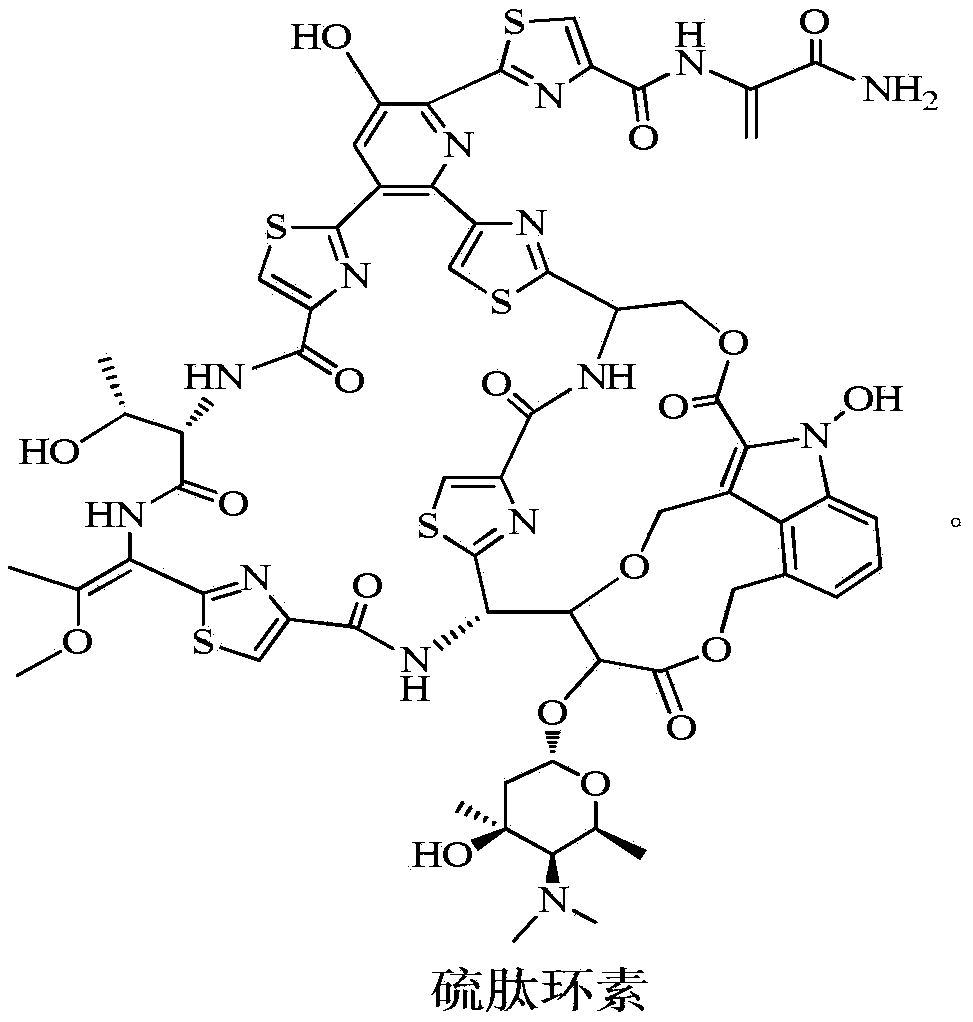
New application of thiopeptcin
A technology of thiopeptidylcycline and antibiotics, which is applied in the directions of inactive medical preparations, cyclic peptide components, and pill delivery, etc., can solve the problems such as the anti-anaerobic bacteria, Clostridium difficile effect and activity of thiopeptidylcycline, etc. The effect of super antibacterial activity, strong antibacterial activity and small safety hazard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
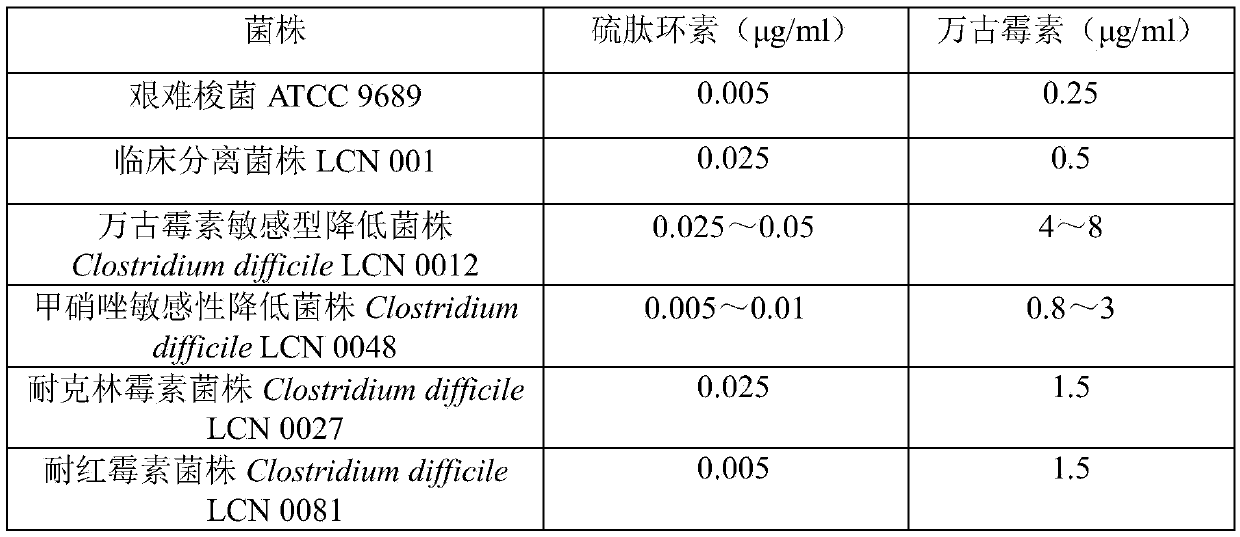
[0027] Example 1 The in vitro anti-Clostridium difficile and its drug-resistant strain activity of thiopeptcycline
[0028] The in vitro antibacterial activity of Thiopeptidecycline was tested against the sensitive Clostridium difficile ATCC9689 strain Clostridium difficile LCN001 and the clinically isolated drug-resistant strain, and compared with vancomycin. According to the broth dilution method recommended by CLSIM11-A7, the minimum inhibitory concentration (MIC) of thiopeptidecycline was determined. According to the measurement needs, the Brooke's broth supplemented with Hemin (5 mg / ml), vitamin K1 (1 μg / ml), lysed horse blood (5%) and oxidase (1:25v / v) was divided into different measurement groups, and then Thiopeptidecycline or vancomycin in DMSO was added. The concentration range of the test drug was 0.025 μg / ml-128 μg / ml. Check the growth of Clostridium difficile in each culture medium, the minimum antibacterial drug concentration at which the experimental bacteria ...
Embodiment 2
[0031] Embodiment 2 Thiopeptidecycline LC-MS / MS detection method
[0032] Add 90 μl of methanol containing 5 ng / ml verapamil to 30 μl of blood or urine sample, vortex for 3 minutes, then centrifuge at 14,000 rpm for 5 minutes, take 80 μl of the supernatant, and inject it into LC-MS / MS for analysis.
[0033] High performance liquid chromatography: Chromatographic column: WatersSymmetry300C183.5μm2.1*100mm;
[0034] Mobile phase A: 10mM ammonium acetate + 0.02% ammonia solution; mobile phase B: methanol;
[0035] Analysis time: 5.2min Injection volume: 5μL Column temperature: 25°C;
[0036] Gradient elution:
[0037] time (min)
Flow rate (mL / min)
B%
0.00
0.4
67
2.40
0.4
67
2.45
0.4
90
3.80
0.4
90
3.85
0.4
67
5.20
0.4
67
[0038] Mass spectrometry: positive ion mode (Agilent6460B)
[0039] Detection ion pair: Thiopeptidecycline: 1437.3→172.0, fragmentor=135, CE=22...
Embodiment 3
[0042] Example 3 Oral Thiopeptidecycline Pharmacokinetics Study
[0043] Thiopeptidecycline liquid preparation method: take a certain amount of thiopeptidecycline, put it in a mixed solvent containing 10% PEG400, 1% Tween 80, and 5mg / ml citric acid at pH 5.0, and shake until it becomes clear to obtain thiopeptidecycline Oral gavage solution.
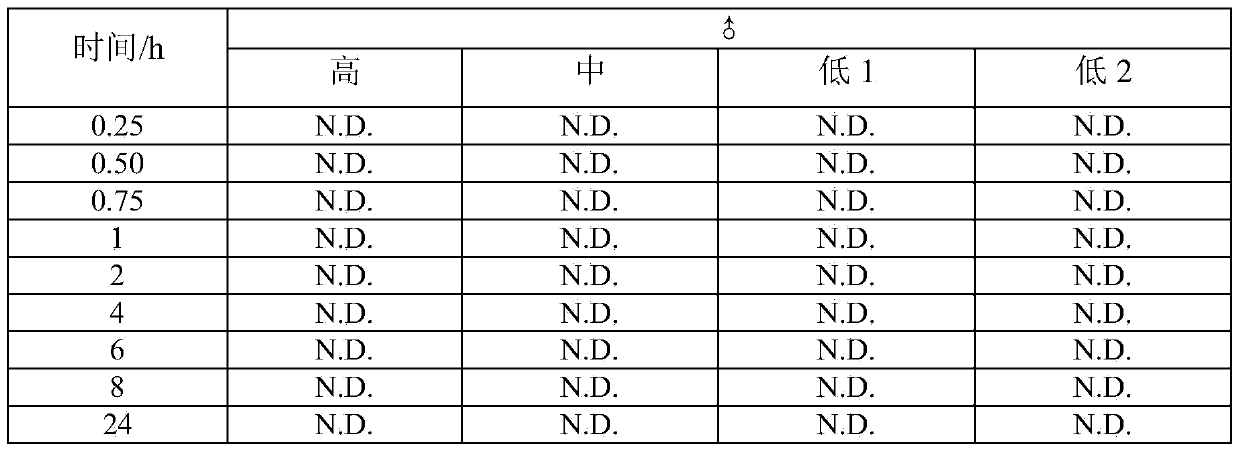
[0044] Male rats were divided into high, medium and low dose groups according to the purpose of the experiment. Among them, the high dose group is 50 mg / Kg, the middle dose group is 25 mg / Kg, the low 1 dose group is 5 mg / Kg, and the low 2 dose group is 1 mg / Kg. About 0.3 ml of blood samples were collected at each time point of about 15 minutes, 30 minutes, 45 minutes, 1, 2, 4, 6, 8 and 24 hours after the administration for the determination of bioavailability. See Example 2 for the method.
[0045] Table 2 Concentration-time data (ng / ml) of thiopeptidecycline in plasma after oral administration of thiopeptidecycline to rats
[0046] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com