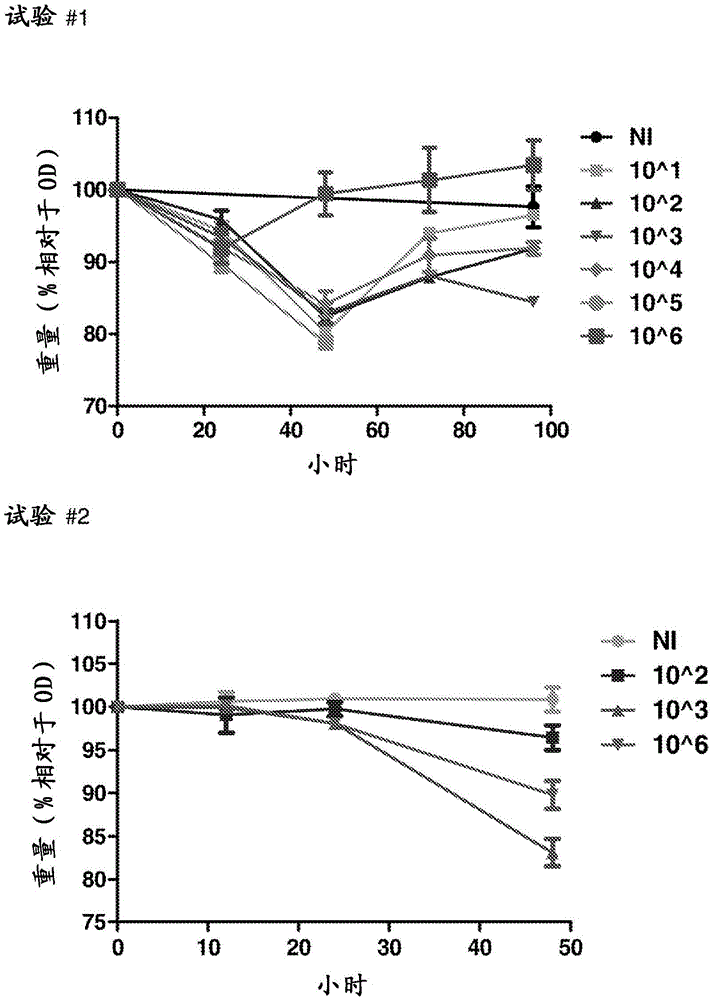
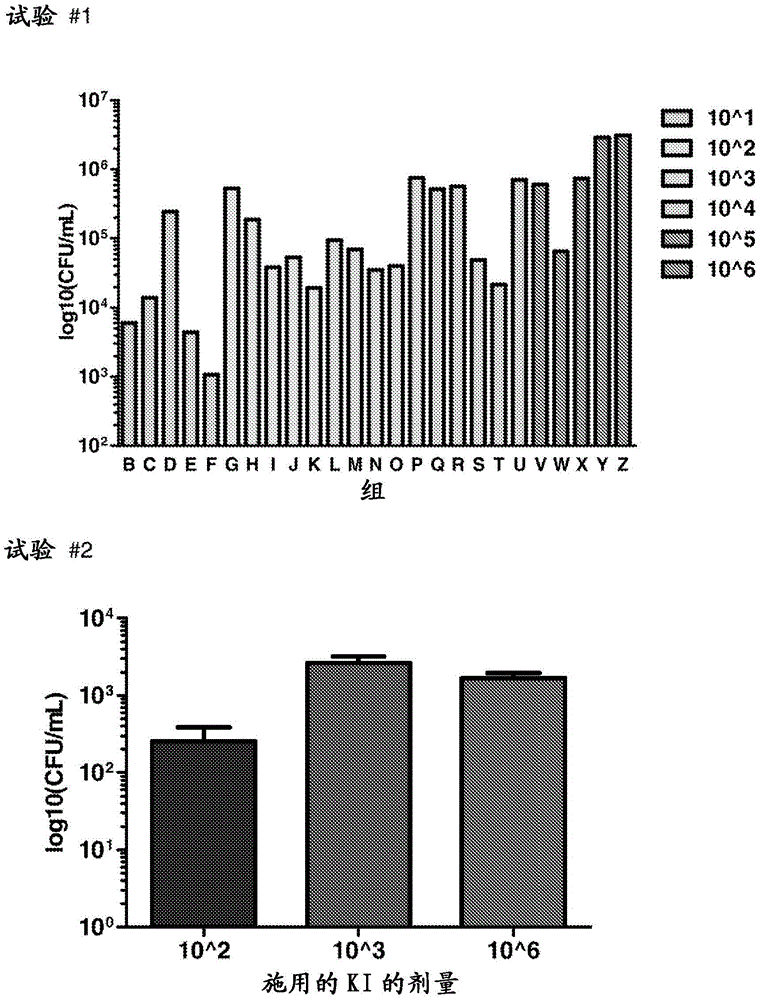
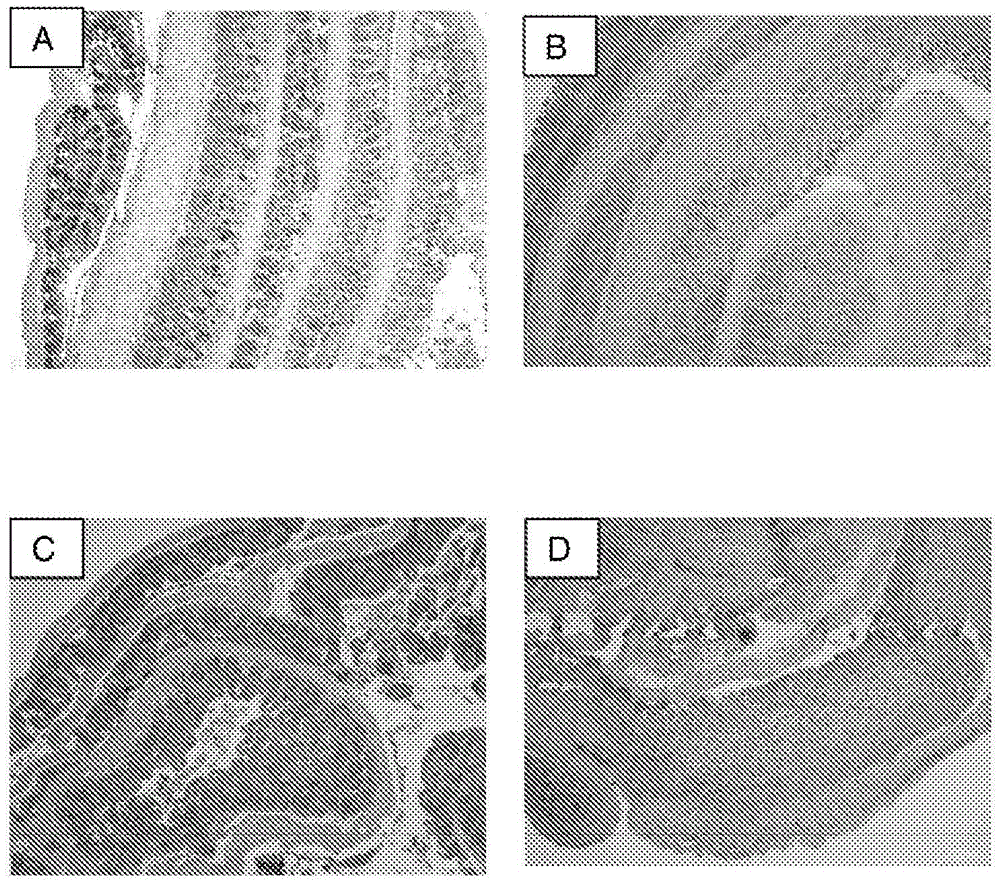
Methods and compositions for the treatment and/or prophylaxis of clostridium difficile associated disease
一种艰难梭菌、艰难梭菌毒素的技术,应用在化学仪器和方法、药物组合、抗细菌药等方向,能够解决治疗失败、复发等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0187] The preparation of pharmaceutical compositions is well known in the art and has been described in many articles and textbooks, see, for example, Remington's Pharmaceutical Sciences, Gennaro A.R. ed., Mack Publishing Co., Easton, PA, 1990, especially pp. 1521- 1712 pages, fully incorporated herein by reference.
[0188] The pharmaceutical compositions of the invention may be administered and administered according to good medical practice.
[0189] The compositions of the invention may contain the active substances in free form and be administered directly to the subject to be treated. Formulations generally comprise at least one active ingredient as defined above together with one or more acceptable carriers thereof. Each carrier should be pharmaceutically and physiologically acceptable in terms of compatibility with the other ingredients and be innocuous to the patient.
[0190] Formulations include those suitable for oral, nasal, or parenteral (including subcutaneou...
Embodiment 10
[0223] Example 10 and Figure 11 demonstrated that administration of antibodies that bind to C. difficile endospores before and during / after infection with C. difficile is effective in treating and preventing C. difficile-associated disease, C. difficile-associated mortality and reducing the shedding of C. difficile and C. difficile spread after bacterial infection.
Embodiment 13
[0224] Example 13 and Figure 15 It is shown that administration of antibodies that bind to C. difficile vegetative cells prior to and during infection with C. difficile is effective in treating and preventing C. difficile-associated disease and C. difficile-associated mortality.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com