Fidaxomicin crystal form I and preparation method thereof
A technology of fidaxomicin and crystal form, applied in the field of fidaxomycin crystal form I and preparation thereof, can solve problems such as poor crystal form stability, shortened product shelf life, etc., achieves low cost, short time consumption, avoids human body the effect of damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Take 1 gram of fidaxomicin and dissolve it ultrasonically with 30 ml of n-heptane-methyl ethyl ketone (1:1) solution. After the dissolution is complete, place it at room temperature for 7 days. After drying for 1 hour, the crystalline form I of Fidaxomycin was obtained.
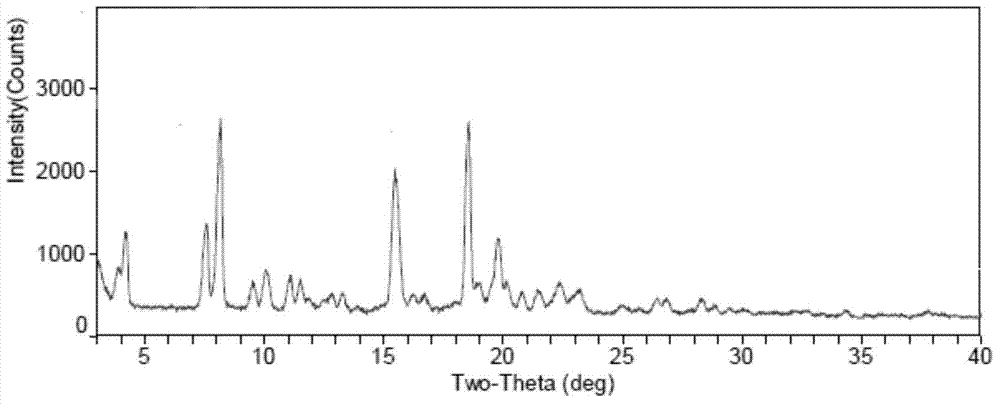
[0038] The XRD value of the prepared Fidaxomycin crystal form I is shown in Table 1, and the spectrum is as follows figure 1 As shown, the figure shows: in the X-ray powder diffraction diagram at 4.22°, 7.60°, 8.17°, 9.51°, 10.07°, 11.07°, 11.51°, 12.85°, 13.28°, 15.47°, 16.24°, 18.55° , 19.78°, 20.15°, 20.80°, 21.47°2θ have X-ray powder diffraction peaks.
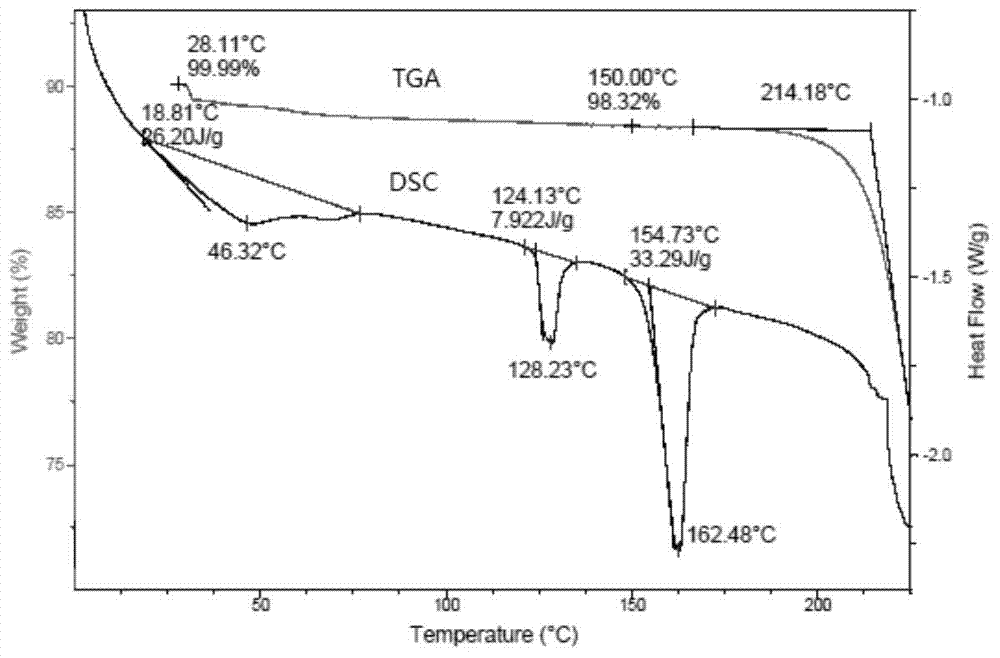
[0039] The TGA and DSC spectra for detecting Fidaxomycin crystal form I are as follows: figure 2 shown.
[0040] Fidaxomycin crystal form I prepared by the present invention has X-ray powder diffraction characteristic peaks at 4.22°, 8.17°, 9.51°, 11.07°, 11.51°, 12.8°, 13.3°, 16.24°, 20.80° and 21.47°2θ , and there are no descriptions of thes...
Embodiment 2
[0042] Take 1 gram of fidaxomicin, dissolve it ultrasonically with 40 ml of 35% acetone aqueous solution, place it at 0°C for 5 days after the dissolution is complete, perform vacuum filtration after crystallization, and vacuum-dry the filter cake in a 40°C oven for 4 hours to obtain Fidaxomicin Form I.
Embodiment 3
[0044] Take 1 gram of fidaxomicin and dissolve it with 100 ml of isopropanol under heating at 50°C. After the dissolution is complete, cool to 10°C and crystallize for 24 hours. , and the filter cake was vacuum-dried in an oven at 40° C. for 6 hours to obtain crystalline form I of Fidaxomycin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com