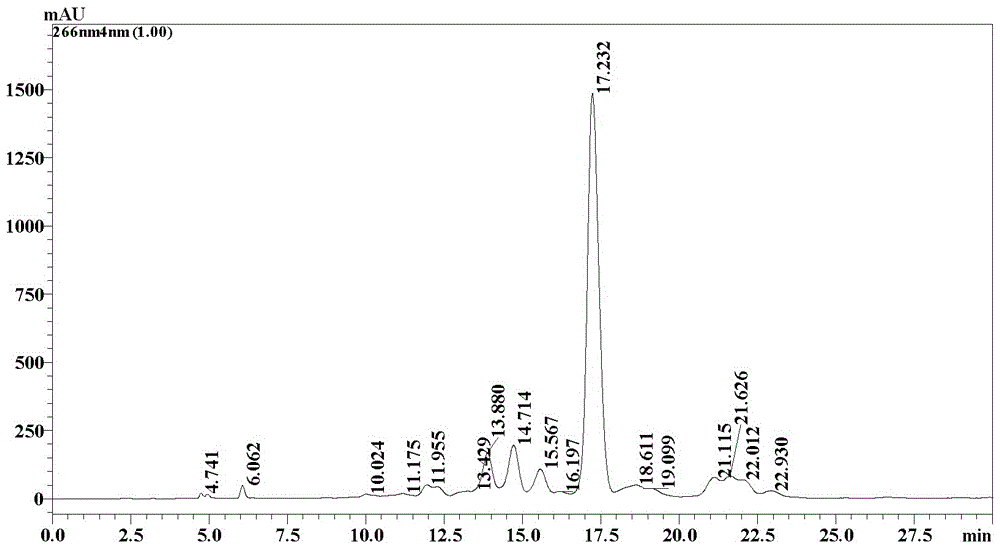
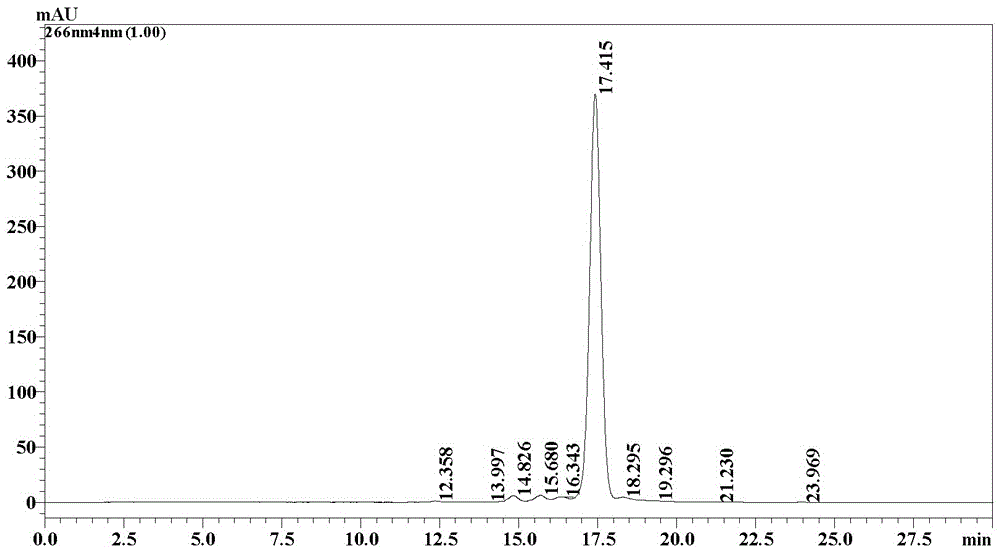
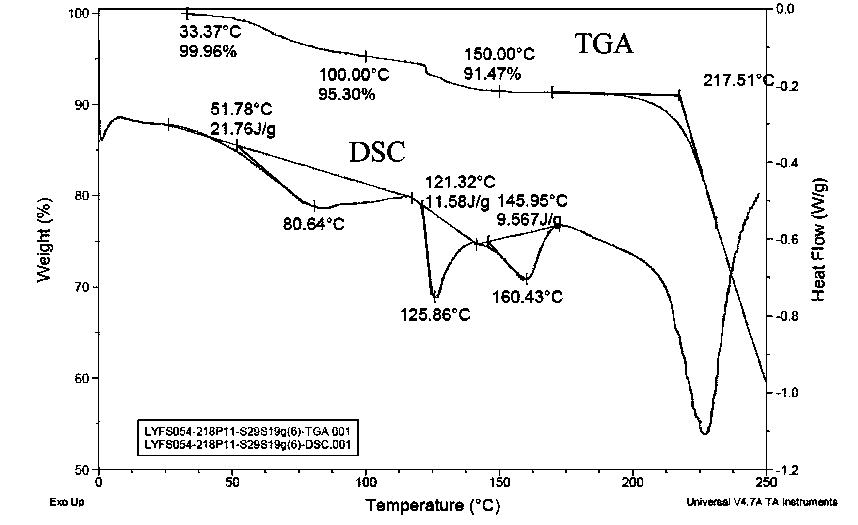
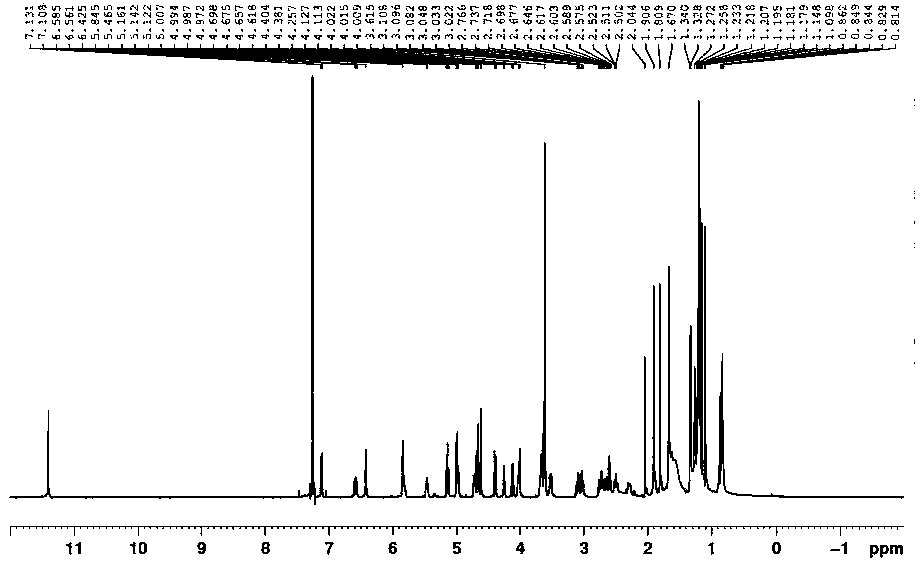
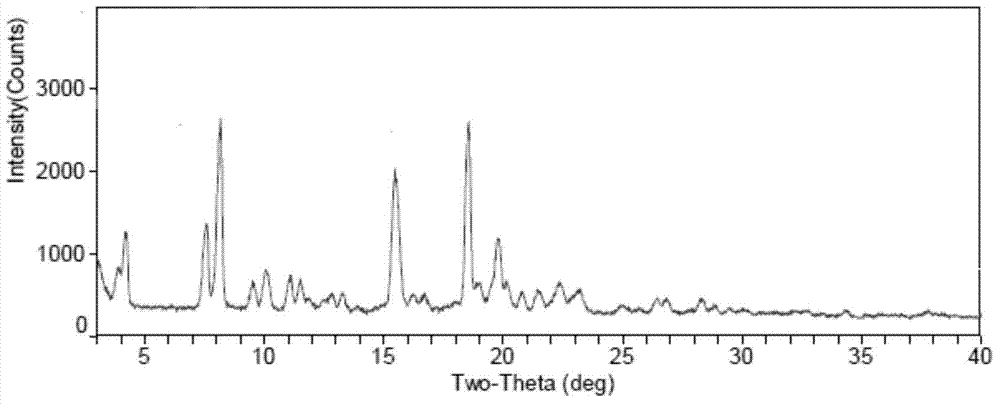
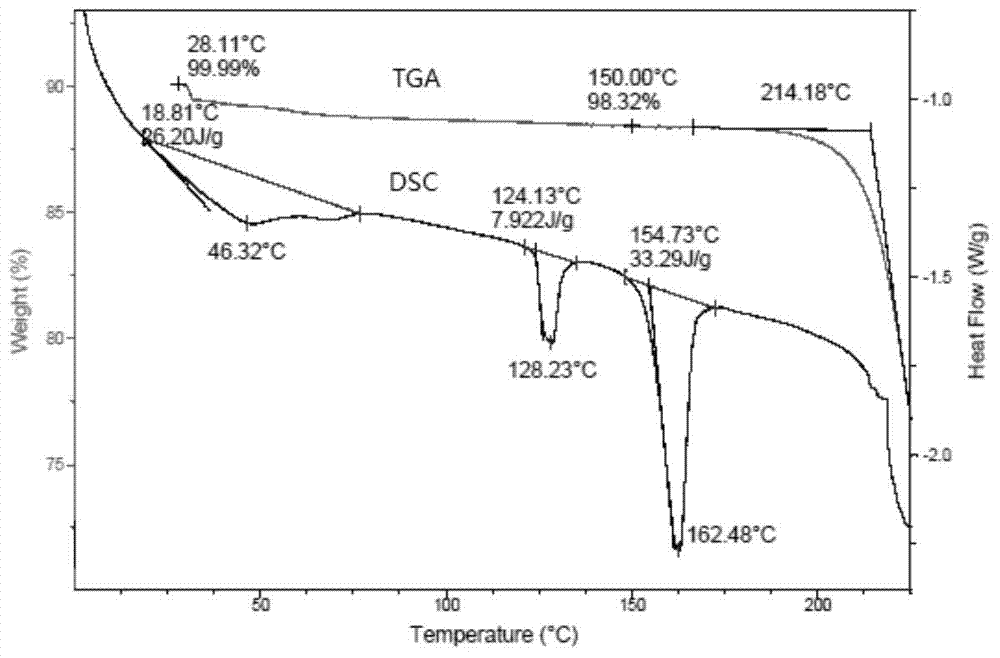
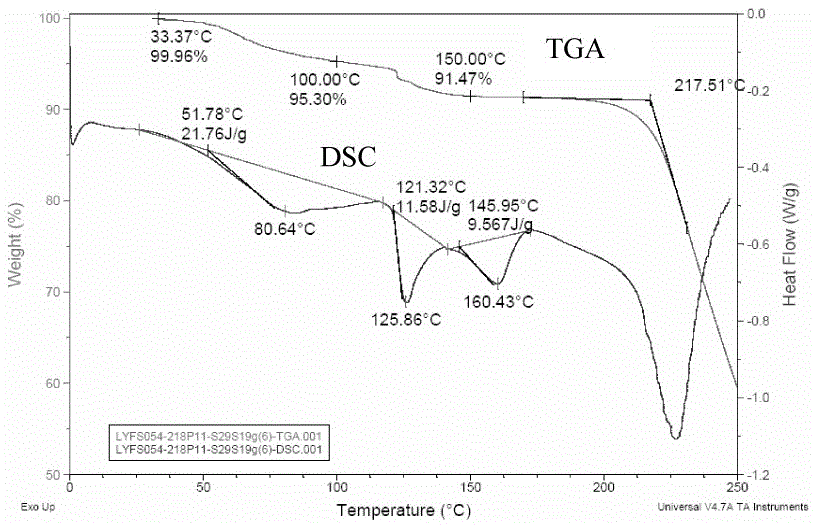
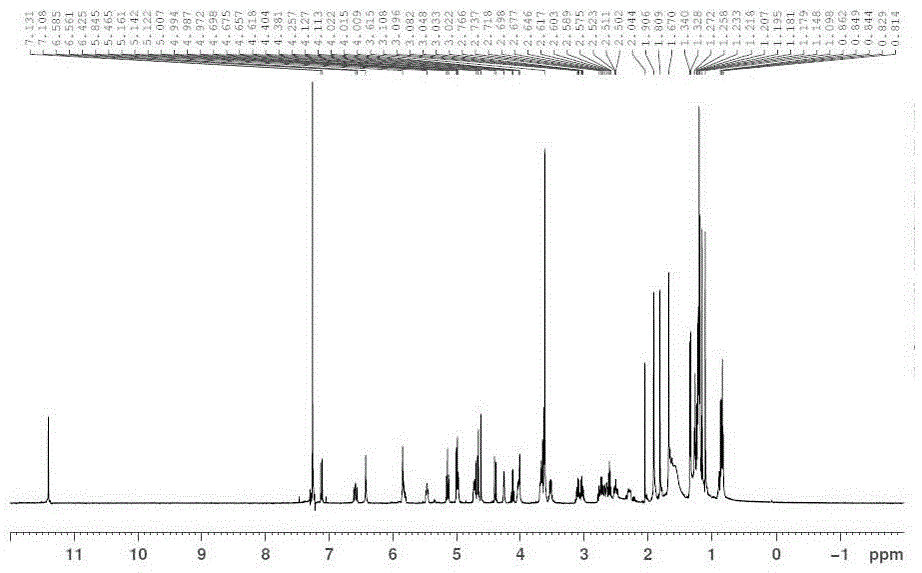
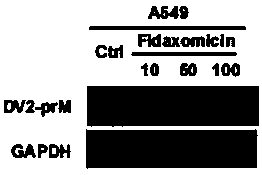
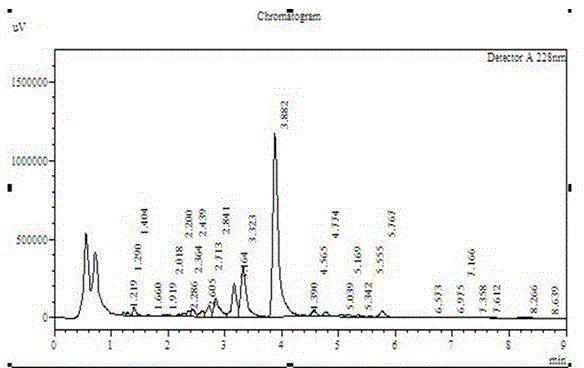
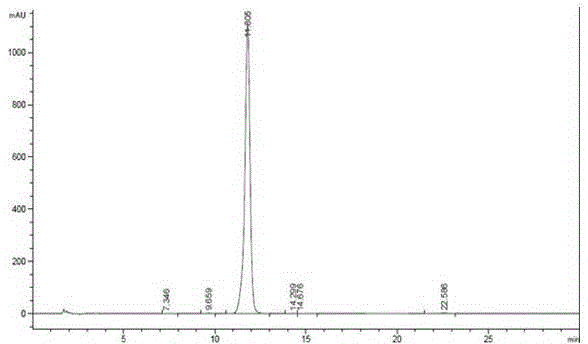
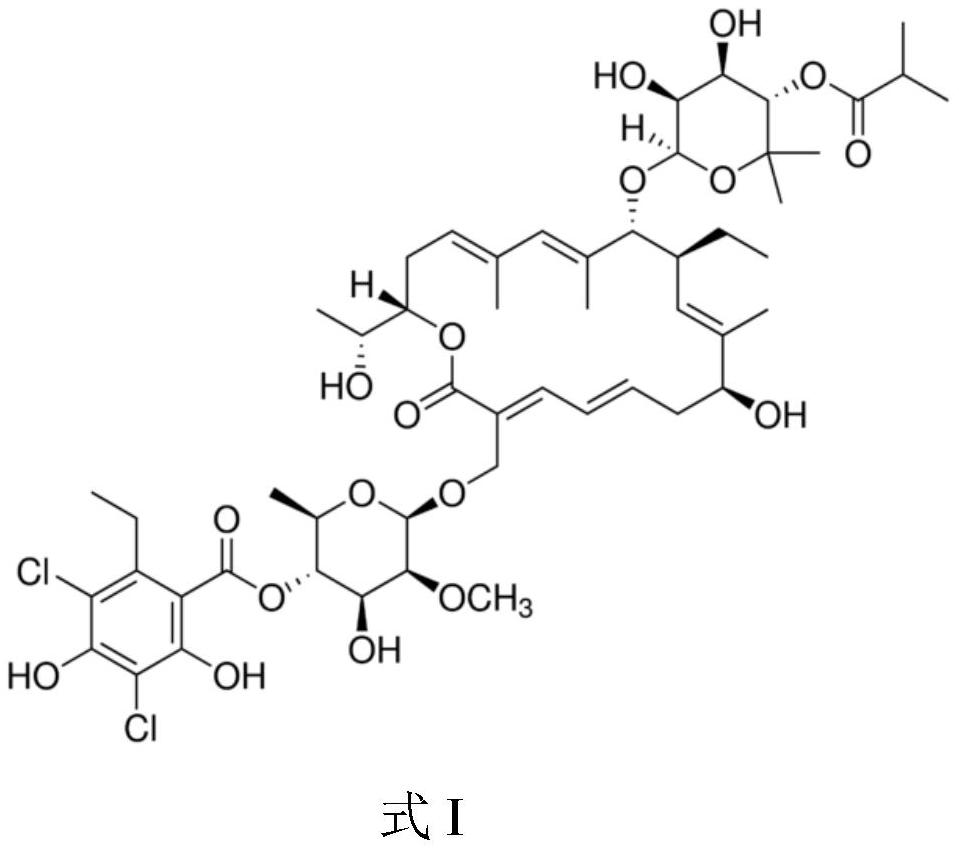
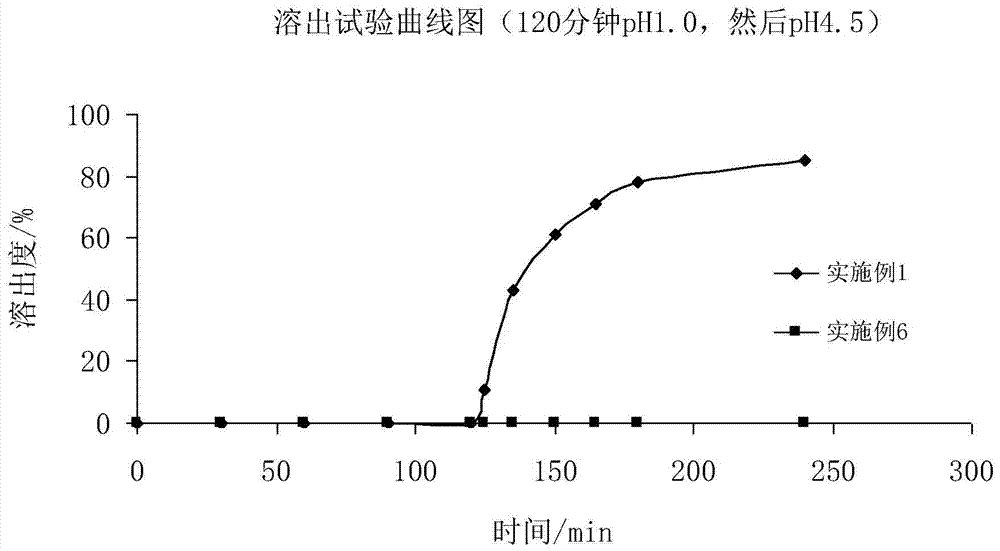
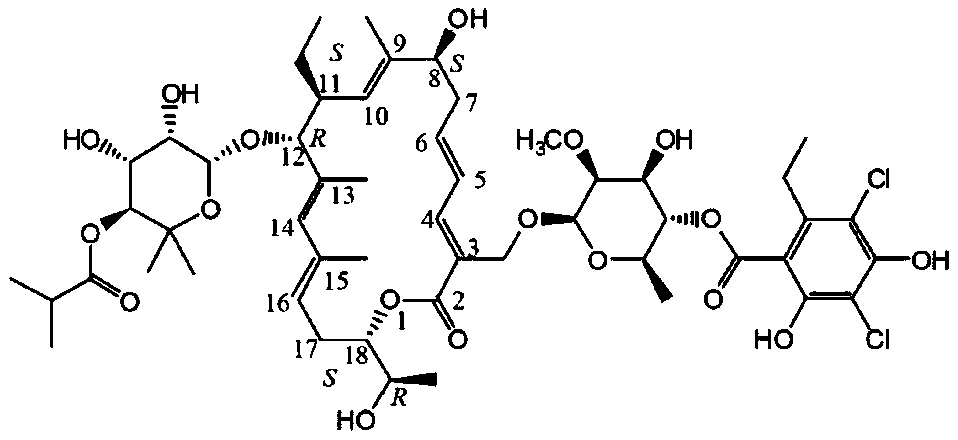
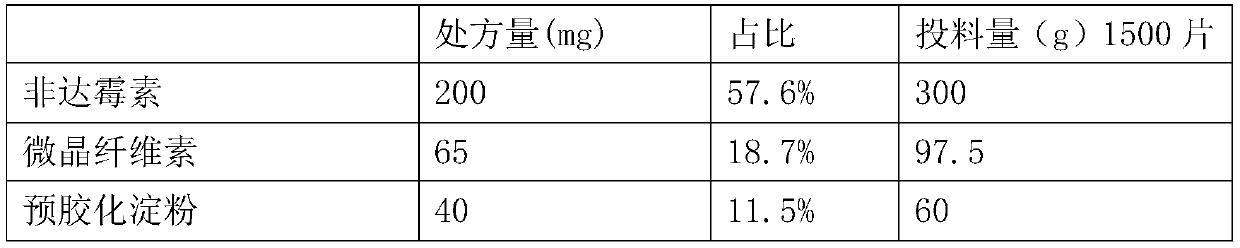
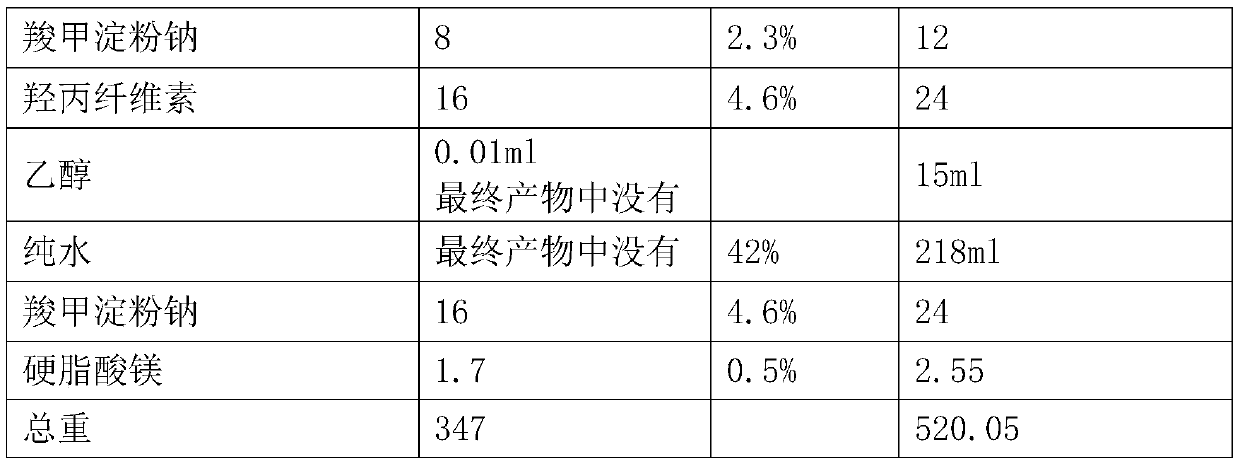
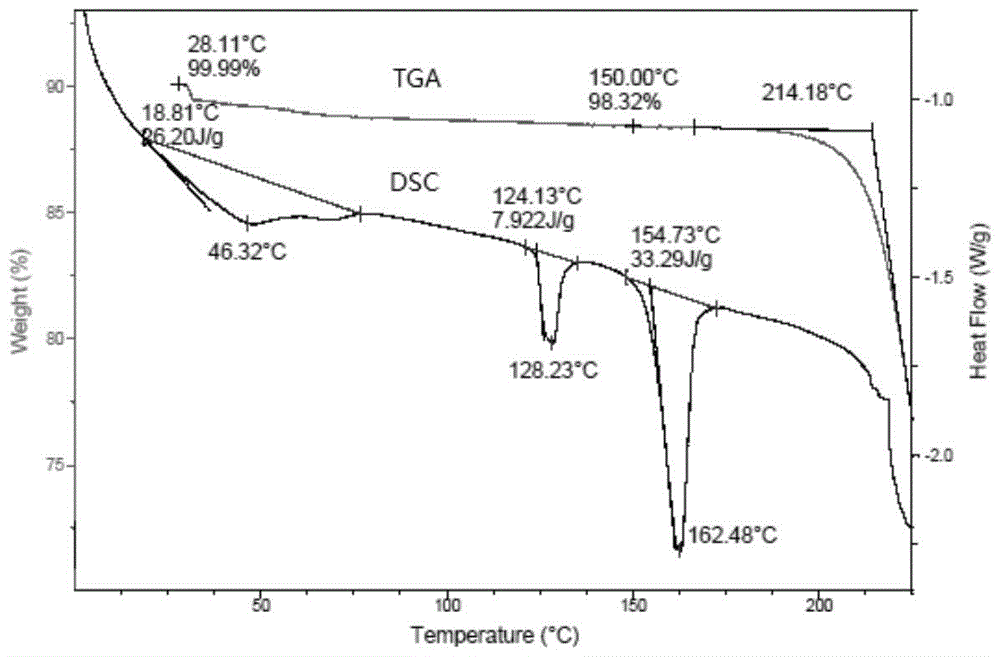
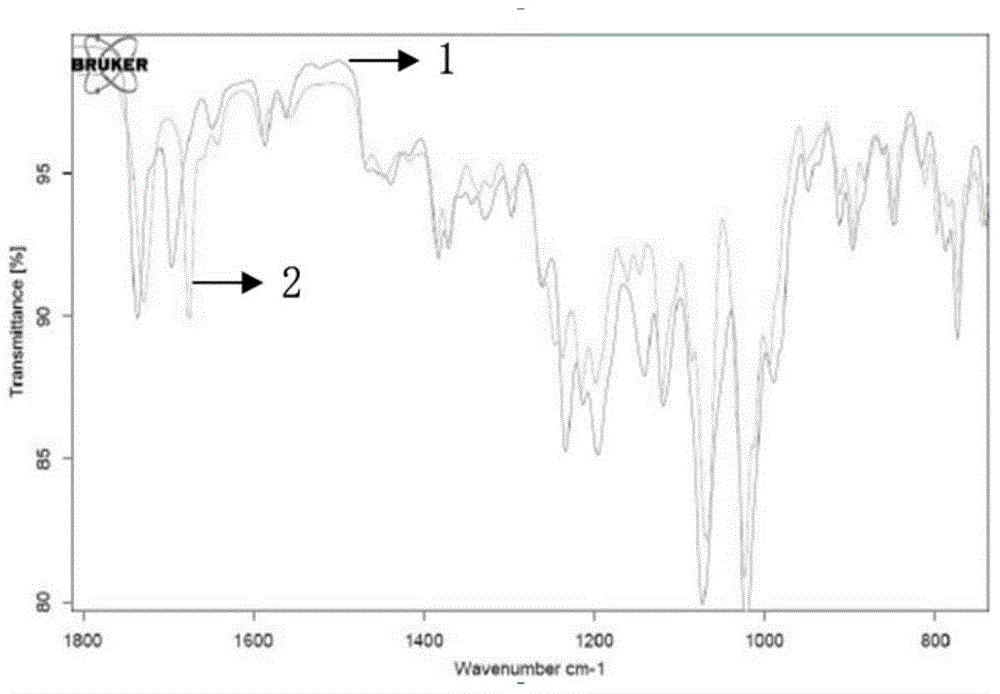
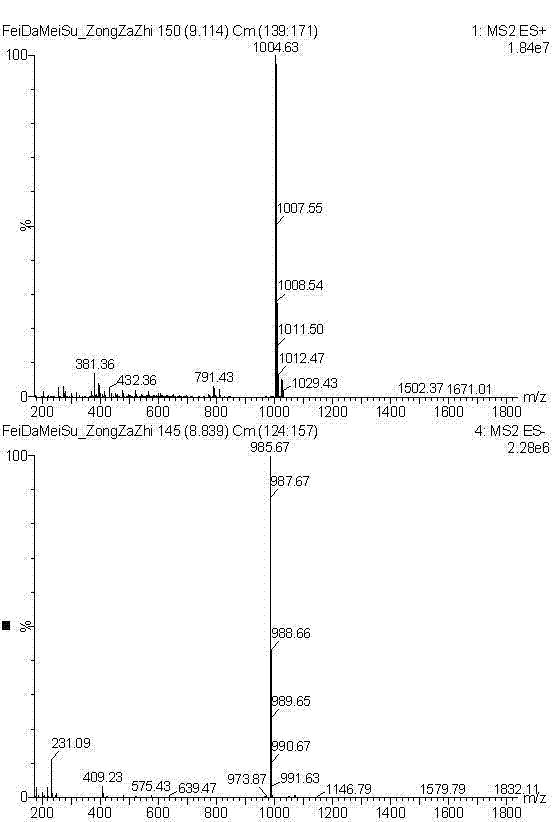Patents
Literature
36 results about "Fidaxomicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
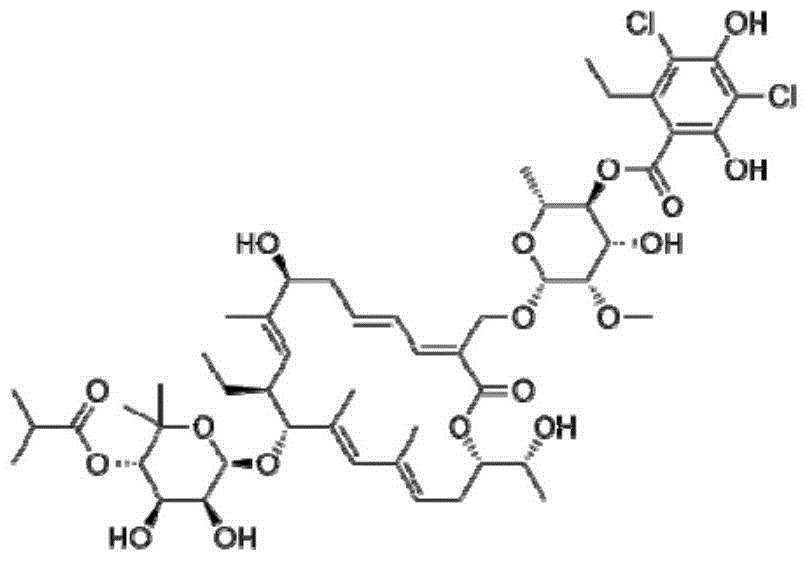
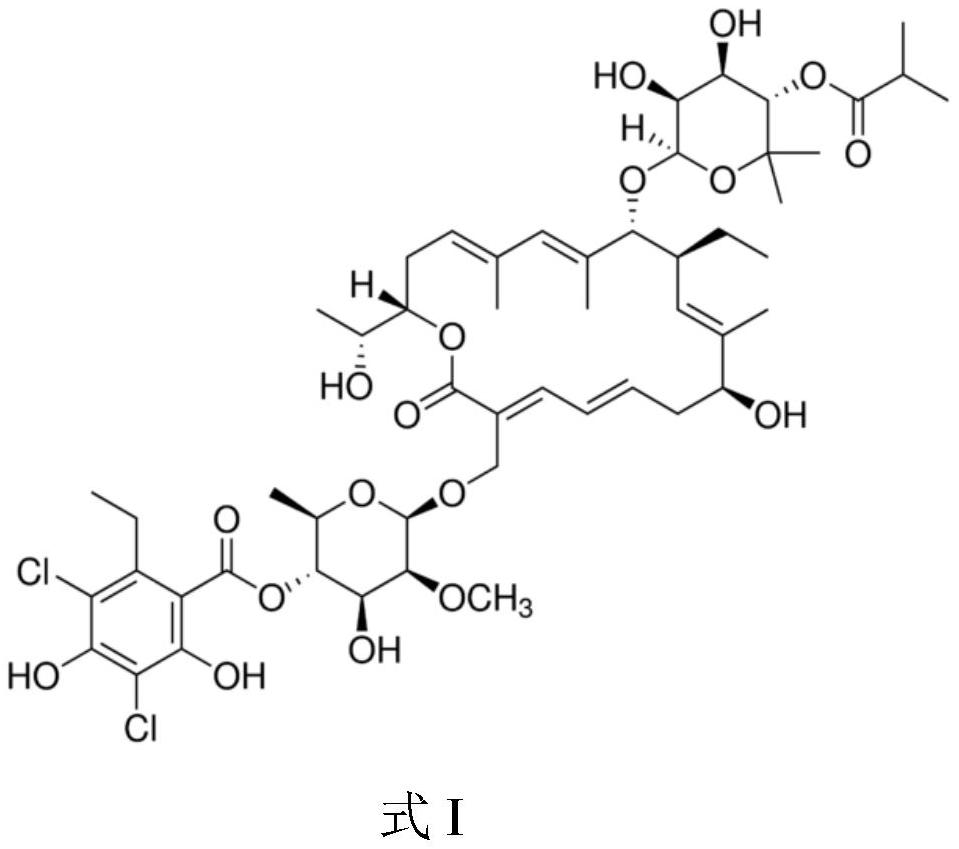
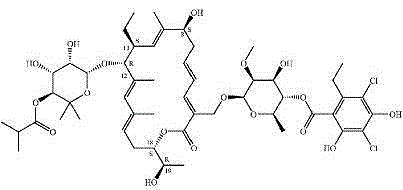
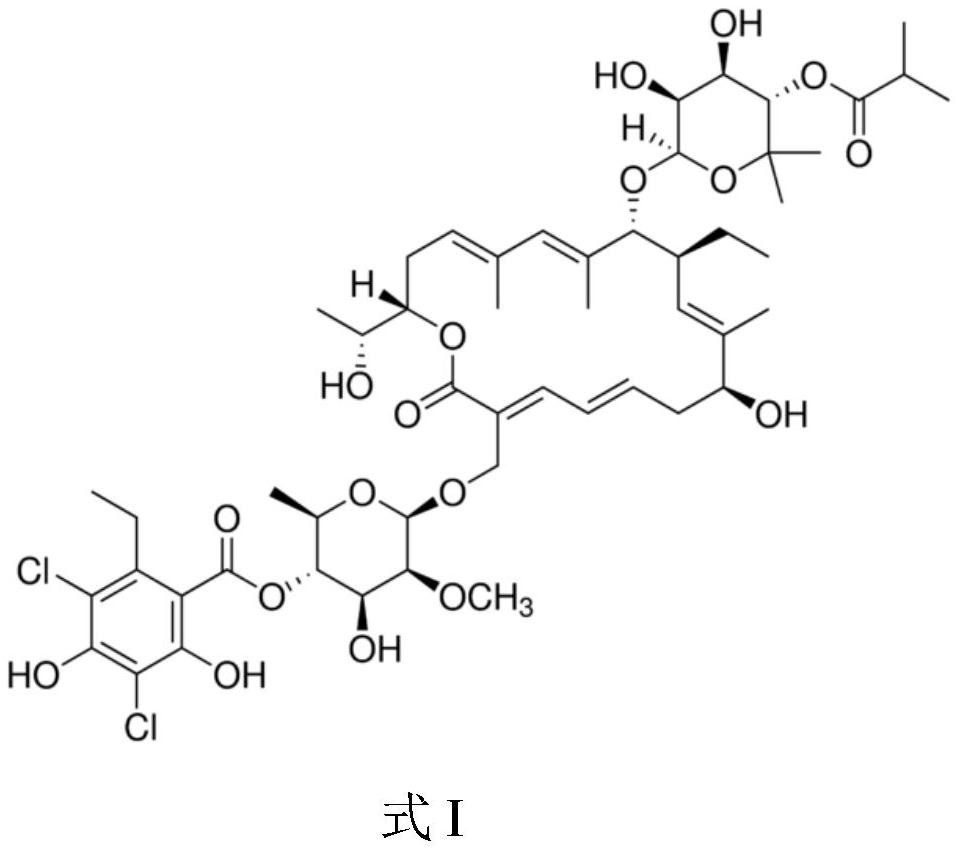
This medication is used to treat a severe intestinal condition (Clostridium difficile-associated diarrhea) due to a type of resistant bacteria.
Actinoplanessp. strain and its use in preparation of fidaxomicin
ActiveCN103320355ARaise the fermentation unitSingle fermentation componentBacteriaMicroorganism based processesChromatographic separationFiltration
The invention discloses an Actinoplanessp. strain and its use in preparation of fidaxomicin. The Actinoplanessp. strain is entophytic Actinoplanessp. N12W0304, is preserved in the China general microbiological culture collection center (CGMCC) on December 26, 2012 and has a preservation number of CGMCC No.7043. A fidaxomicin production process comprises the following steps of preparing an Actinoplanessp. N12W0304 seed solution and fermentation broth, carrying out centrifugation of the fermentation broth to obtain mycelia, carrying out extraction, condensation, dissolution, filtration, resin adsorption and drying of the mycelia to obtain a fidaxomicin crystal crude product, dissolving the fidaxomicin crystal crude product, and carrying out liquid chromatographic separation and pressure-reduction drying of the solution to obtain a fidaxomicin refined product. Under the fermentation conditions, the Actinoplanessp. strain has a fidaxomicin fermentation unit which is more than 1400mg / L and is higher than the fidaxomicin fermentation yield reported at present. The fidaxomicin fermentation process provided by the invention is simple, is suitable for industrial production and realizes a total product yield of 60%.
Owner:NCPC NEW DRUG RES & DEV
Preparation method of high-purity fidaxomicin
ActiveCN103275152AEliminate distractionsImprove qualitySugar derivativesSugar derivatives preparationChromatographic separationMetabolite
The invention discloses a method for separating and purifying fidaxomicin from fidaxomicin fermented mycelia. The method comprises the following steps of first filtering and extracting the fidaxomicin fermentation liquid, diluting the extracted liquor with water, then decoloring, adsorbing and analyzing the extracted liquor through macroporous resin, and concentrating, extracting and drying the analyzed liquor to obtain a fidaxomicin crude product; and dissolving the fidaxomicin crude product in a polarity organic solvent, injecting polymer microsphere columns to carry out the chromatographic separation to obtain the high-purity fidaxomicin fine powder. By adopting the method, the interference of fermented secondary metabolite can be effectively eliminated, and the quality and the yield of the fidaxomicin can be improved. The entire process is simple and easy to operate; the prepared fidaxomicin can be used for drugs; and the purity of the prepared fidaxomicin is more than 95 percent, and the whole-coarse total yield is more than 45 percent. The used solvent is a conventional solvent, small in toxicity and applicable to the industrial production.
Owner:NCPC NEW DRUG RES & DEV
Method for separating and purifying fidaxomicin
ActiveCN104513286AHigh purityHigh recovery rateSugar derivativesSugar derivatives preparationAcetic acidPhysical chemistry
The invention provides a method for separating and purifying fidaxomicin. The method comprises the following steps: taking the C8 reverse phase silica gel as the filling material, carrying out column chromatography, dissolving fidaxomicin by acetic acid, loading the solution into the column, eluting the solution by a mobile phase, and collecting the sample; after the separation and purification, the HPLC purity of fidaxomicin is not less than 99.5%, and the content of a single impurity is not larger than 0.10%. The provided purification method of fidaxomicin adopts a novel medium having high selectivity and high capacity, thus the column has a high efficiency, the resolution is high, the separation and purification effect is better, the retention time is shorter, the elution agent using amount is less, and moreover the shortages that the fidaxomicin purity is low and the fidaxomicin quality is bad due to the low column efficiency and bad separation degree are overcome.
Owner:JIANGSU SENRAN CHEM +1
Fidaxomicin solid dispersion and preparation method thereof
InactiveCN104546666AImprove solubilityPrevent precipitationAntibacterial agentsOrganic active ingredientsClostridium difficile (bacteria)Disease cause
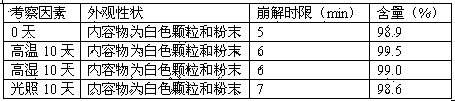
The invention relates to the field of pharmaceutic preparations, in particular to a solid dispersion of fidaxomicin serving as a treating medicament for a disease caused by clostridium difficile and a preparation method for the solid dispersion. The fidaxomicin solid dispersion is solid powder obtained by highly dispersing fidaxomicin into enteric-soluble or water-soluble carrier material, so that the solubility of the fidaxomicin at the small intestine part is greatly improved; compared with a commercially available oral tablet, the fidaxomicin solid dispersion has the advantages of good antibacterial effect, long acting time and little side effect. A medicine composition containing the fidaxomicin solid dispersion provided by the invention is high in solubility and high in stability; the preparation process is simple, the quality is stable, and the fidaxomicin solid dispersion is suitable for industrial production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Actinoplanes strain and application thereof
ActiveCN104560766AIncrease productionRaise the fermentation unitAntibacterial agentsBacteriaClostridium difficile (bacteria)Bacterial strain
Owner:ZHEJIANG HISUN PHARMA CO LTD
Preparation method of fidaxomicin crystal
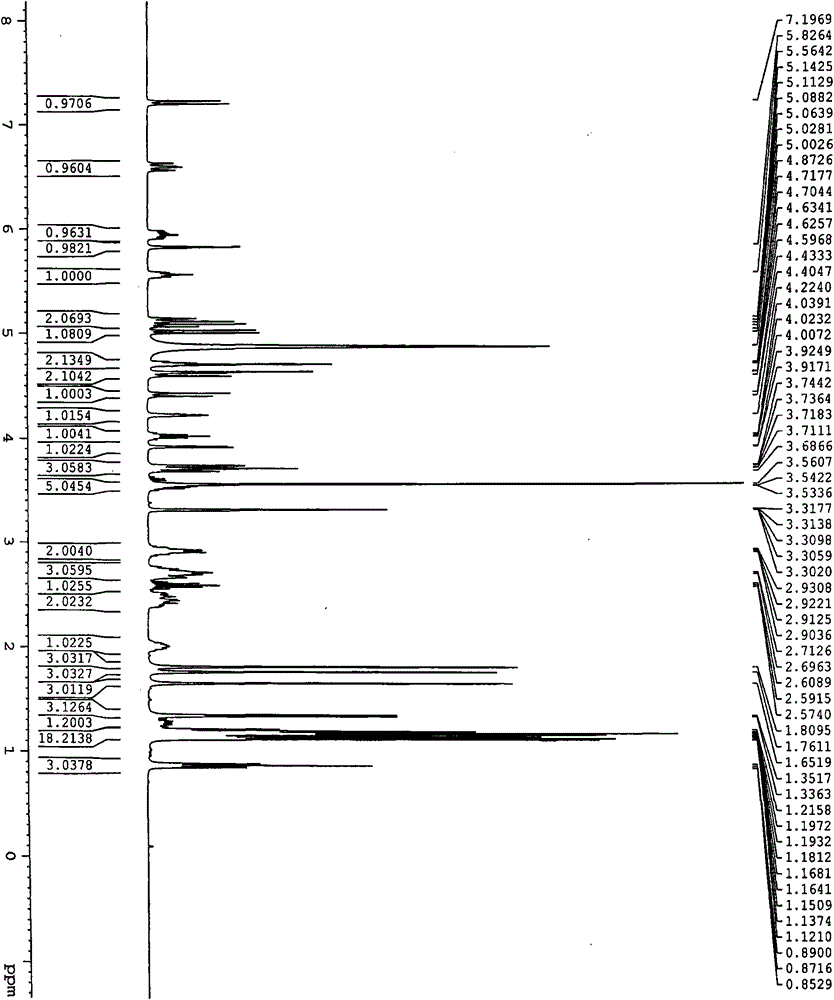
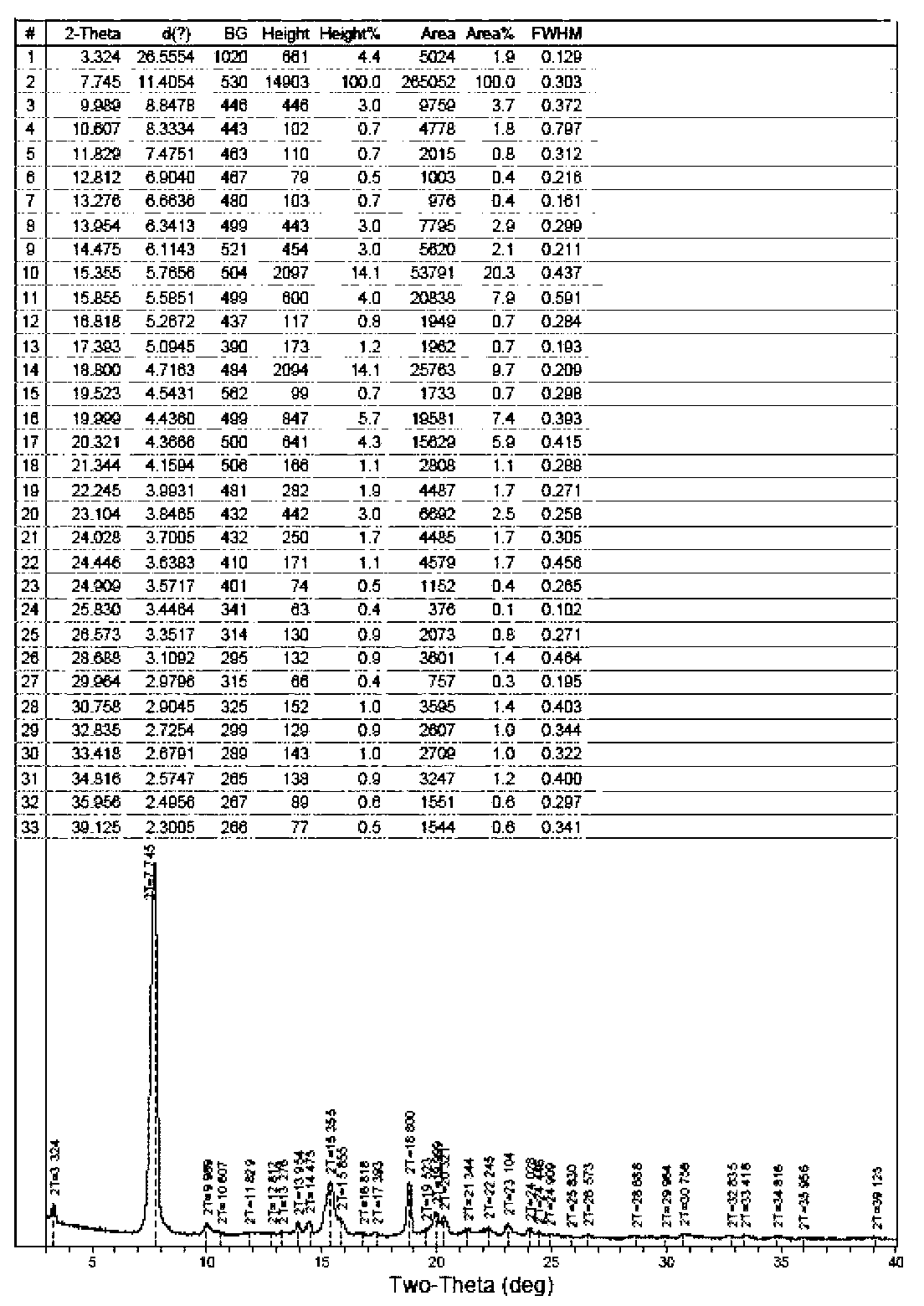
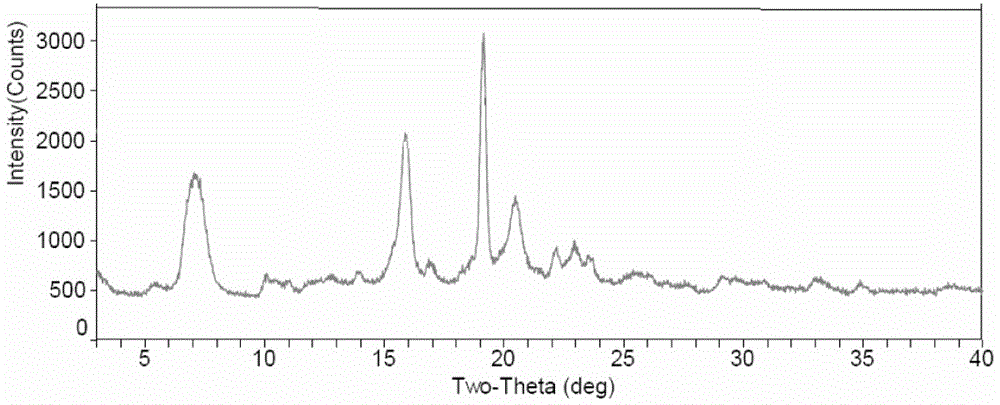
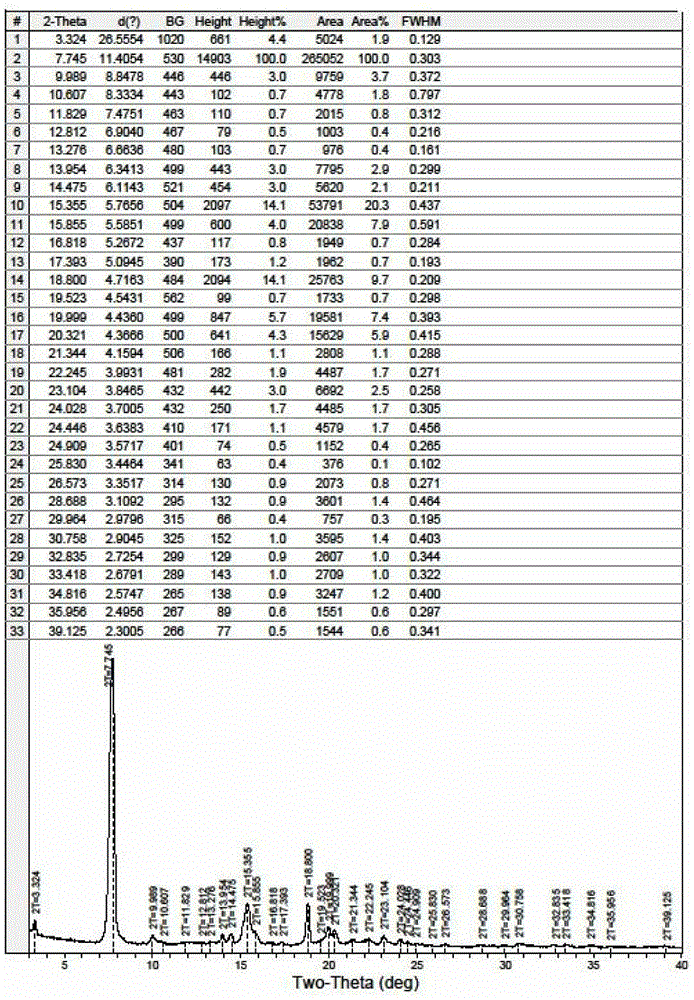
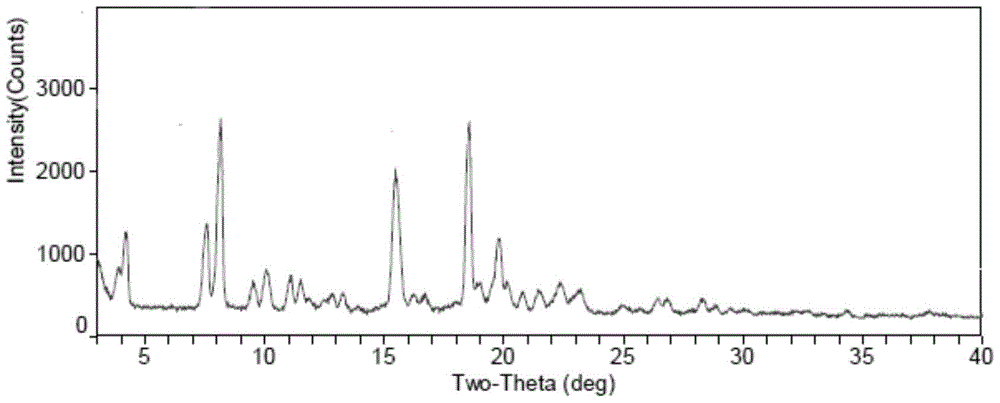
ActiveCN103275153ALow costReduce dosageSugar derivativesSugar derivatives preparationFiltrationX-ray
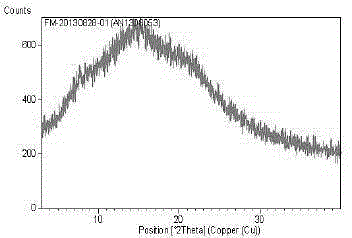
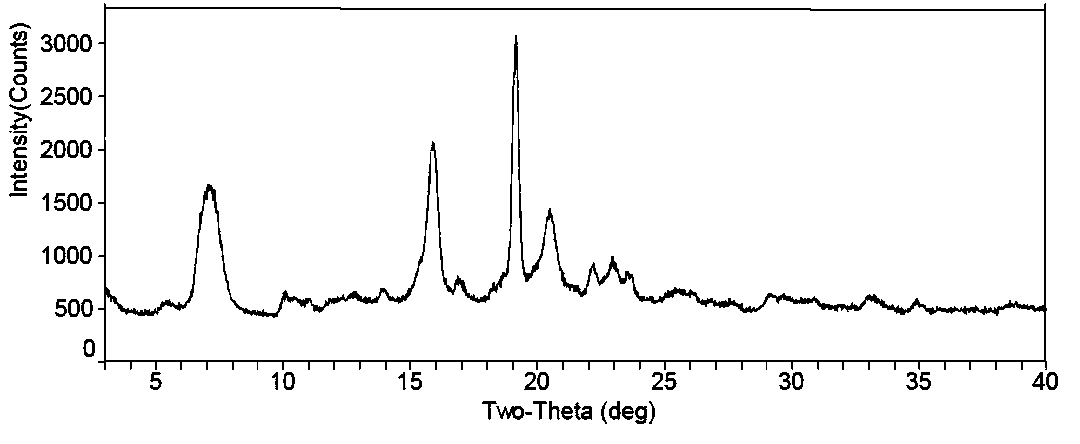
The invention discloses a fidaxomicin crystal and a preparation method of the crystal. Characteristic peaks, expressed by 2 theta, of an X-ray powder diffraction spectrum of the crystal are located at 3.324, 7.745, 9.989, 1.607, 13.954, 14.475, 15.355, 15.855, 18.800, 19.999, 20.321, 22.245, 23.104, 24.028 and 24.446. The method comprises the preparation steps of dissolving fidaxomicin in an aqueous solution of alcohol, crystallizing, conducting suction filtration, drying, and obtaining the fidaxomicin crystal. The preparation method has the advantages that the preparation method is stable in process and simple and quick to operate, facilitates environmental protection, and is appropriate to industrial production. The obtained fidaxomicin is stable in crystal form and uniform in particle, and facilitates subpackage and storage.
Owner:NCPC NEW DRUG RES & DEV
Pharmaceutical Compositions for Rectal Administration
InactiveUS20160002278A1Good spreadabilityProcess stabilityAntibacterial agentsBiocideClostridium difficileRectal use
The present invention relates to pharmaceutical compositions for rectal administration comprising fidaxomicin and to a process for preparing the pharmaceutical compositions for rectal administration. The invention also relates to an aerosol canister comprising a foamable pharmaceutical composition comprising fidaxomicin for rectal administration and to the treatment or maintenance of remission of infections such as diarrhea caused by Clostridium difficile.
Owner:CIPLA LTD
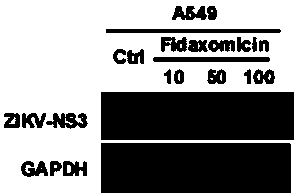
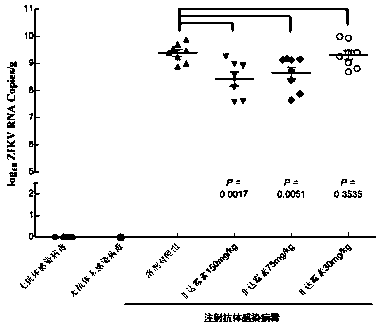
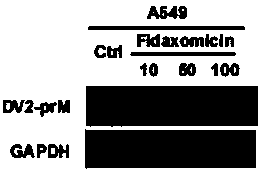
Application of Fidaxomicin in preparation of medicines treating related diseases and/or symptoms caused by Zika virus infection
ActiveCN107737133AImprove securityInhibitory activityOrganic active ingredientsAntiviralsZika virusDisease
The invention discloses application of Fidaxomicin in preparation of medicines treating related diseases and / or symptoms caused by Zika virus infection. Fidaxomicin is approved in clinic to serve as amedicine resisting clostridium difficile infection, is highly safe, has high activity in inhibiting Zika virus, and can relatively strongly bond with non-structural protein NS5 of the Zika virus, thereby being expected to be a new, effective medicine resisting Zika virus infection.
Owner:SUN YAT SEN UNIV
Stable fidaxomicin medicine composition
InactiveCN105640973AAvoid degradationKeep dryAntibacterial agentsOrganic active ingredientsCurative effectCroscarmellose sodium
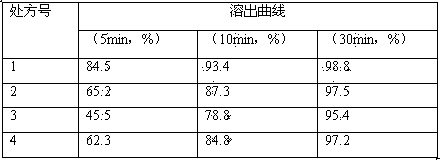
The invention discloses a stable fidaxomicin medicine composition, which is characterized in that a recipe for preparing 1000 tablets comprises the following ingredients including 200 to 400g of fidaxomicin, 70 to 140g of microcrystalline cellulose, 0.5 to 1g of superfine silica gel powder, 50 to 100g of croscarmellose sodium and a proper amount of 10-percent amylum pregelatinisatum solution. The invention also relates to a preparation method of the stable fidaxomicin medicine composition. The fidaxomicin prepared by the recipe and the preparation method has the advantages of high dissolution rate, high bioavailability and good curative effect.
Owner:TIANJIN HANKANG PHARMA BIOTECH
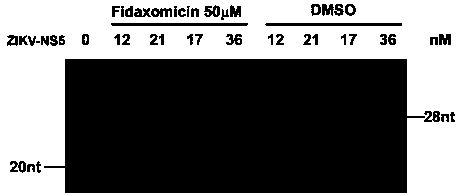
Application of fidaxomicin in preparing medicines for treating related diseases and/or symptoms caused by dengue virus infection
ActiveCN107929300AImprove securityHigh activityOrganic active ingredientsAntiviralsDiseaseClostridium difficile infections
The invention discloses an application of fidaxomicin in preparing medicines for treating related diseases and / or symptoms caused by dengue virus infection. The fidaxomicin, as a clinically approved drug for resisting clostridium difficile infection, is high in safety; and in addition, the fidaxomicin, which is high in activity of inhibiting the dengue virus infection and relatively strong in binding capacity with dengue virus non-structural protein NS5, is expected to function as a novel drug for resisting the dengue virus infection.
Owner:SUN YAT SEN UNIV
Method for preparing fidaxomicin by flash chromatography
InactiveCN106632551AEasy accessHigh puritySugar derivativesSugar derivatives preparationChromatographic separationElution
The invention discloses a method for preparing fidaxomicin by flash chromatography. The method comprises the following steps: firstly, extracting fidaxomicin fermentation mycelia with 60 to 80 percent ethanol, roughly separating extract liquid through polystyrene resin, and collecting a fraction which contains fidaxomicin; then, performing reverse-phase bonding silica gel rapid column chromatography separation twice on the fraction, wherein elution solvents of different selectivity are adopted; finally obtaining a non-fidaxomicin refined product of which the purity is over 98.5 percent. By adopting the method, the purity and yield of the obtained fidaxomicin are high; moreover, the method is relatively easy to operate, is relatively low in cost, and is suitable for industrial production.
Owner:FUJIAN INST OF MICROBIOLOGY
Fidaxomicin crystal form II and preparation method thereof
ActiveCN103897003AImprove stabilityLow costAntibacterial agentsOrganic active ingredientsX-rayPowder diffraction
The invention discloses a fidaxomicin crystal form II and a preparation method thereof. An X-ray powder diffraction spectrum of the crystal formula has X-ray powder diffraction peaks at 2theta, namely 7.04 degrees, 15.87 degrees, 19.14 degrees, 20.46 degrees and 22.95 + / -0.2 degrees. The crystal form obtained by the method is stable, simple in preparation method, strong in maneuverability and applicable to industrial production.
Owner:NCPC NEW DRUG RES & DEV
Fidaxomicin crystal form I and preparation method thereof
ActiveCN103880904ALow costReduce dosageAntibacterial agentsOrganic active ingredientsPowder diffractionFidaxomicin
The invention discloses a fidaxomicin crystal form I and a preparation method thereof. An X-ray powder diffraction spectrum of the crystal form has X-ray powder diffraction peaks at 2theta of 4.22 degrees, 7.60 degrees, 8.17 degrees, 9.51 degrees, 10.07 degrees, 11.07 degrees, 11.51 degrees, 12.85 degrees, 13.28 degrees, 15.47 degrees, 16.24 degrees, 18.55 degrees, 19.78 degrees, 20.15 degrees, 20.80 degrees and 21.47 degrees. The crystal form provided by the invention is stable, simple in preparation method, strong in maneuverability, and applicable to industrial production.
Owner:NCPC NEW DRUG RES & DEV
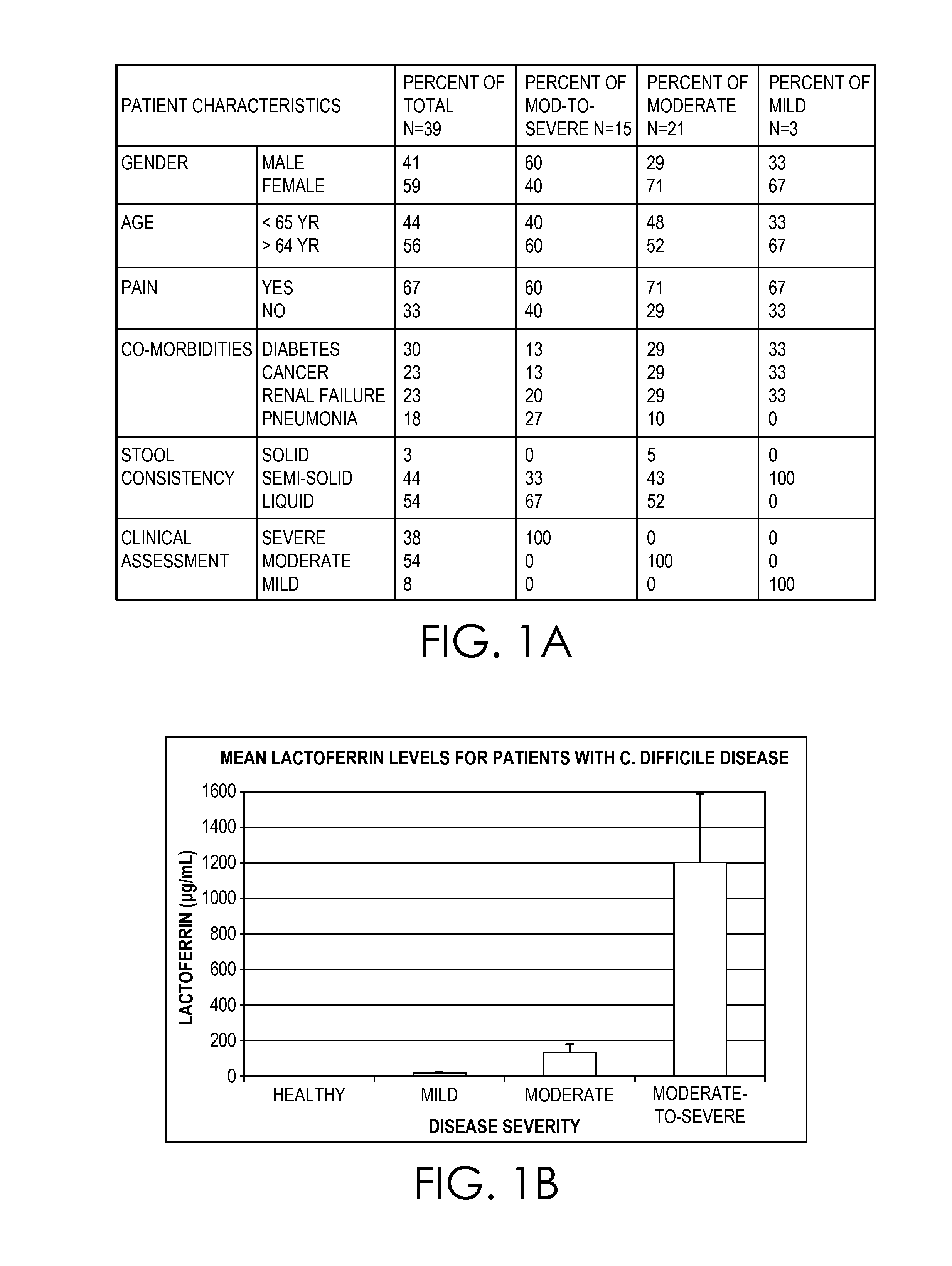
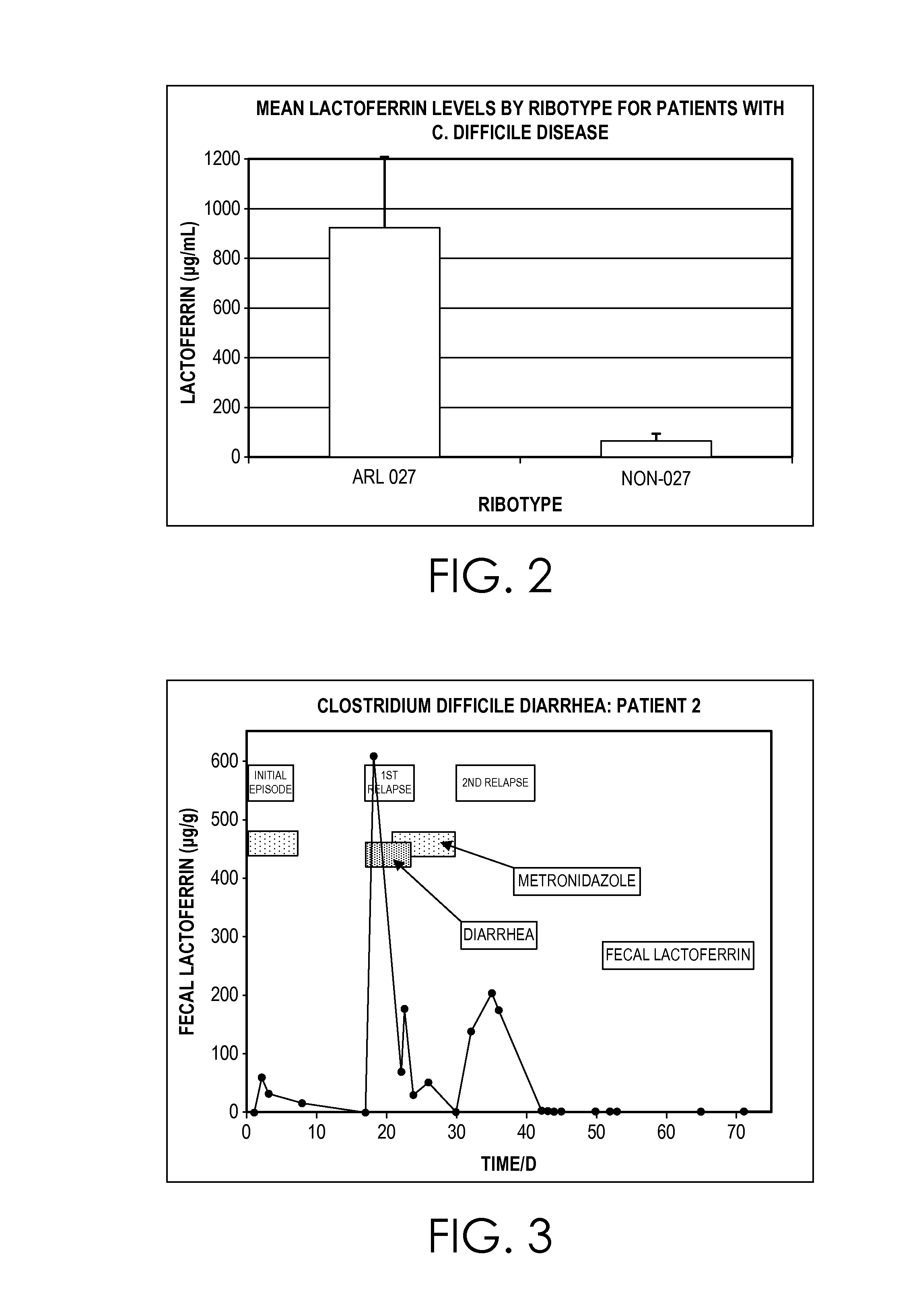
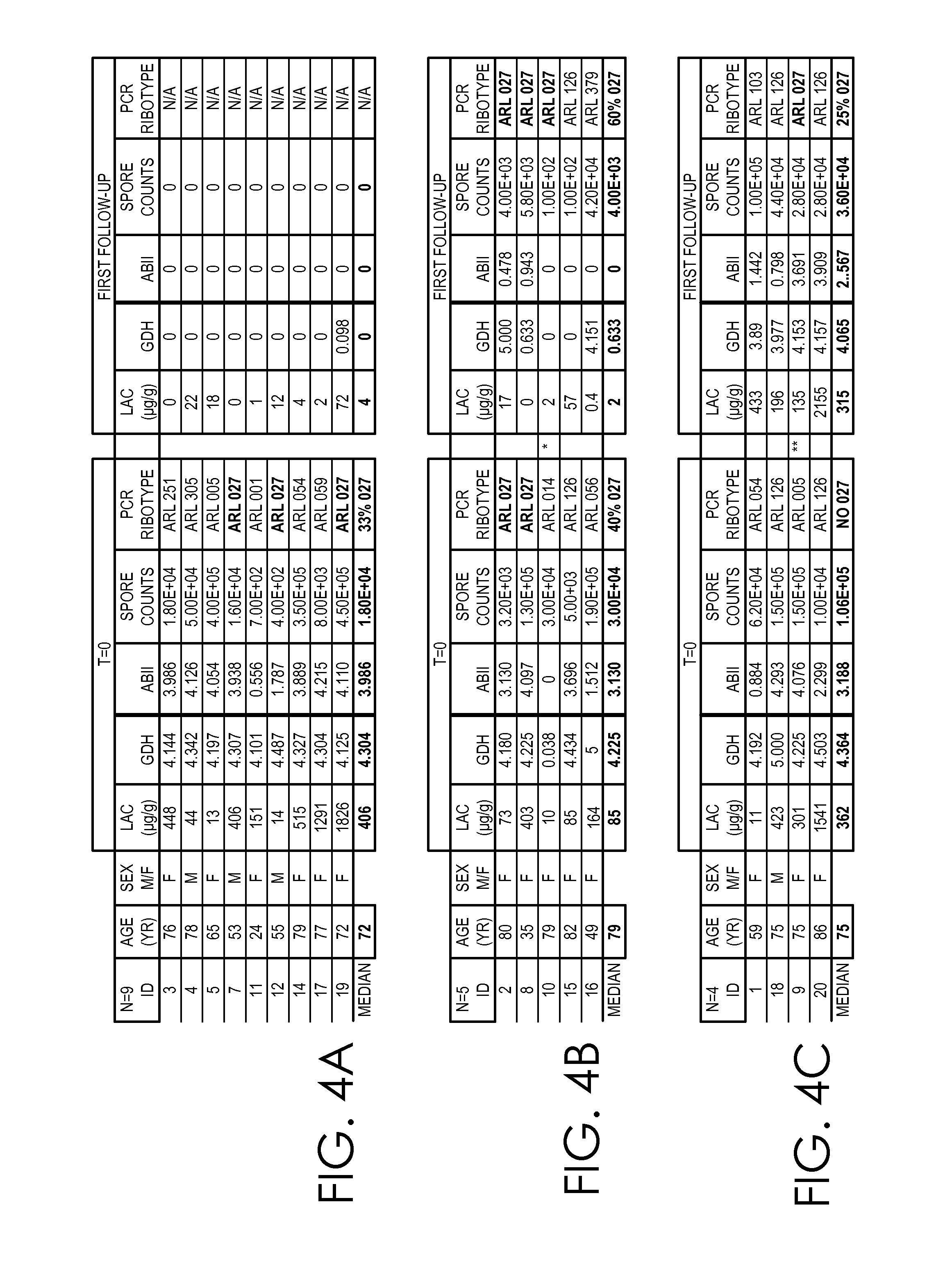
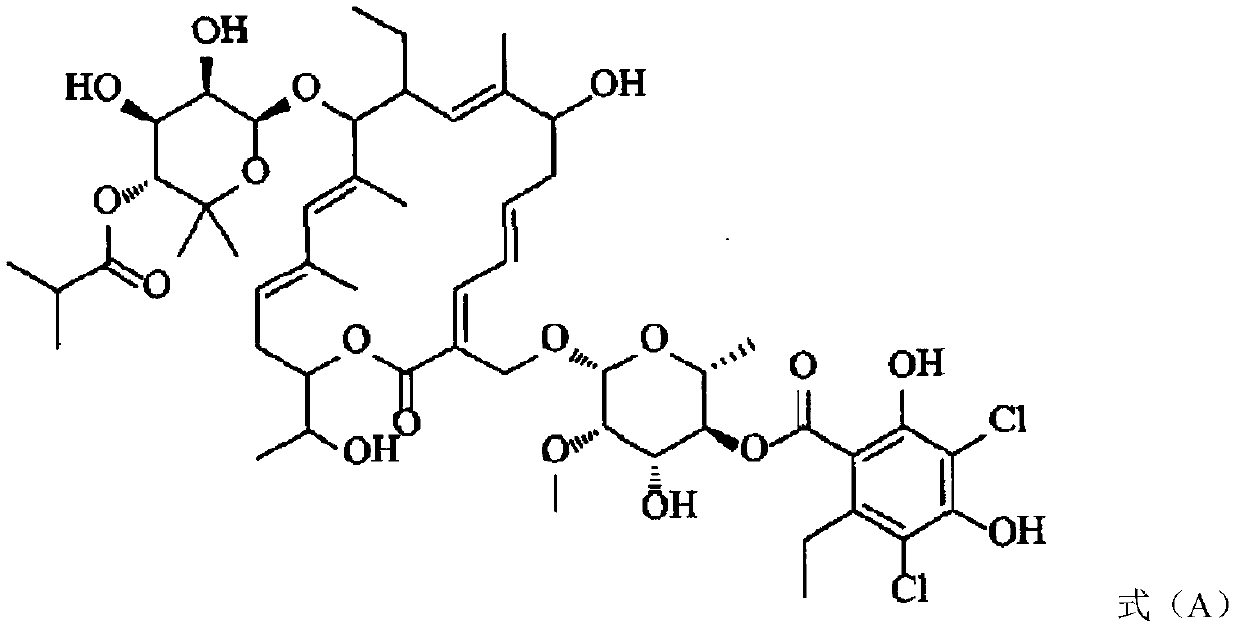
Clostridium difficile dehydrogenase and toxin as a biomarker for monitoring infection in patients with clostridium difficile disease and differentiating carrier state from active disease
Clostridium difficile disease involves a range of clinical presentations ranging from carrier status with other causes of symptoms to mild and self-limiting diarrhea to life-threatening pseudomembranous colitis and megacolon. Cases of C. difficile are treated differently depending on the presence and then the severity of disease. Patients that are carriers may not receive treatment with concern of causing the disease. Mild to moderate cases may be treated with metronidazole while severe and relapsing cases are often treated with vancomycin or fidaxomicin. Current molecular assays are highly sensitive for detecting toxigenic C. difficile and cannot rule out carrier status. Utilization of a biomarker panel that includes C. difficile antigen (GDH), toxins A and B, and fecal lactoferrin allows clinicians to differentiate between a carrier state and active state of C. difficile and allows for monitoring to evaluate the effectiveness of treatment.
Owner:TECH LAB
Fidaxomicin impurities and preparation method thereof
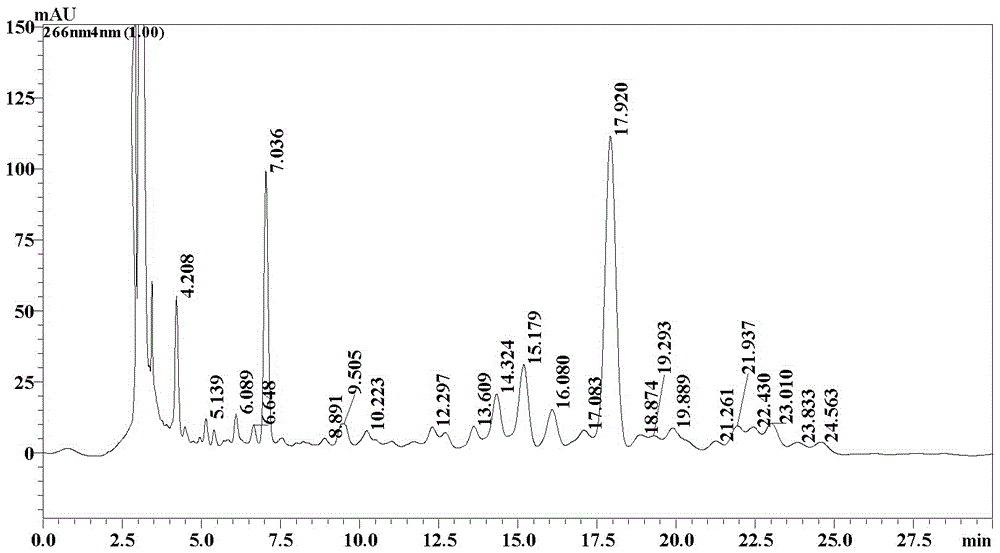
InactiveCN110183502AImprove quality controllabilityQuality improvementSugar derivativesSugar derivatives preparationQuality controlImpurity
The invention provides fidaxomicin new impurities and a preparation method thereof. The impurities can be applied to quality control of fidaxomicin raw materials and / or preparations, and is used for impurity contrast positioning, quality determination or quantity determination in the fidaxomicin raw materials and / or preparations, and improvement of the quality of the fidaxomicin raw materials and / or preparations is facilitated.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Pharmaceutical compositions for rectal administration
InactiveCN105142612ACooperate wellEasy to useAntibacterial agentsOrganic active ingredientsClostridium difficilePharmaceutical drug
The present invention relates to pharmaceutical compositions for rectal administration comprising fidaxomicin and to a process for preparing the pharmaceutical compositions for rectal administration. The invention also relates to an aerosol canister comprising a foamable pharmaceutical composition comprising fidaxomicin for rectal administration and to the treatment or maintenance of remission of infections such as diarrhoea caused by Clostridium difficile..
Owner:CIPLA LTD
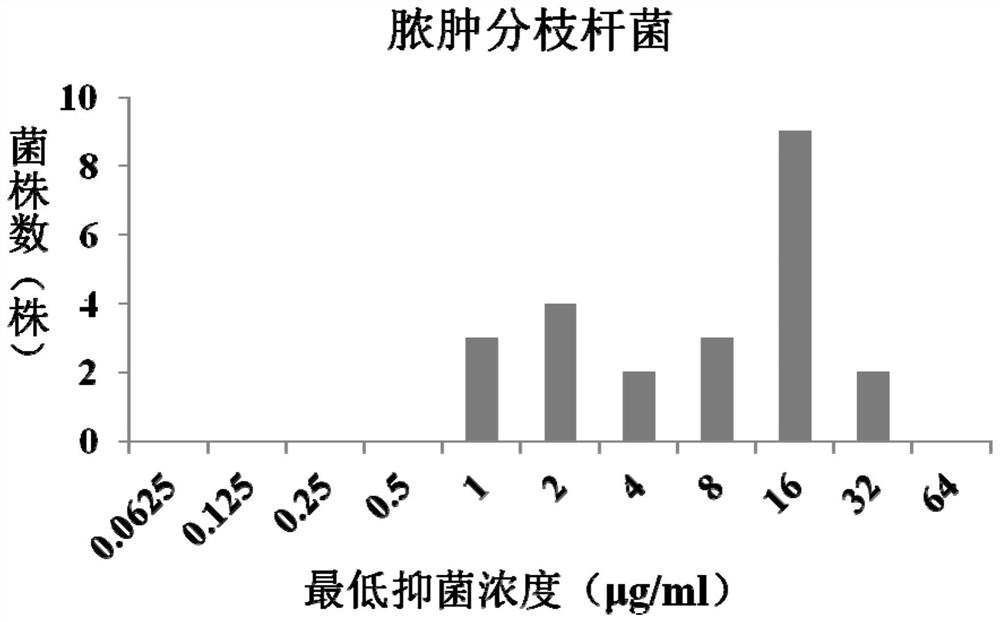
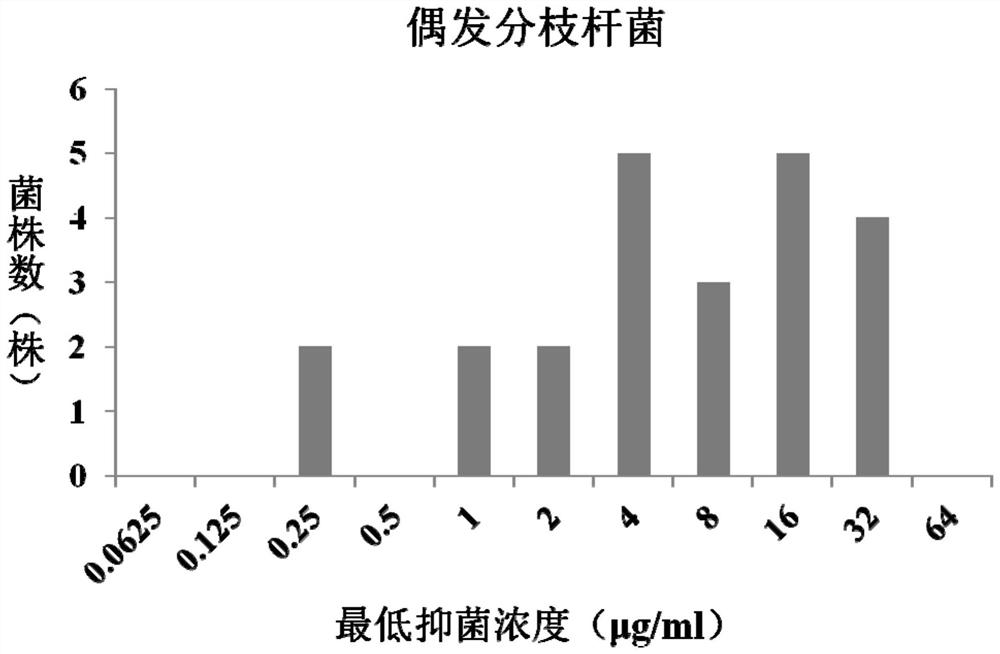
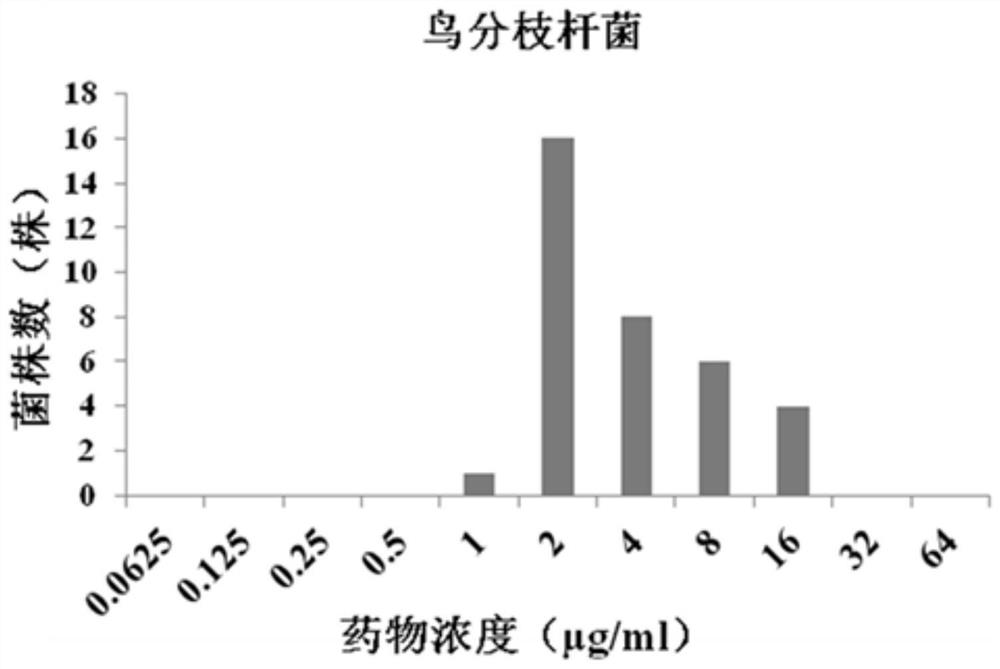
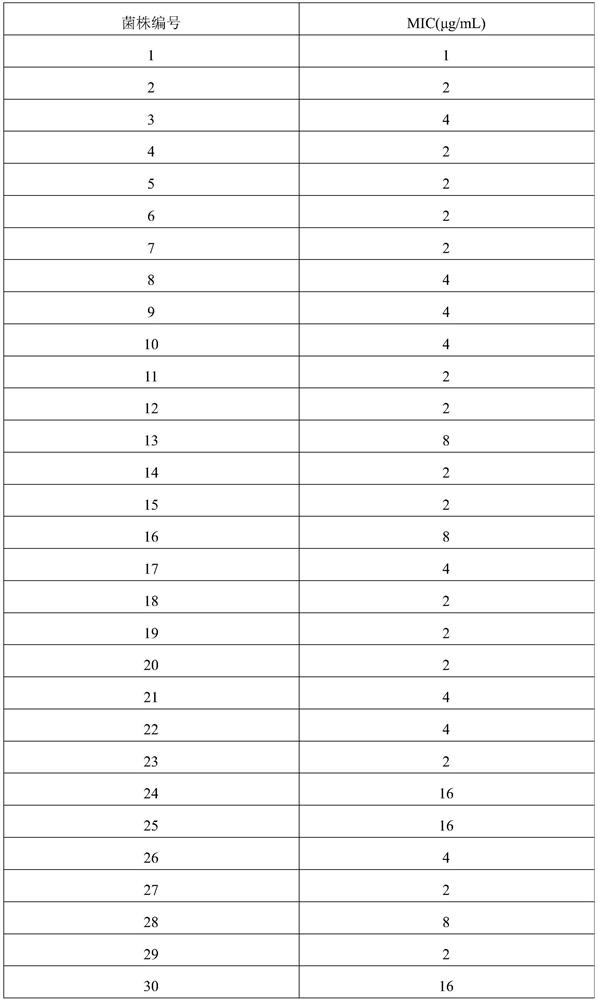
Application of fidaxomicin to preparation of product for inhibiting activity of mycobacterium abscessus
PendingCN114224899AAntibacterial agentsOrganic active ingredientsDiseaseMycobacterium abscessus Infections
The invention discloses application of fidaxomicin to preparation of a product for inhibiting activity of mycobacterium abscessus. The invention provides application of fidaxomicin or a pharmaceutically acceptable salt thereof or a substance taking the fidaxomicin or the pharmaceutically acceptable salt thereof as an active ingredient in preparation of a product for inhibiting activity of mycobacterium abscessus. According to the invention, the anti-mycobacterium abscessus activity of fidaxomicin is determined by adopting a microwell plate double dilution method, and the result shows that fidaxomicin has better bacteriostatic activity on a mycobacterium abscessus standard strain and clinically separated mycobacterium abscessus; new application of fidaxomicin in prevention and treatment of mycobacterium abscessus infection diseases is expected to be explored.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV +3
Fidaxomycin crystal form II and preparation method thereof
ActiveCN103897003BImprove stabilityLow costAntibacterial agentsOrganic active ingredientsX-rayPowder diffraction
Owner:NCPC NEW DRUG RES & DEV
Application of fidaxomicin in the preparation of medicines for treating related diseases and/or symptoms caused by dengue virus infection
ActiveCN107929300BImprove securityHigh activityOrganic active ingredientsAntiviralsDiseaseClostridium difficile infections
The invention discloses an application of fidaxomicin in preparing medicines for treating related diseases and / or symptoms caused by dengue virus infection. The fidaxomicin, as a clinically approved drug for resisting clostridium difficile infection, is high in safety; and in addition, the fidaxomicin, which is high in activity of inhibiting the dengue virus infection and relatively strong in binding capacity with dengue virus non-structural protein NS5, is expected to function as a novel drug for resisting the dengue virus infection.
Owner:SUN YAT SEN UNIV
Preparationmethod of macrolide compound
ActiveCN104497078AImprove conversion rateImprove antibacterial propertiesSugar derivativesSugar derivatives preparationOrganic solventMacrolide resistance
The invention discloses a preparation method of a macrolide compound OP-1118. The method comprises the following steps: a, reacting fidaxomicin in alkaline solution to obtain a reaction product; b, dissolving the reaction product obtained in the step a in an organic solvent to obtain a sample liquid; and c, conducting chromatography on the sample solution in a C18 medium pressure column to obtain a macrolide compound OP-1118 with purity more than 98.5%. The preparation method of the invention has the advantages of simple operation and high sample recovery rate, and is applicable to industrialization production.
Owner:NCPC NEW DRUG RES & DEV
Method for separating and purifying Fidaxomycin
ActiveCN109251229BHigh purityLow puritySugar derivativesSugar derivatives preparationOrganic solventDesiccant
Owner:CHENGDU UNIV
Actinoplanessp. strain and its use in preparation of fidaxomicin
The invention discloses an Actinoplanessp. strain and its use in preparation of fidaxomicin. The Actinoplanessp. strain is entophytic Actinoplanessp. N12W0304, is preserved in the China general microbiological culture collection center (CGMCC) on December 26, 2012 and has a preservation number of CGMCC No.7043. A fidaxomicin production process comprises the following steps of preparing an Actinoplanessp. N12W0304 seed solution and fermentation broth, carrying out centrifugation of the fermentation broth to obtain mycelia, carrying out extraction, condensation, dissolution, filtration, resin adsorption and drying of the mycelia to obtain a fidaxomicin crystal crude product, dissolving the fidaxomicin crystal crude product, and carrying out liquid chromatographic separation and pressure-reduction drying of the solution to obtain a fidaxomicin refined product. Under the fermentation conditions, the Actinoplanessp. strain has a fidaxomicin fermentation unit which is more than 1400mg / L and is higher than the fidaxomicin fermentation yield reported at present. The fidaxomicin fermentation process provided by the invention is simple, is suitable for industrial production and realizes a total product yield of 60%.
Owner:NCPC NEW DRUG RES & DEV
Fermentation production method of fidaxomicin
ActiveCN104561198AWide variety of sourcesMeet the needs of fermentationMicroorganism based processesFermentationBiotechnologyEngineering
The invention discloses a fermentation production method of fidaxomicin. In the fermentation production process of fidaxomicin, edible oil is added to a fermentation liquid, and the productivity of target products is improved; when dissolved oxygen of the fermentation liquid starts to rise from the lowest point, edible oil is intermittently or continuously added. According to the method, the operation is simple, the condition is mild, the raw material source is wide, the equipment requirement is low, the production cost is reduced, the fermentation yield is greatly increased, and the method is suitable for industrial production.
Owner:CHONGQING QIANTAI BIOLOGICAL MEDICINE +1
Application of fidaxomicin in preparation of product for resisting mycobacterium fortuitum infection
The invention discloses an application of fidaxomicin in preparation of a product for resisting mycobacterium fortuitum infection. The invention provides an application of fidaxomicin or a pharmaceutically acceptable salt thereof or a substance taking the fidaxomicin or the pharmaceutically acceptable salt thereof as an active ingredient in preparation of a mycobacterium fortuitum bacteriostatic agent. According to the invention, the anti-mycobacterium fortuitum activity of the fidaxomicin is determined by adopting a microwell plate double dilution method, and the result shows that the fidaxomicin has relatively good bacteriostatic activity on mycobacterium fortuitum standard strains and clinically separated mycobacterium fortuitum; new application of fidaxomicin in prevention and treatment of accidental mycobacterium infection diseases is expected to be explored.
Owner:TSINGHUA UNIV +3
A kind of preparation method of fidaxomicin crystal
ActiveCN103275153BLow costReduce dosageSugar derivativesSugar derivatives preparationSolventAqueous solution
Owner:NCPC NEW DRUG RES & DEV
A kind of Fidaxomycin enteric-coated preparation
ActiveCN104546672BAvoid degradation consumptionQuick releaseAntibacterial agentsOrganic active ingredientsIntestinal structureTime lag
Owner:NCPC NEW DRUG RES & DEV
Stable fidaxomicin composition
ActiveCN110037992AReduce the overall heightRaise quality standardsAntibacterial agentsOrganic active ingredientsGranulation procedureAnoxomer
The invention discloses a pharmaceutical composition containing fidaxomicin and a preparation method thereof. The preparation method comprises the following steps: preparing granules containing fidaxomicin by adopting wet process granulation; and compacting the granules into the pharmaceutical composition. According to the composition containing the fidaxomicin disclosed by the invention, throughimproving the water addition amount in a wet process granulation procedure, the stirring velocity during granulation and the time required for wet process granulation, even if under the case of no adding an antioxidant, the prepared composition containing the fidaxomicin has high stability, less increase of related substances, meets the quality standard of drugs, can reduce a requirement on a temperature in a storage step and is stable in dissolution.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
Method for separating and purifying fidaxomicin
ActiveCN109251229AHigh purityLow puritySugar derivativesSugar derivatives preparationOrganic solventGradient elution
The invention provides a method for separating and purifying fidaxomicin. The method solves the problems of a great number of separation steps, high production cost and low recovery rate of conventional methods for separating and purifying fidaxomicin. The method of the invention comprises the following steps: (1) dissolving a crude product containing fidaxomicin in an organic solvent, adding an alkali solution for washing, drying an organic phase with a drying agent, and then performing concentration under reduced pressure to obtain a concentrate; (2) allowing the concentrate obtained in thestep (1) to pass through a polyamide column, carrying out gradient elution, collecting an eluate containing fidaxomicin, and then performing concentration under reduced pressure and crystallization successively to obtain a white powdery pure product of fidaxomicin. The obtained fidaxomicin has a purity of 97% or more and total yield of 75% or more. The method of the invention can prepare high-purity fidaxomicin, has the advantages of fewer steps, simple and practicable process, high yield and environmental friendliness, and is very suitable for commercial production.
Owner:CHENGDU UNIV
Application of fidaxomicin in preparation of product for inhibiting activity of mycobacterium avium
The invention discloses application of fidaxomicin in preparation of a product for inhibiting the activity of mycobacterium avium. The invention provides application of fidaxomicin or pharmaceuticallyacceptable salt thereof or a substance taking fidaxomicin or pharmaceutically acceptable salt thereof as an active ingredient in preparation of a product for inhibiting the activity of mycobacteriumavium. According to the application of the fidaxomicin, the fidaxomicin anti-mycobacterium avium activity determination is carried out by adopting a microwell plate double dilution method, the resultshows that the fidaxomicin has good antibacterial activity on mycobacterium avium standard strains and clinically separated mycobacterium avium, and the new application of the fidaxomicin in prevention and treatment of mycobacterium avium infection diseases is expected to be explored.
Owner:BEIJING CHEST HOSPITAL CAPITAL MEDICAL UNIV +3
Fidaxomycin crystal form I and preparation method thereof
ActiveCN103880904BLow costReduce dosageAntibacterial agentsOrganic active ingredientsX-rayPowder diffraction
The invention discloses a fidaxomicin crystal form I and a preparation method thereof. An X-ray powder diffraction spectrum of the crystal form has X-ray powder diffraction peaks at 2theta of 4.22 degrees, 7.60 degrees, 8.17 degrees, 9.51 degrees, 10.07 degrees, 11.07 degrees, 11.51 degrees, 12.85 degrees, 13.28 degrees, 15.47 degrees, 16.24 degrees, 18.55 degrees, 19.78 degrees, 20.15 degrees, 20.80 degrees and 21.47 degrees. The crystal form provided by the invention is stable, simple in preparation method, strong in maneuverability, and applicable to industrial production.
Owner:NCPC NEW DRUG RES & DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com