Mutant strain for producing sisomicin
A technology of sisomicin and mutant strains, applied in bacteria, microorganisms, fermentation, etc., can solve the problems of slow progress in the application of Micromonospora
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The acclimation screening of bacterial strain HP388 of the present invention needs at least more than 10 cycles, and each cycle mainly includes the following processes:
[0024] Take out the cultivated slant surface of T125 strain T125 (M. inuuuumosa var. Chengjiang varietal T125), add 3ml of sterile water, gently scrape off the spores with an inoculation needle, take out the spore suspension, and shake it fully on the shaker, so that Disperse into single spores, followed by serial dilution (10 0 、10 -1 、10 -2 、10 -3 、10 -4 、10 -5 、10 -6 、10 -7 ), spread the flat plate, and after ultraviolet irradiation (10, 20, 30, 40, 50 seconds), culture at 37°C to isolate a single colony. When a single colony grows enough to distinguish its characteristics (25 days), pick colonies with different morphological characteristics and carry out submerged fermentation culture (6 days).
[0025] Take the above-mentioned high-quality strains, culture mycelia, and prepare protoplasts....
Embodiment 2
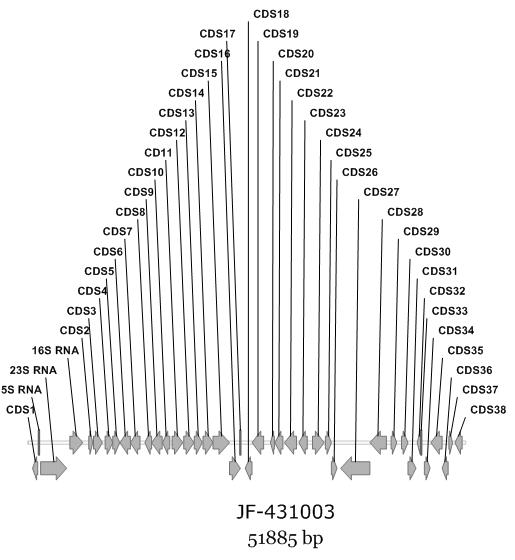
[0028] Example 2 Obtaining and DNA Sequencing of Sisomicin Biosynthetic Gene Cluster in Mutant Strain HP388
[0029] 1 material
[0030] 1.1 Strains and plasmids E. coli DH5α and cosmid carrier Supercos-1 (purchased from Shanghai Sangong); E. coli LE392 was purchased from Stratagene Company.
[0031] 1.2 Reagents Restriction endonuclease, proteinase K, calf intestinal alkaline phosphatase (CIAP enzyme), T4 DNA ligase and Taq enzyme were purchased from TAKARA company; lysozyme was purchased from Shanghai Sangong; phage packaging protein extract (Gigapack Ⅲ XL packaging extract) was purchased from Stratagene.
[0032] 1.3 Culture medium Bacterial culture medium LB, add ampicillin (final concentration: 100 μg / ml) and kanamycin (final concentration: 25 μg / ml) as needed; Micromonospora einuatus culture medium.
[0033] library construction method
[0034] (a) Construction of Micromonas eugenus gene library: cosmid vector Supercos-1 with restriction endonuclease Nhe ...
Embodiment 3
[0043] Example 3: Preparation of sisomicin, a metabolite of Micromonas eugenus HP388
[0044] The high-yield and stable-yield engineering bacterium Micromonospora eneurica HP388 provided by the invention is directly used to produce sisomicin as an antibacterial drug and to synthesize netilmicin.
[0045] 1. Fermentation and Culture of Micromonospora eneurica HP388 Strain
[0046] Slant medium (%): by weight percentage, soluble starch 1.5%, bran 1.5%, MgSO 4 ·6H 2 O 0.03%, KNO 3 0.3%, K 2 HPO 4 3H 2 O 0.03%, NaCl 0.05%, CaCO 3 0.4%, agar 2%, pH7.5.
[0047] Seed medium: glucose 0.1%, sweet potato starch 1.0%, corn flour 1.5%, peptone 0.2%, soybean cake powder 1.0%, KNO 3 0.05%, CaCO 3 0.5%, pH7.0.
[0048] Fermentation medium: sweet potato starch 6.0%, corn flour 1.0%, peptone 0.4%, soybean cake powder 2.0%, KNO 3 0.01%, (NH 4 ) 2 SO 4 0.1%, CaCO 3 0.5%, amylase 0.025%, pH7.5.
[0049] Fermentation of Micromonas eneurica HP388. Before fermentation, h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com