Methods and Compositions for Treating Clostridium difficile Associated Disease
a technology of clostridium difficile and composition, which is applied in the field of methods for preventing or treating clostridium difficile associated disease (cdad), can solve the problems of toxic megacolon, cd resistant to such antibiotics to colonize and overpopulate the gut of some patients, and natural gut flora
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
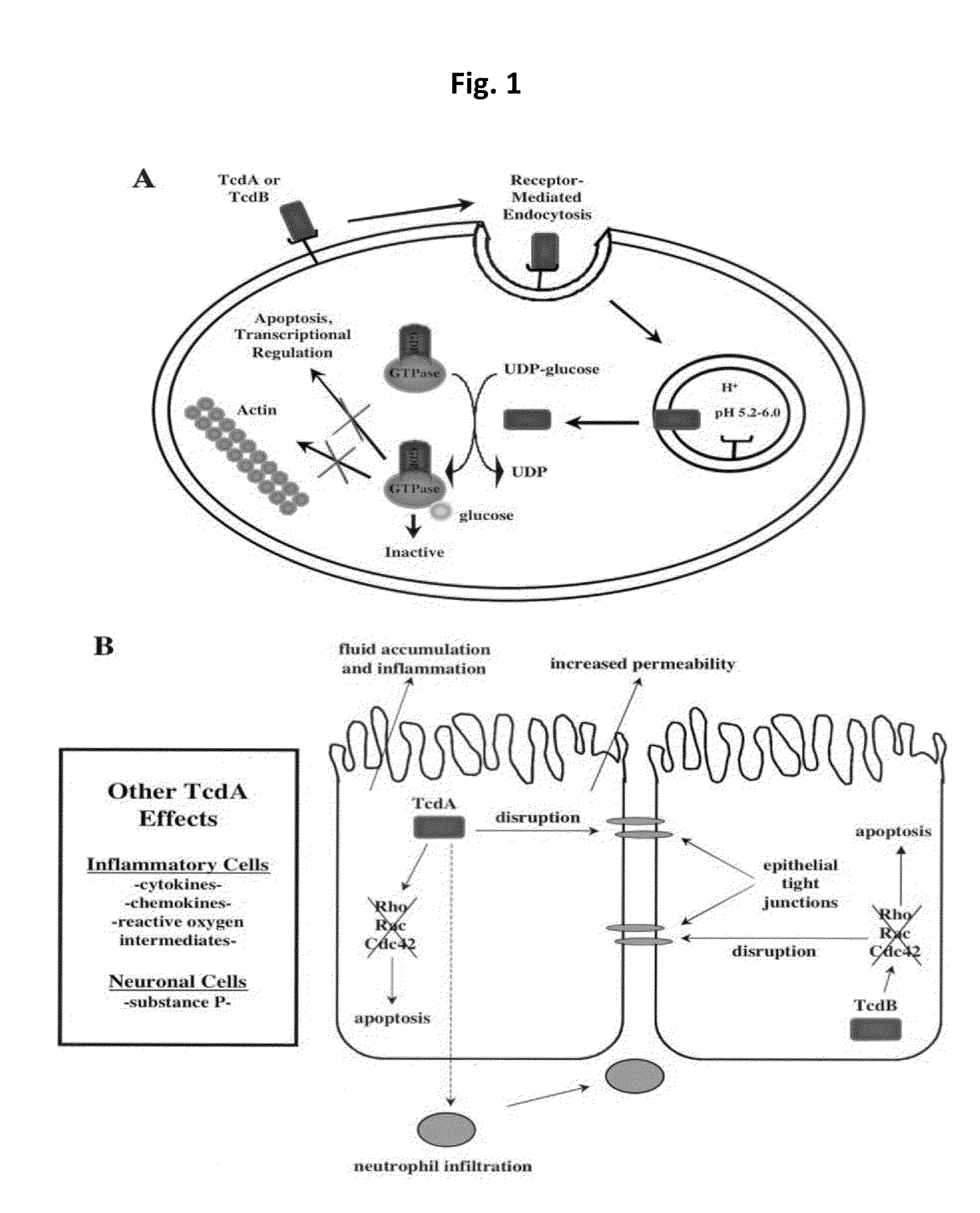
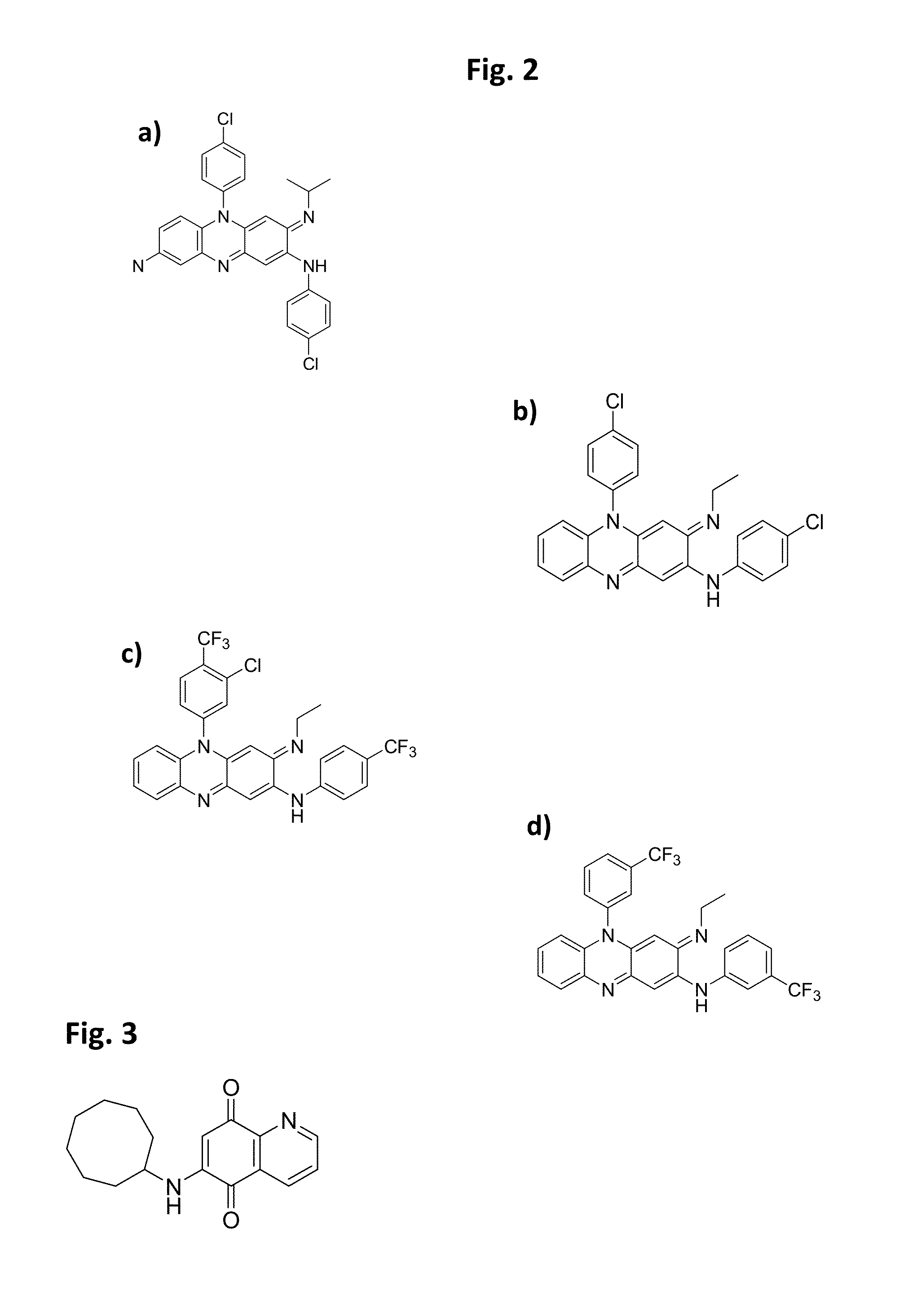
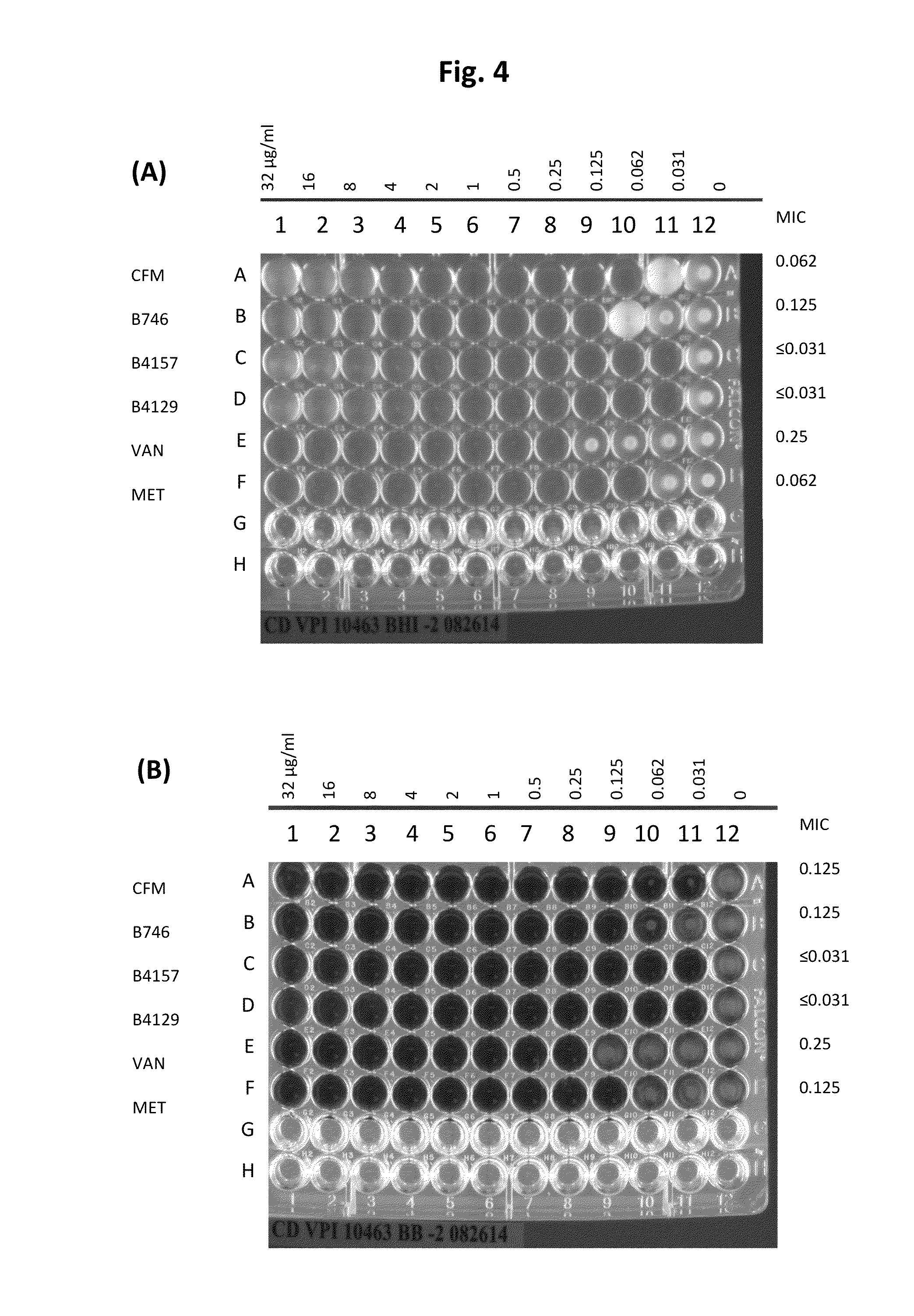
[0037]The present invention is directed to pharmaceutical compositions and methods for preventing or treating CDI and / or CDAD. According to embodiments of the present invention, pharmaceutical compositions for treating CDI and / or CDAD comprise clofazimine (CFM) and / or a CFM analogue(s), either as the primary or sole active compound or in combination with one or more additional therapeutic agents. According to other embodiments, pharmaceutical compositions for treating CDI and / or CDAD additionally or alternatively comprise azaquinone (AZQ) (also known as Gangamicin, NSC 186017, and BRN 0407295).
[0038]CFM (see FIG. 2(a)), 3-(p-chloroanilino)-10-(p-chlorophenyl)-2,10-dihydro-2-isopropyliminophenazine, is a fat-soluble riminophenazine dye, which was discovered and developed as a treatment for tuberculosis (TB) in the 1950s. However, because of its low activity against TB in guinea pig and simian models, the interest in the drug as an effective treatment for TB quickly diminished, althou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com