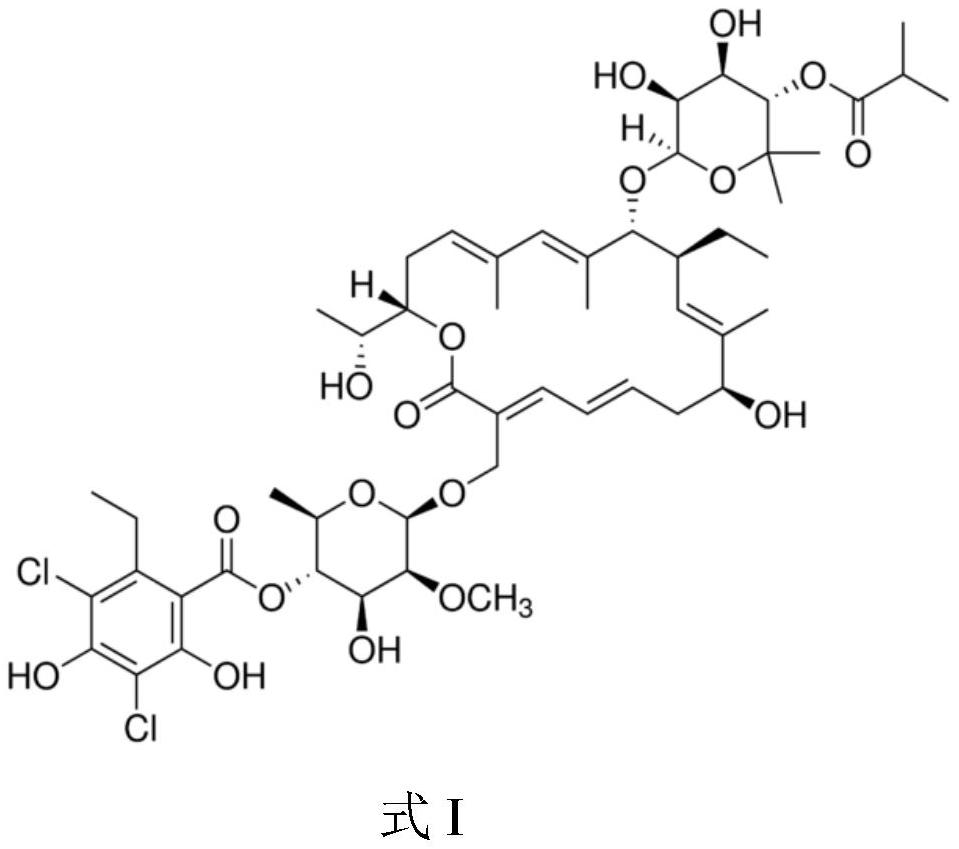
Application of fidaxomicin to preparation of product for inhibiting activity of mycobacterium abscessus
A technology of fidaxomicin and mycobacteria, applied in the field of medicine, can solve the problems that fidaxomicin has not yet inhibited mycobacterium abscessus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, Fidaxomycin is detected to the antibacterial activity of Mycobacterium abscessus standard strain
[0045] Drug to be tested: Fidaxomicin
[0046] 1. Add 100 μl of Mueller Hinton (MH) medium to each well of a 96-well plate;
[0047] 2. After completing step 1, take the 96-well plate and add 100 μl of the drug solution to be tested (prepared in DMSO) with a concentration of 128 μg / mL in the 12th column. After the second column, take out 100 μl and discard it, and the first column does not contain drug, which is the positive control well. Three replicate wells were set up for each concentration.
[0048] 3. After completing step 2, take the 96-well plate and add 100 μl of the bacterial suspension of Mycobacterium abscessus standard strain ATCC19977 to each well, so that the final volume in each well is 200 μl, and the final concentration of the bacterial solution is 2.5×10 5 CFU / mL; see Table 1 for the final drug concentration in each column well.
[0049...
Embodiment 2
[0060] Example 2, Fidaxomycin is detected for the antibacterial activity of clinically isolated Mycobacterium abscessus bacterial strains
[0061] Clinically isolated strains: 23 strains were isolated and cultured from sputum specimens of patients with Mycobacterium abscessus infection, and were identified as Mycobacterium abscessus by sequencing of 16SrRNA, hsp65, rpoB, and 16-23S rRNA region.
[0062] Drug to be tested: Fidaxomicin.
[0063] According to the method in Example 1, the bacteriostatic activity of the drug to be tested against 23 clinically isolated Mycobacterium abscessus strains was detected.
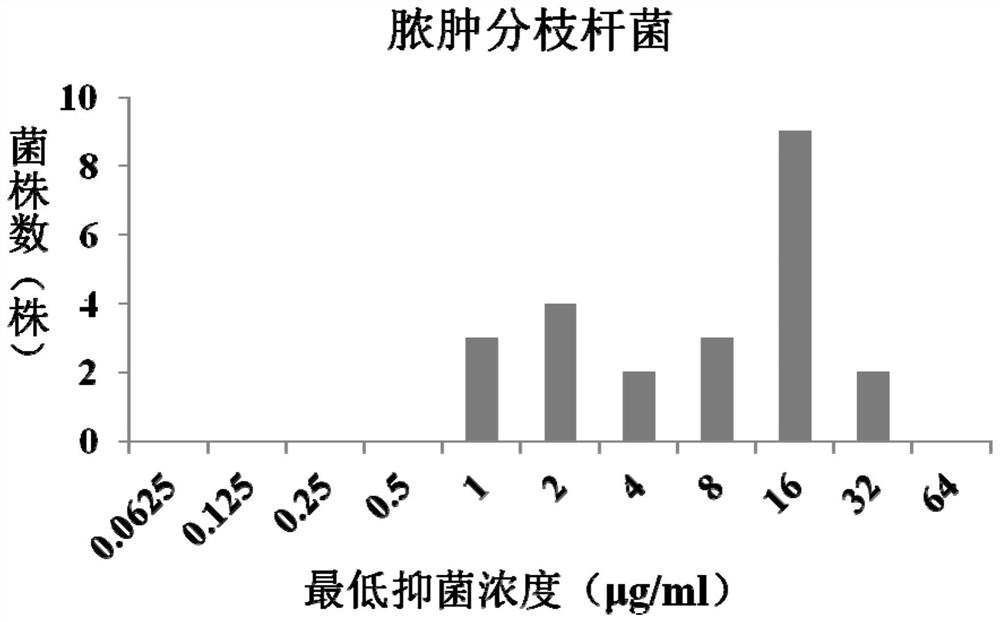
[0064] The MIC results are shown in Table 2. The statistical results of MIC concentration distribution are shown in Table 3 and figure 1 shown.
[0065] Table 2 Bacteriostatic activity of fidaxomicin to clinical isolates of Mycobacterium abscessus strains
[0066]
[0067]
[0068] Table 3 Statistical results of the MIC concentration distribution of fidaxomicin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com