Actinoplanessp. strain and its use in preparation of fidaxomicin
A technology of swimming actinomycetes and fidaxomicin, applied in the directions of bacteria, microorganism-based methods, biochemical equipment and methods, etc., to achieve the effects of single fermentation component, high fermentation unit, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Isolation and identification of embodiment 1 bacterial strain
[0040] 1. Strain isolation
[0041] Agrimony samples were collected from Yunnan (23°22′39.648" north latitude, 104°47′17.0808" east longitude, 1498.7432m above sea level). The samples were stored at 4°C after collection, and the strains were isolated quickly.
[0042]Agrimony samples were rinsed with tap water several times to remove the surface soil, and then the following procedures were used for surface disinfection under sterile conditions: 0.01% Tween-20 treatment for 1 min, sodium hypochlorite with an available chlorine content of 4.5%-5% for 3 min, sterile Treat with 2.5% sodium thiosulfate for 10 minutes, 75% ethanol for 5 minutes, wash with sterile water 3-4 times, soak in sterile 10% sodium bicarbonate for 10 minutes. The surface-sterilized Agrimony samples were fully blotted dry with sterile filter paper, crushed under aseptic conditions and sprinkled on the separation medium plate, and cultivat...
Embodiment 250L
[0059] The preparation of embodiment 250L tank fermentation Fidaxomycin
[0060] (a) Preparation of Actinomycetes mobilis strain N12W0304 seed solution
[0061] The seed medium was inoculated with Actinomycetes mobilis strain N12W0304, and cultured at 32° C. at 220 rpm for 48 hours to obtain seed liquid.
[0062] The preparation method of the seed medium is: 40.0 grams of cornstarch, 4.0 grams of glucose, 8.0 grams of peptone, 5.0 grams of beef extract, 5.0 grams of yeast extract powder, add tap water to dissolve, dilute to 1000ml, pH value 7.0-7.2, extinguish at 121 °C Bacteria 30min.
[0063] (b) Preparation of fermented broth of actinomycetes
[0064] Inoculate the 50L fermentation tank with the fermentation medium with the inoculum amount of 10% by volume of the seed solution. The temperature of the tank is 28°C, the tank pressure is 0.05±0.01Mpa, the ventilation rate is 20L / min, stirring at 400rpm, and fermented for 120h.
[0065] The preparation method of the fermenta...
Embodiment 3
[0071] Embodiment 3 Refining Fidaxomycin
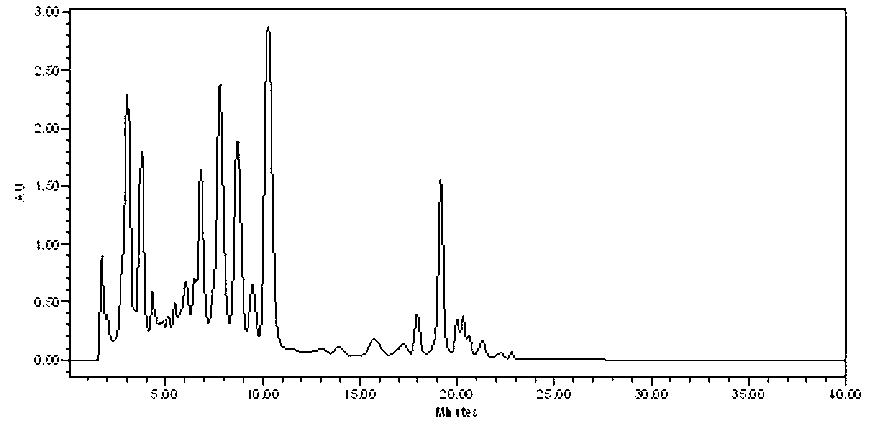
[0072] 51.8 g of the crude fidaxomicin prepared in Example 2 was dissolved in 400 ml of methanol, filtered through a 0.45 μm membrane, and separated by HPLC. Preparative column, Daisogel-C1830×250mm 10μm; mobile phase, 57% acetonitrile water, containing 0.05% trifluoroacetic acid; flow rate 40ml / min; UV254nm detection. The effluents connected to the chromatographic peak of fidaxomicin (RT≈15~17min) were combined and concentrated under reduced pressure to obtain 26.6 g of the product—fine product of fidaxomicin, with a content of 97.0% and a total yield of 61.4%.
[0073] Product is identified, and its result is as follows:
[0074] (1) Appearance: The prepared product exists in the state of white powder, glassy transparent solid, and crystalline powder.
[0075] (2) Solubility: The prepared product is soluble in methanol, ethanol, chloroform, ethyl acetate, and difficult to use in water and n-hexane.
[0076] (3) UV spectrum
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com