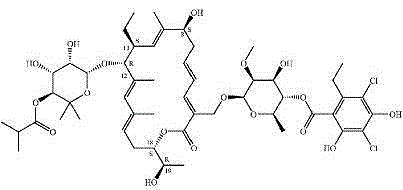
A kind of preparation method of fidaxomicin crystal
A technology for fidaxomicin and crystals, applied in the field of preparation of fidaxomycin crystals, can solve problems such as limiting industrial application, increasing production cost, adverse environmental impact, etc., achieving low cost, avoiding human injury, and solvent dosage. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] Preparation of Fidaxomycin fine powder:
[0022] Take 10 liters of fidaxomicin fermentation broth, and the fermentation unit is 510 micrograms / ml. The fidaxomicin fermentation broth was filtered to obtain 2.5 kg of mycelia. Add 5 liters of 80% methanol aqueous solution to the above-mentioned mycelia, stir and extract for 1 hour, then filter, and collect the fidaxomicin extract. The extract was diluted with water to a methanol concentration of 30%, and then introduced into a macroporous resin LX98 column for decolorization. The resin loading capacity was 500 ml, and the flow rate was 1000 ml / hour. The decolorization solution is introduced into the macroporous resin HZ816 column again for adsorption, the resin loading capacity is 500 milliliters, and the flow rate is 1000 milliliters / hour. The saturated resin was desorbed successively with 40% and 80% methanol aqueous solution at a flow rate of 500 ml / hour, and the desorption solution was collected in sections. The f...
Embodiment 1
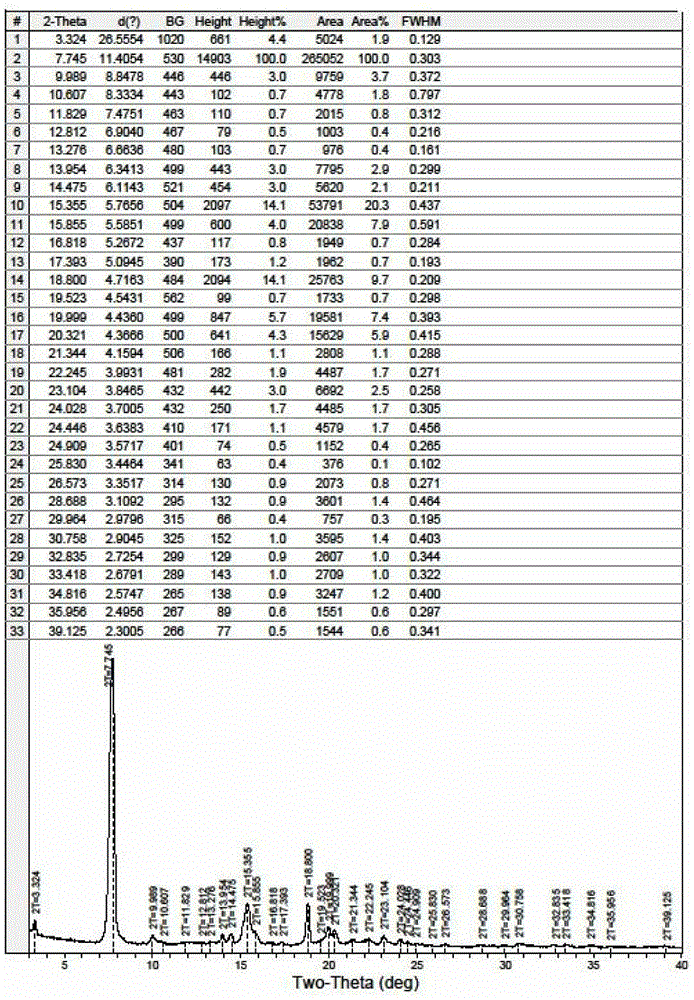
[0024] Take 20 grams of fidaxomicin, heat and dissolve it with 600 ml of 60% ethanol aqueous solution at 45°C, cool to 0°C and crystallize for 24 hours after the dissolution is complete, perform vacuum filtration after crystallization, and place the filter cake in an oven at 45°C After vacuum drying for 2 hours, 15.2 g of fidaxomicin crystals were obtained, with a yield of 76%. The XRPD pattern of the made Fidaxomycin crystal is as follows figure 1 shown.
Embodiment 2
[0026] Take 20 grams of fidaxomicin and dissolve it with 200 ml of 70% ethanol aqueous solution under heating at 40°C. After the dissolution is complete, cool to 20°C for crystallization for 48 hours. Vacuum drying in an oven for 3 hours yielded 16 g of fidaxomicin crystals, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com