Preparation method of high-purity fidaxomicin
A fidaxomicin, high-purity technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of large damage to the downstream purification medium, less refined powder, and more impurities. The effect of improving quality and product yield, eliminating interference and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of Fidaxomycin fermentation broth:
[0030] The seed medium was inoculated with Actinomycetes mobilis strain N12W0304, and cultured at 32 °C and 220 rpm for 48 h to obtain seed liquid. The seed solution was inoculated with an inoculum volume of 10% by volume in a 50 L fermenter equipped with fermentation medium, the tank temperature was 28 °C, the tank pressure was 0.05±0.01 Mpa, the ventilation rate was 20 L / min, stirred at 400 rpm, and fermented for 120 h to obtain Fidaxomycin fermentation broth.
[0031] Wherein the preparation method of the seed medium is: 40.0 grams of cornstarch, 4.0 grams of glucose, 8.0 grams of peptone, 5.0 grams of beef extract, 5.0 grams of yeast extract powder, add tap water to dissolve, dilute to 1000 ml, pH value 7.0-7.2 , sterilized at 121°C for 30 min.
[0032] The preparation method of the fermentation medium is: 30.0 grams of glucose, 15.0 grams of defatted soybean powder, 3.0 grams of beef extract, K 2 HPO 4 0.6 g, Fe...
Embodiment 2
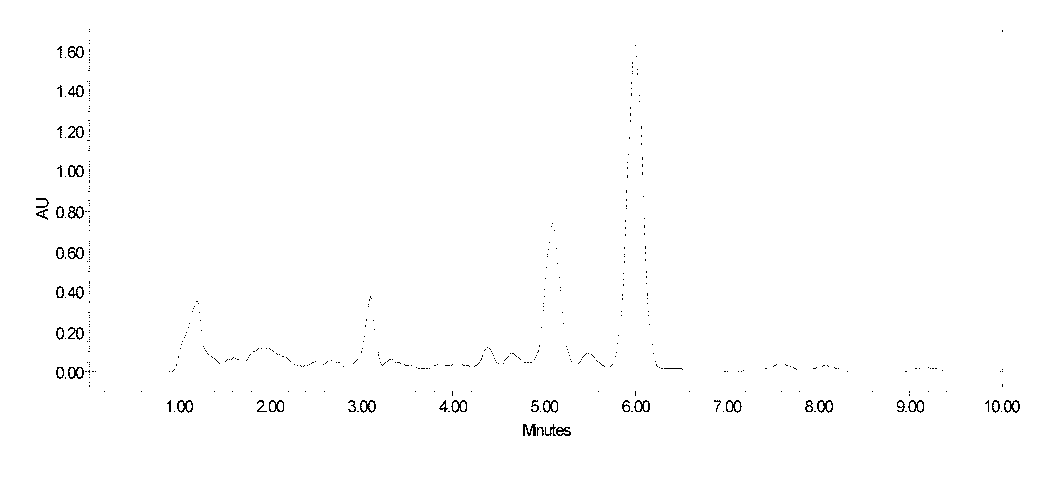
[0036] Fidaxomycin fermentation broth 10L, fermentation unit 620μg / mL. The fidaxomicin fermentation broth was filtered to obtain 2.6kg mycelium. Add 10 L of ethanol with a volume ratio concentration of 95% to the above-mentioned mycelia, stir and extract for 1 hour, then filter, and collect the fidaxomicin extract. The extract was diluted with water to an ethanol concentration of 35%, and then introduced into a macroporous decolorization resin LX98 column for decolorization. The resin loading capacity was 500mL, and the flow rate was 1000mL / h. The decolorization solution is introduced into the macroporous adsorption resin D312 column again for adsorption, the resin loading capacity is 500mL, and the flow rate is 1000mL / h. The saturated resin was eluted successively with 40% and 80% ethanol solutions at a flow rate of 500mL / h, and the eluent was collected in sections. The fidaxomicin analysis solution was concentrated, then extracted with butyl acetate, and the extract was co...
Embodiment 3
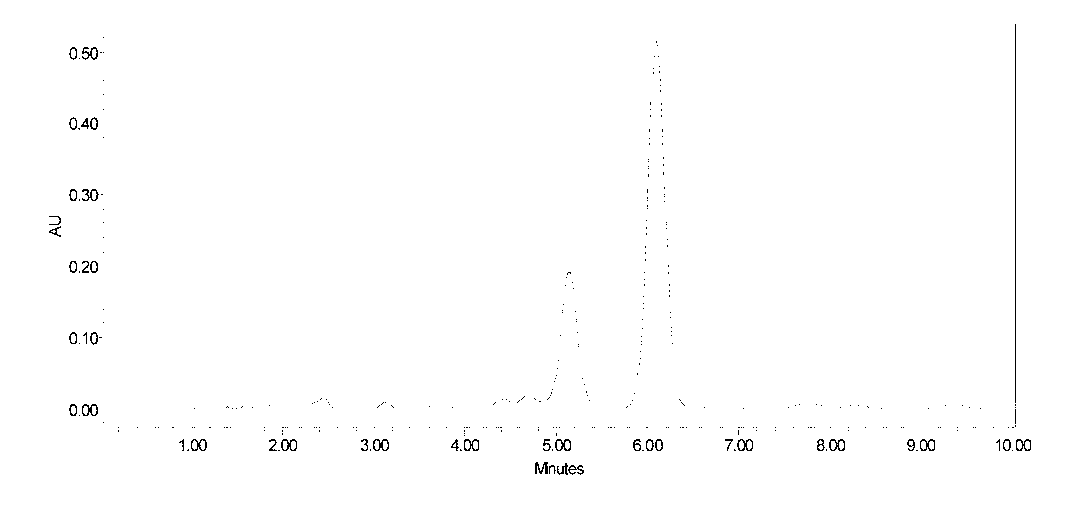
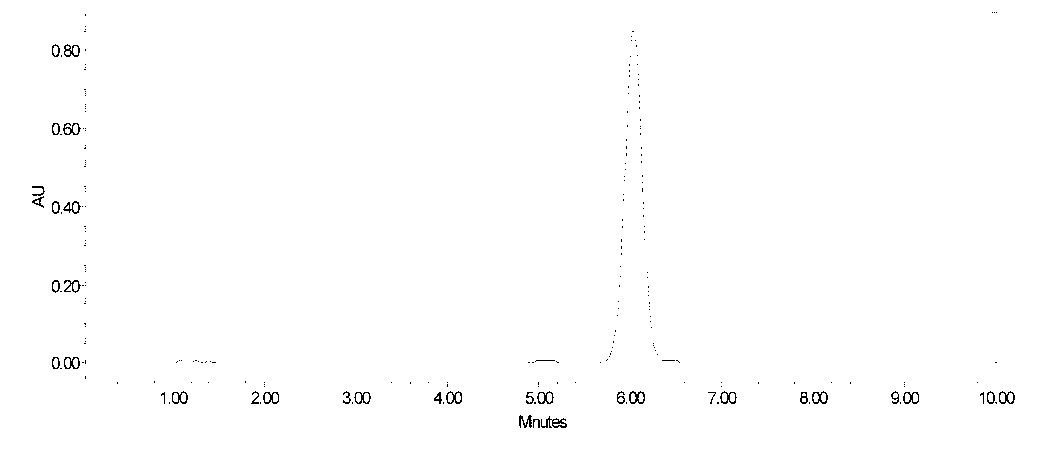
[0039] 30L of fidaxomicin fermentation broth, the fermentation unit is 570μg / mL. The fidaxomicin fermentation liquid was filtered to obtain 6.1 kg of mycelia. Add 18L of 90% ethanol to the above-mentioned mycelia, stir and extract for 1 hour, then filter, and collect the fidaxomicin extract. The extract was diluted with water to an ethanol concentration of 35%, and then introduced into a macroporous decolorizing resin D301 column for decolorization. The resin loading capacity was 1500mL, and the flow rate was 3000mL / h. The decolorization solution is introduced into the macroporous adsorption resin HZ816 column again for adsorption, the resin loading capacity is 1500mL, and the flow rate is 3000mL / h. The saturated resin was eluted successively with 40% and 80% ethanol solutions at a flow rate of 1500mL / h, and the eluent was collected in sections. The above fidaxomicin eluate was concentrated, then extracted with ethyl acetate, the extract was concentrated and dried to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com