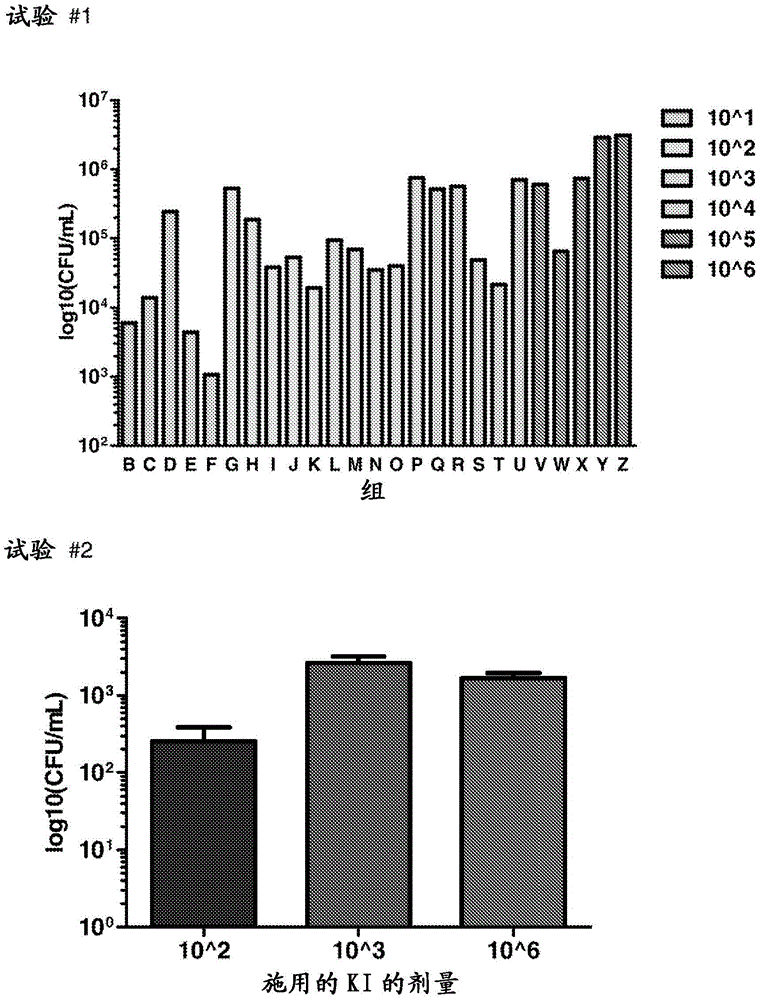
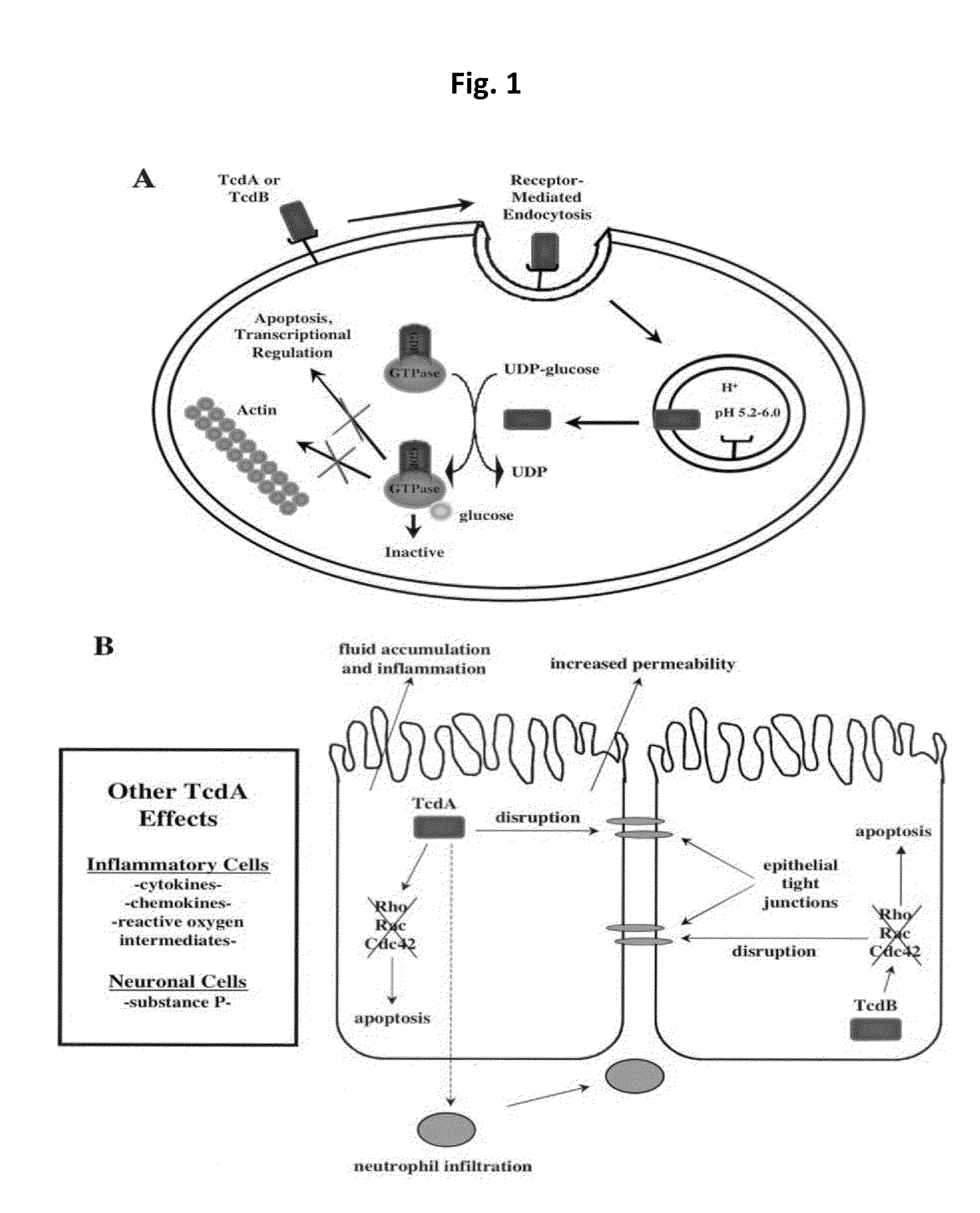
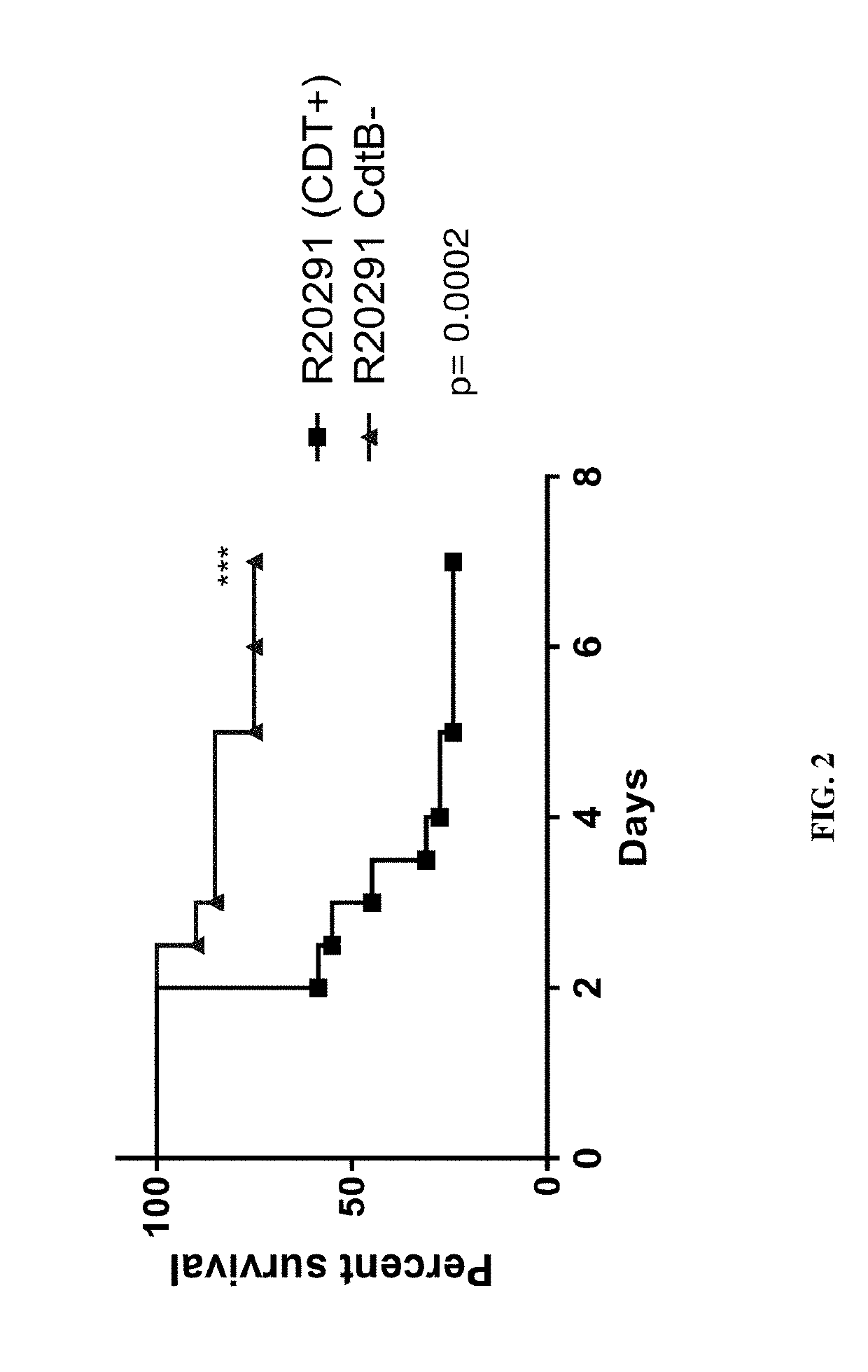
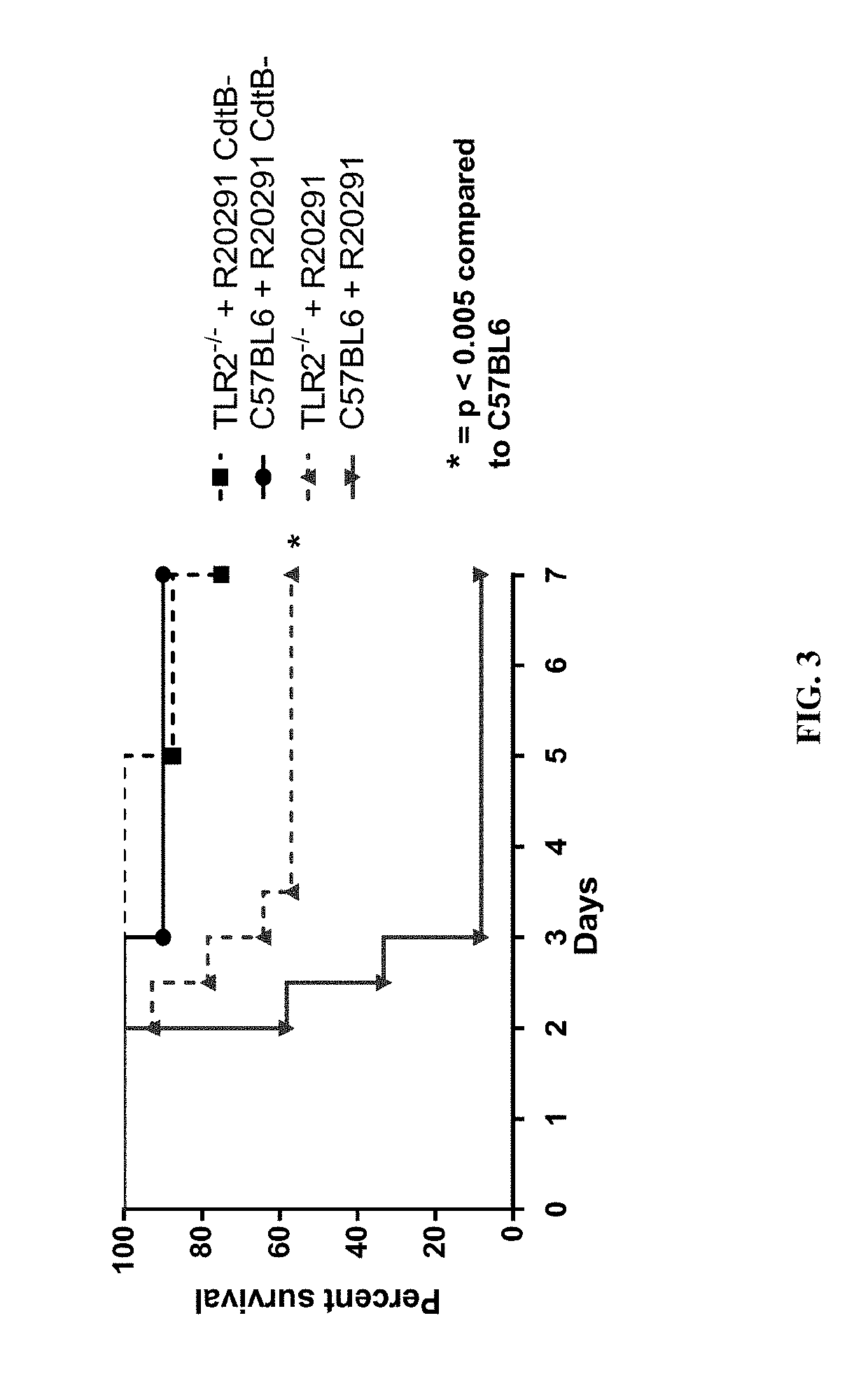
Clostridium difficile infection is the leading cause of hospital acquired antibiotic-associated diarrhea in the US (Bartlett, in 2006). The increased prevalence of circulating C. difficile strains poses a significant health threat to US health care facilities. Strains expressing the toxin C. difficile Transferase (CDT), in addition to Toxins A and B (TcdA and TcdB), are more virulent and are associated with higher mortality rates (Bacci et al., 2011). We recently identified a protective role for eosinophils against C. difficile pathogenesis (Buonomo et al., 2016). We have also defined CDT's ability to increase host inflammation and suppress protective eosinophils through a TLR2 dependent mechanism (Cowardin et al., 2016). How CDT promotes virulence and eosinophil suppression via TLR2 is still under investigation. We employed a genome-wide microarray approach to reveal divergent transcriptional profiles between protected (TLR2− / −) and unprotected (WT) mice infected with either CDT expressing or CDT mutant strains of C. difficile. This work revealed novel host mediated TLR2-dependent inflammatory pathways to CDT. We provide an unbiased framework for understanding the host immune response to the binary toxin CDT produced by C. difficile and how TLR2 signaling enhances virulence.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Prevention of Clostridium Difficile Infection in High Risk Patients
InactiveUS20150157653A1Avoid problemsPrevent proliferationBiocideCarbohydrate active ingredientsClostridial infectionClostridium difficile infections
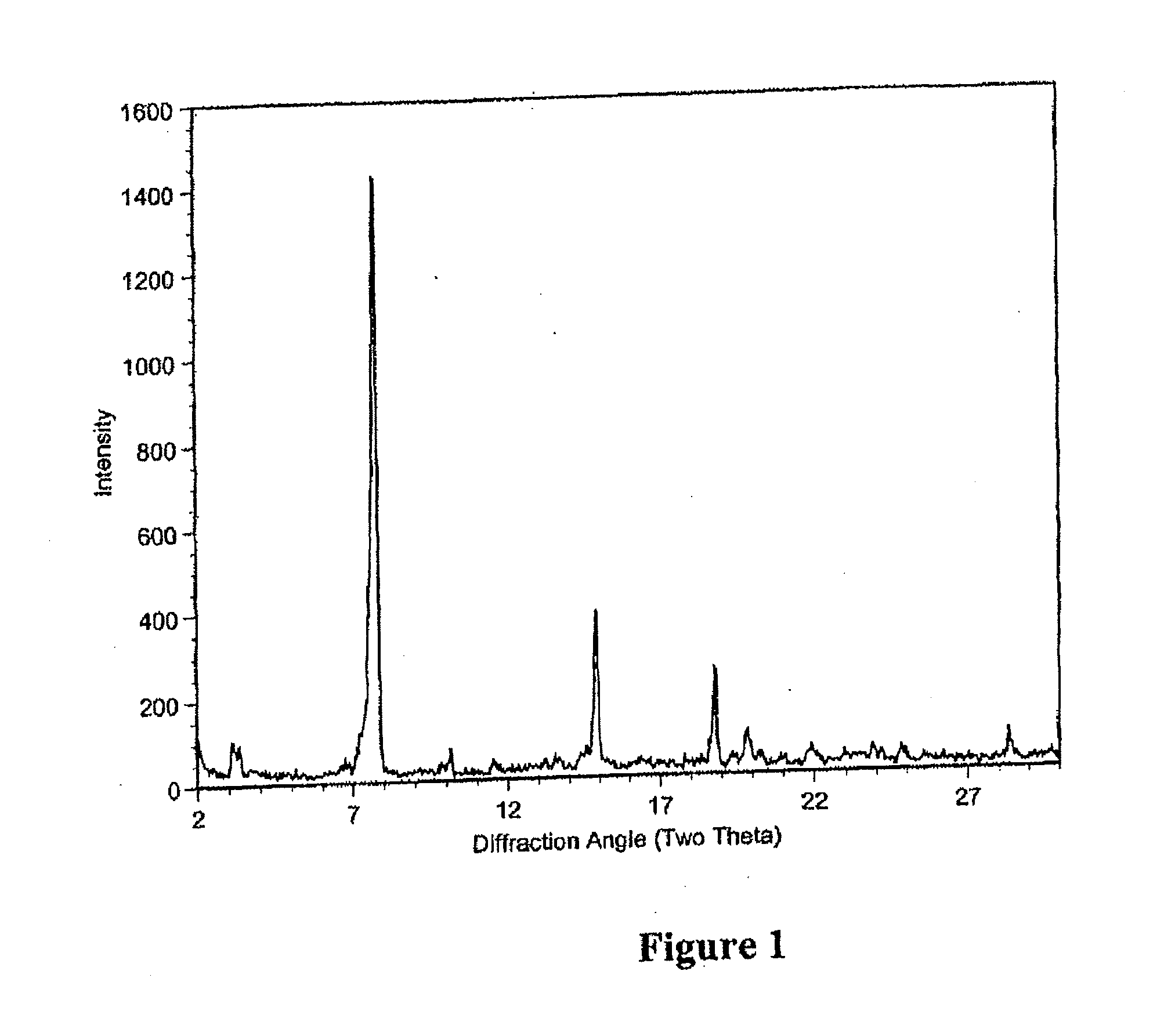
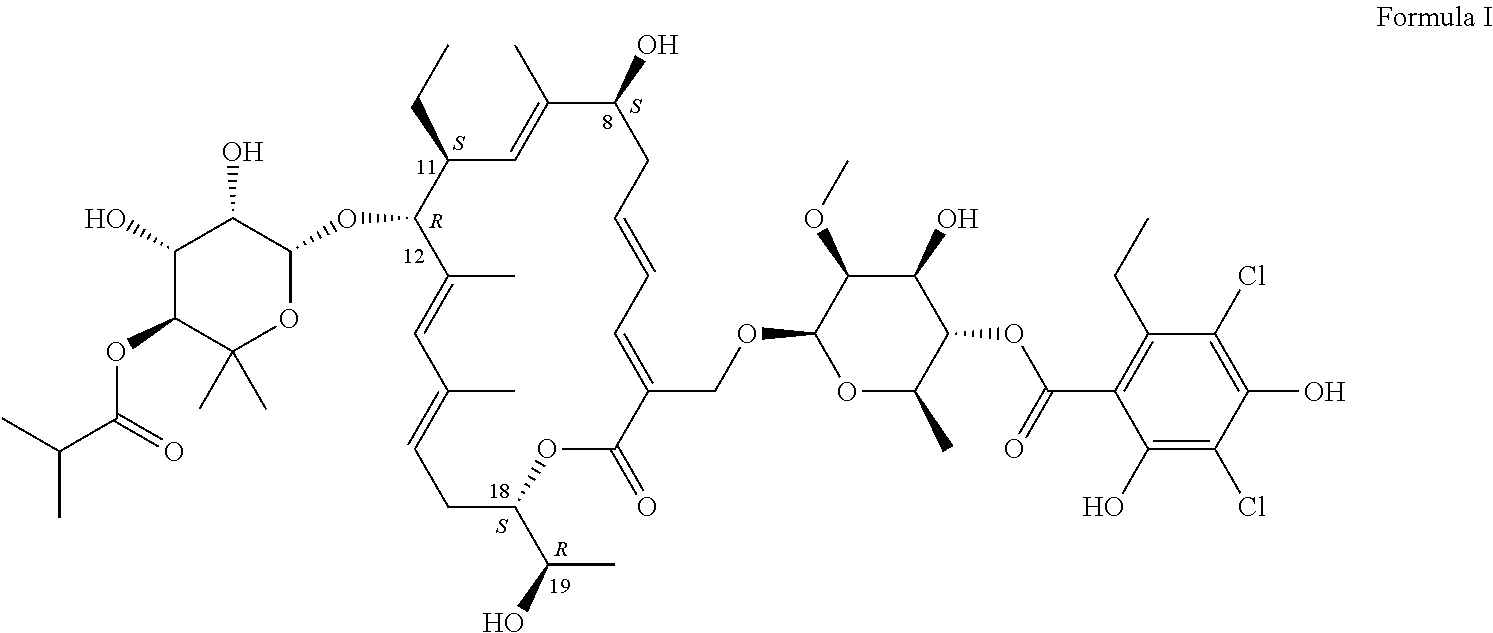
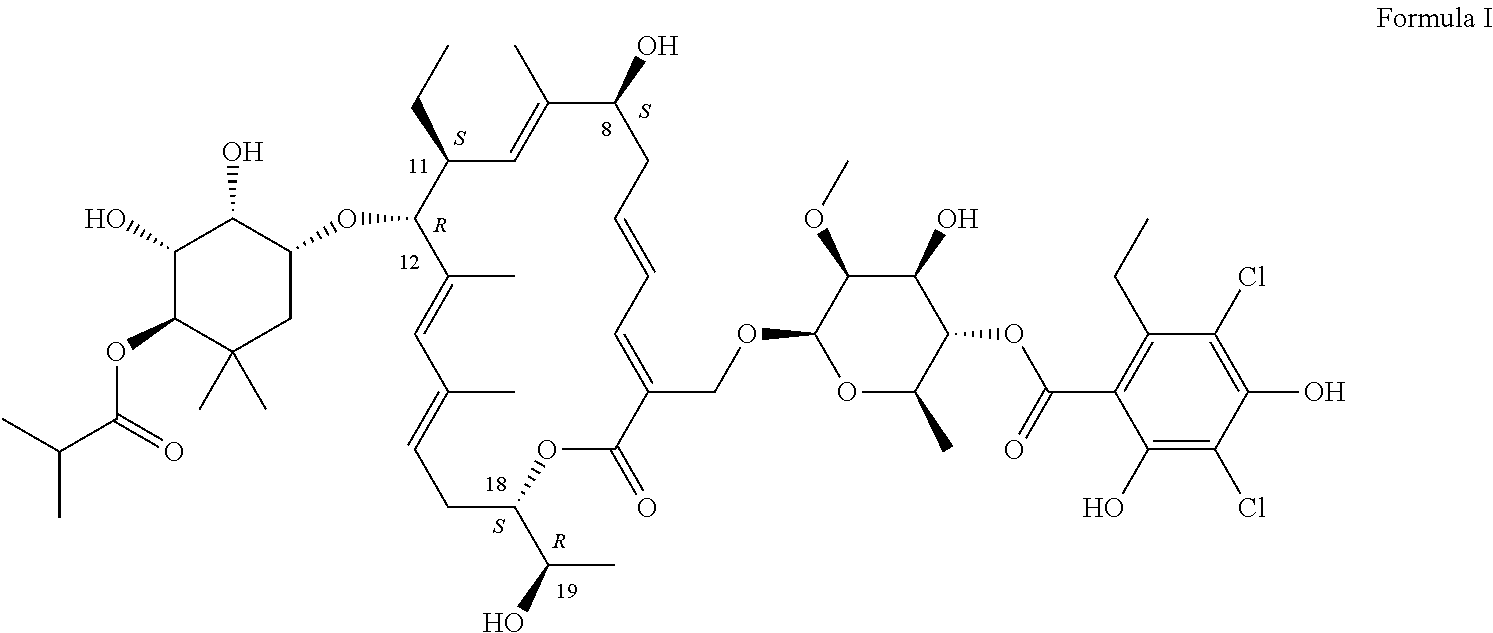
The present invention relates to methods of preventing Clostridium difficile infection (COI) in a human subject who is at high risk of developing this infection, comprising administering to the subject an effective amount of the compounds described herein. In some embodiments, the subject is further receiving concomitant antibiotic therapy to prevent and / or treat an infection other than Clostridium. In some embodiments, the subject previously was treated for a Clostridium infection. In some embodiments, the compound of formula I prevents the Clostridium infection.
Owner:MERCK SHARP & DOHME CORP
Proteins that interact with Clostridium difficile cytotoxin b
ActiveCN103665141BAvoid cytotoxicityAvoid toxicityAntibacterial agentsPeptide/protein ingredientsClostridial infectionClostridium difficile cytotoxin B
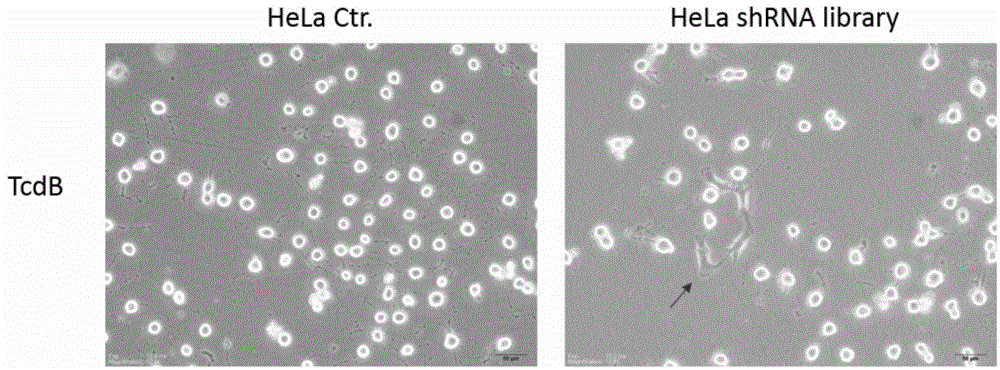
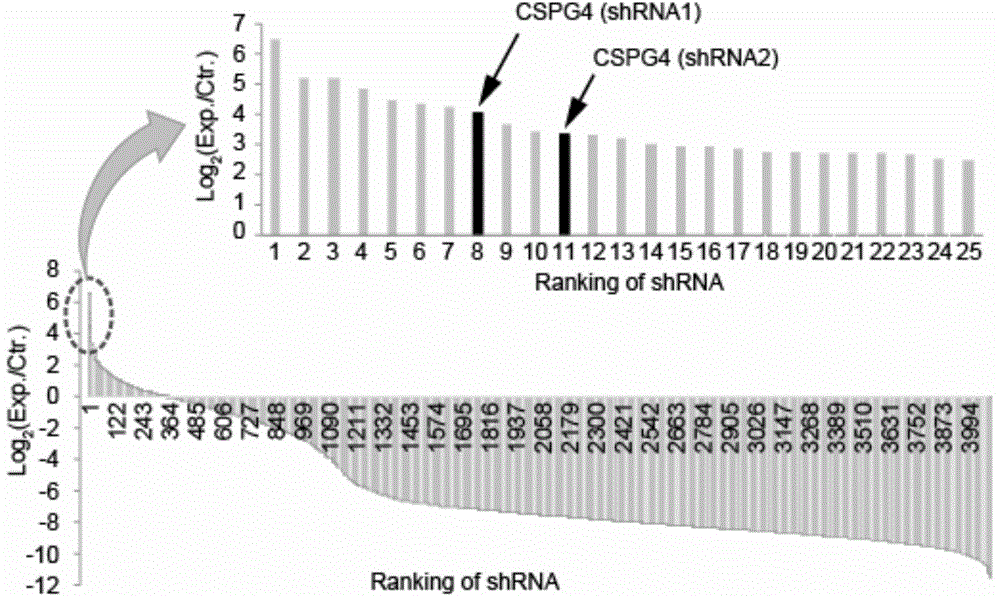
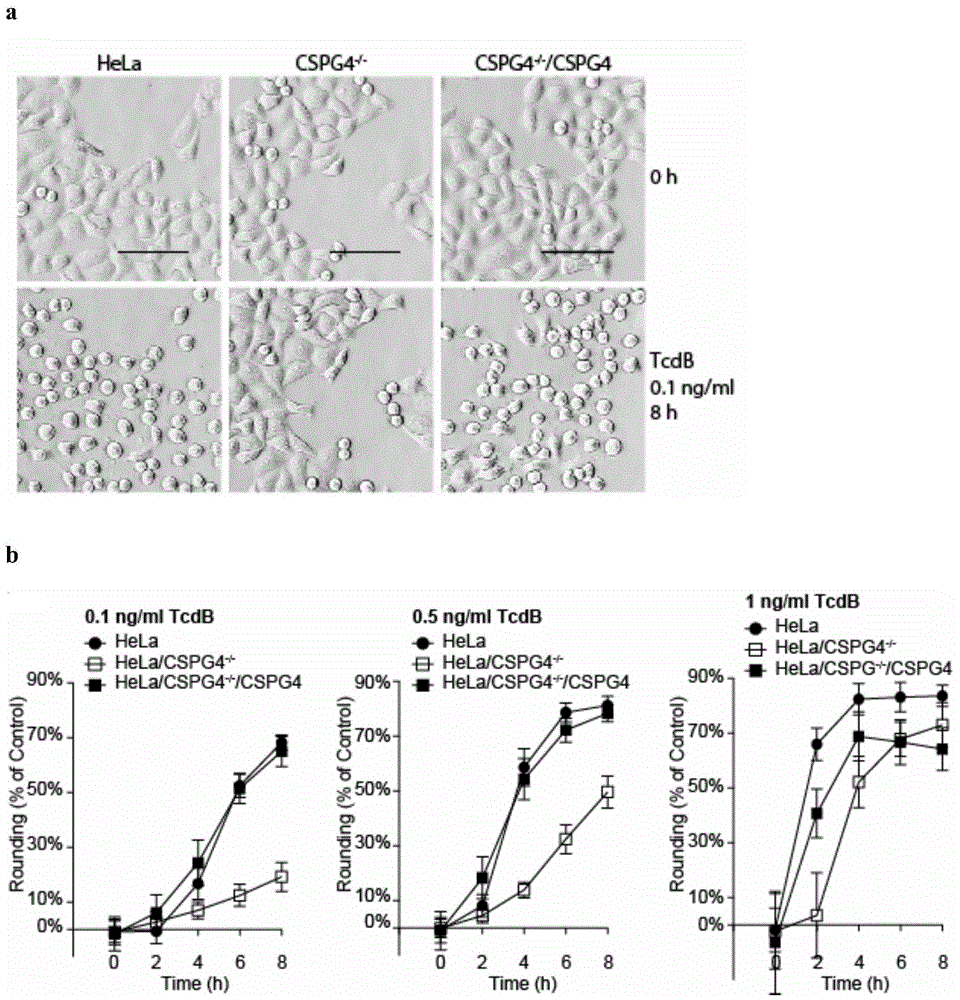
The invention provides a protein interacted with clostridium difficile cytotoxin B. The protein is chondroitin sulfate protein polysaccharide CSPG4 or a polypeptide formed by 640 amino acids at the N end of the CSPG4 protein. The invention firstly finds that the CSPG4 has the function of a clostridium difficile cytotoxin B cell receptor, the toxicity of the clostridium difficile cytotoxin B can be obviously reduced by restraining the expression or the function of the CSPG4, and a new thought is provided for treating the clostridium difficile infection.
Owner:PEKING UNIV
Use of nifuratel to treat infections caused by clostridium species
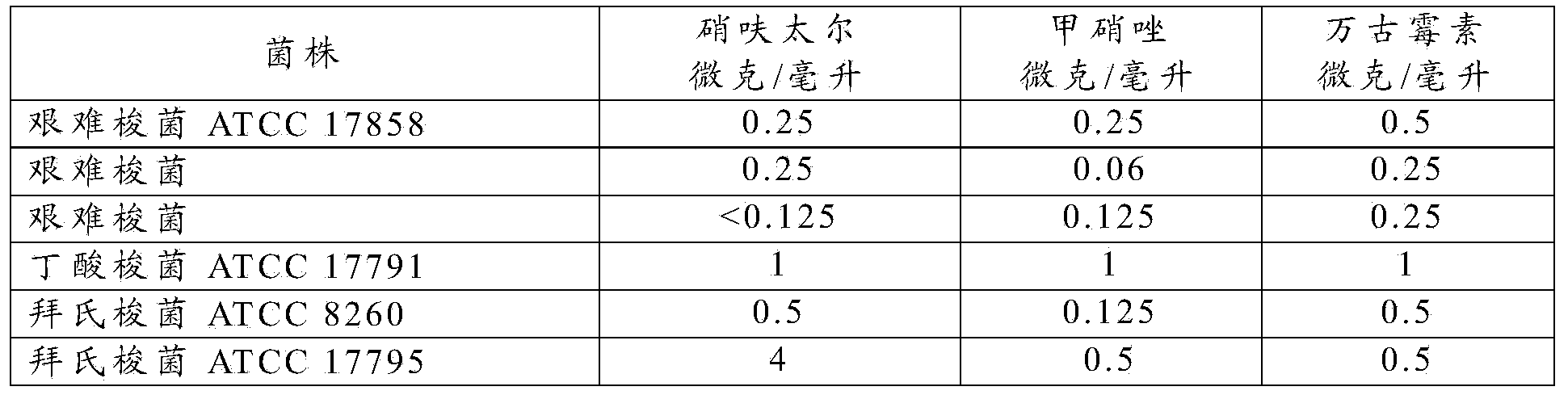
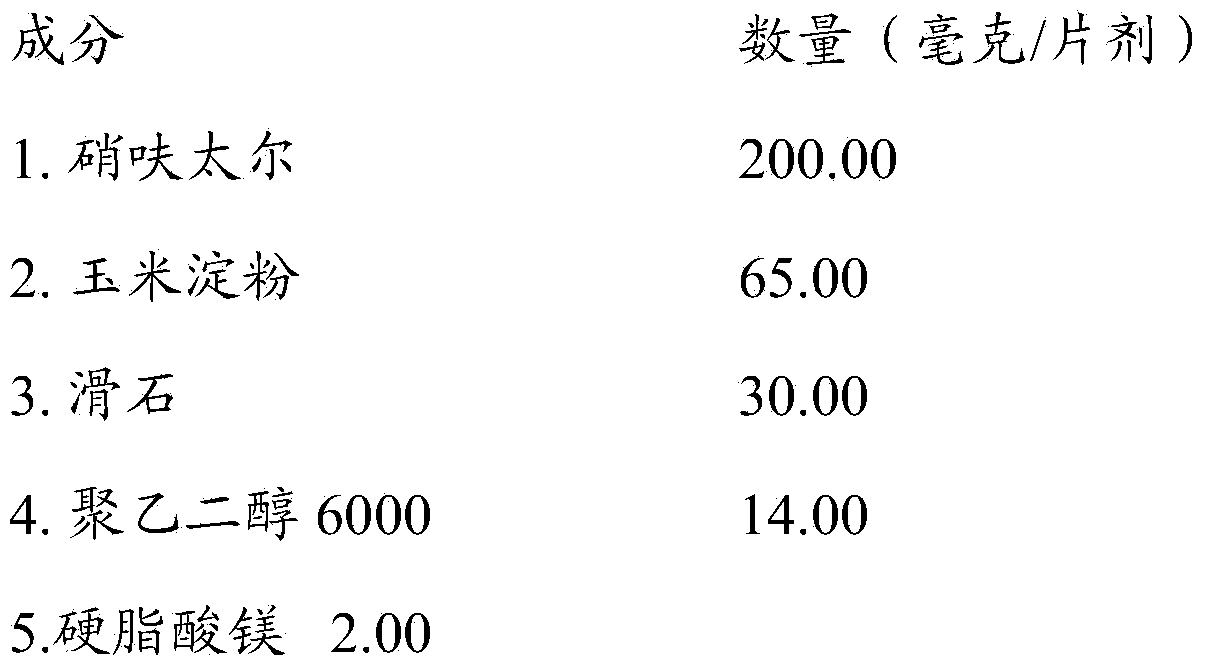
InactiveCN103384523AAntibacterial agentsOrganic active ingredientsClostridial infectionClostridium difficile infections
The present invention is directed to the use of nifuratel, or a physiologically acceptable salt thereof, to treat infections caused by bacteria species of the genus Clostridium. The invention is further directed to the use of nifuratel to treat Clostridium difficile infection (CDI) and in particular Clostridium difficile associated diarrhoea (CDAD).
Owner:POLICHEM SA
Methods of diagnosing clostridium difficile infection or recurrence in a subject
ActiveUS20180164282A1Improve accuracyImprove reliabilityMicrobiological testing/measurementScattering properties measurementsClostridial infectionBacteroides
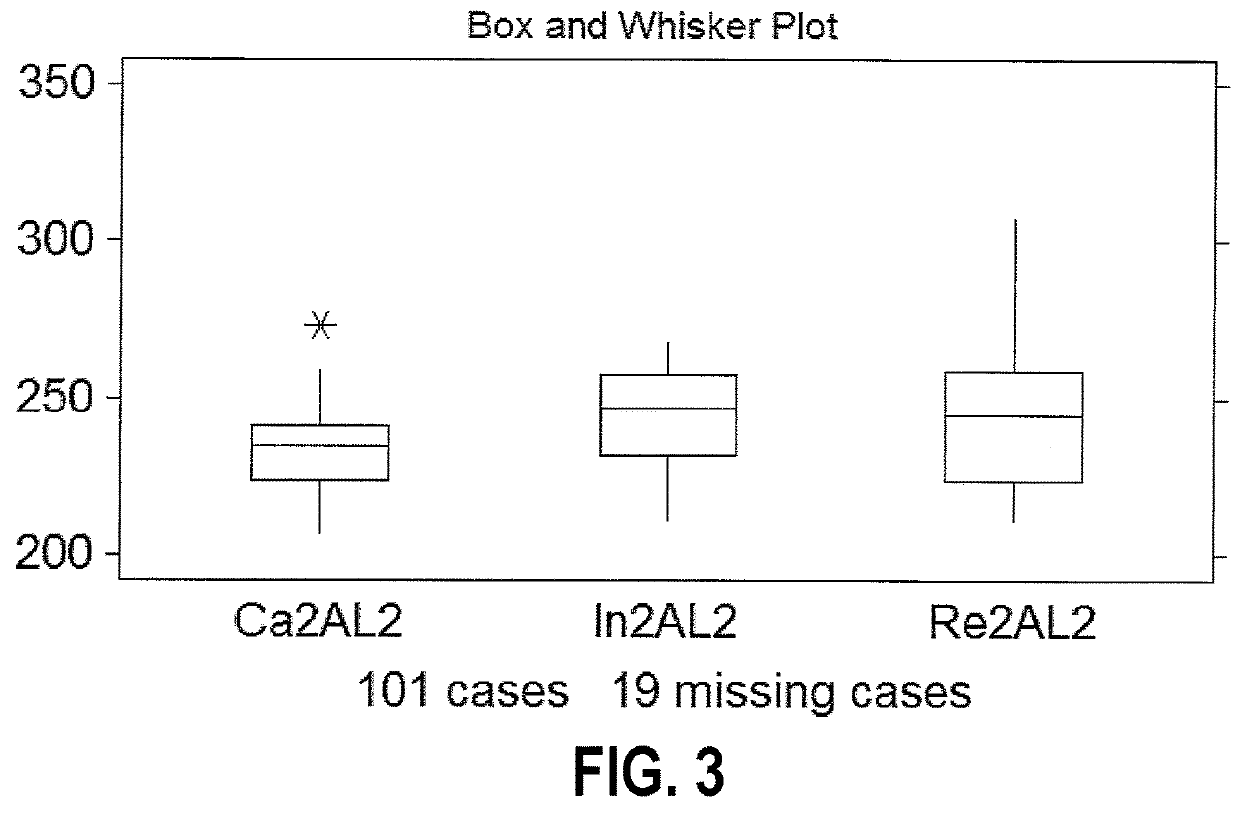
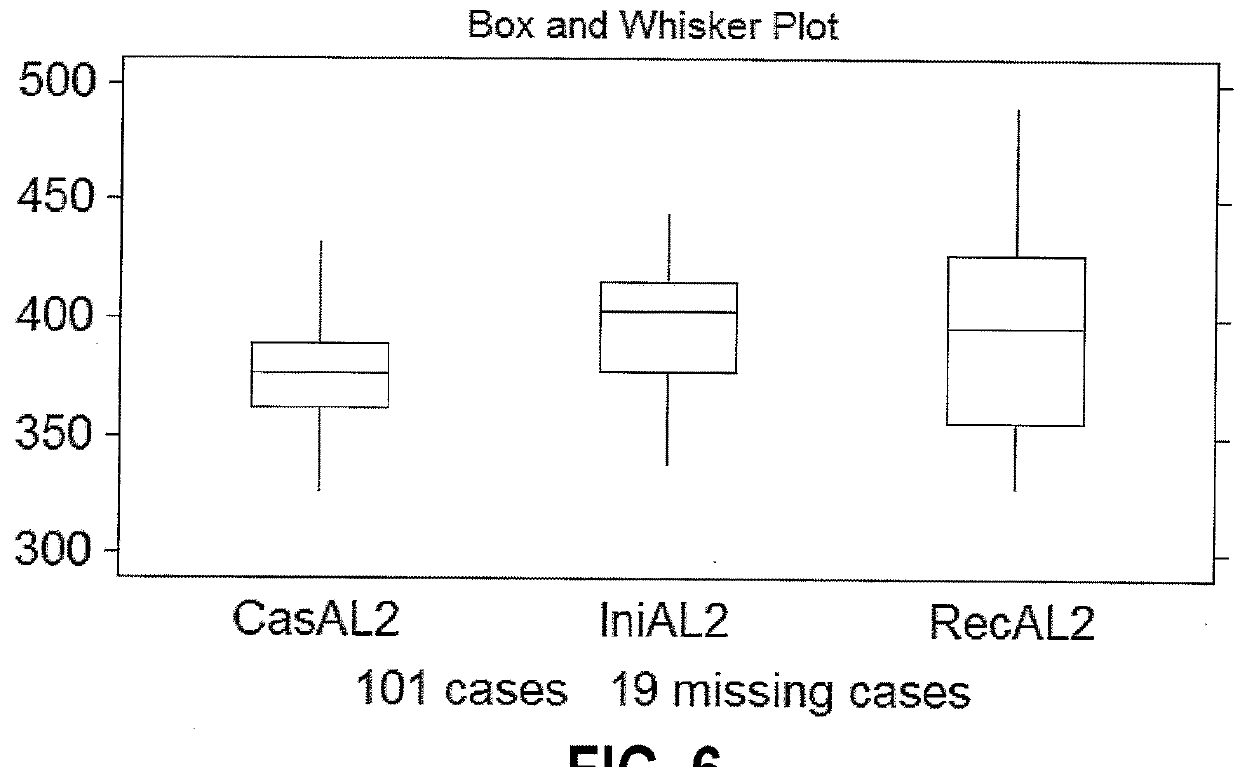
Methods are described for identifying CDI patients as well as CDI patients at risk for recurrence. Embodiments include: (1) flow cytometry of circulating peripheral blood mononuclear cells (PBMC) to phenotype for the less efficient immunoglobulin response to bacterial toxins and surface antigens that characterizes patients who will become recurrent; (2) stratification by means of complete blood count (CBC) using a Coulter counter to detect the differences in lower angle light scatter (LAL), which has a larger range in the recurrent population; and (3) stratification by means of complete blood count (CBC) using a Coulter counter to detect the lower axial light loss (AL2) exhibited in recurrent patients.
Owner:MUSIDORA BIOTECH LLC +1
A new application of Thiopeptidecycline
ActiveCN105363018BUnique structureNew mechanism of actionAntibacterial agentsPowder deliveryClostridial infectionThiopeptin
The invention discloses the application of thiopeptidecycline in the preparation of medicines for treating Clostridium difficile infectious diseases and its complications. Thiopeptidecycline has significant activity against Clostridium difficile and its drug-resistant strains, and has important clinical application value. In vivo experiments have verified that the thiopeptidecycline does not enter the blood circulation after oral administration. In view of the characteristics of intestinal administration, the present invention also provides an oral preparation of the thiopeptidecycline containing a safe and effective dose and a preparation method thereof.
Owner:NANJING BIOTICA PHARMA
Synergistic clofazimine/metronidazole combination for treating clostridium difficile
ActiveUS20170143707A1Organic active ingredientsPharmaceutical delivery mechanismDiseaseClostridial infection
Methods for treating a subject infected with Clostridium difficile comprises administering to the subject a synergistic combination of clofazimine and metronidazole. Pharmaceutical compositions comprising clofazimine and metronidazole are provided for treating Clostridium difficile infection and diseases or symptoms associated therewith.
Owner:ELONA PHARM LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com