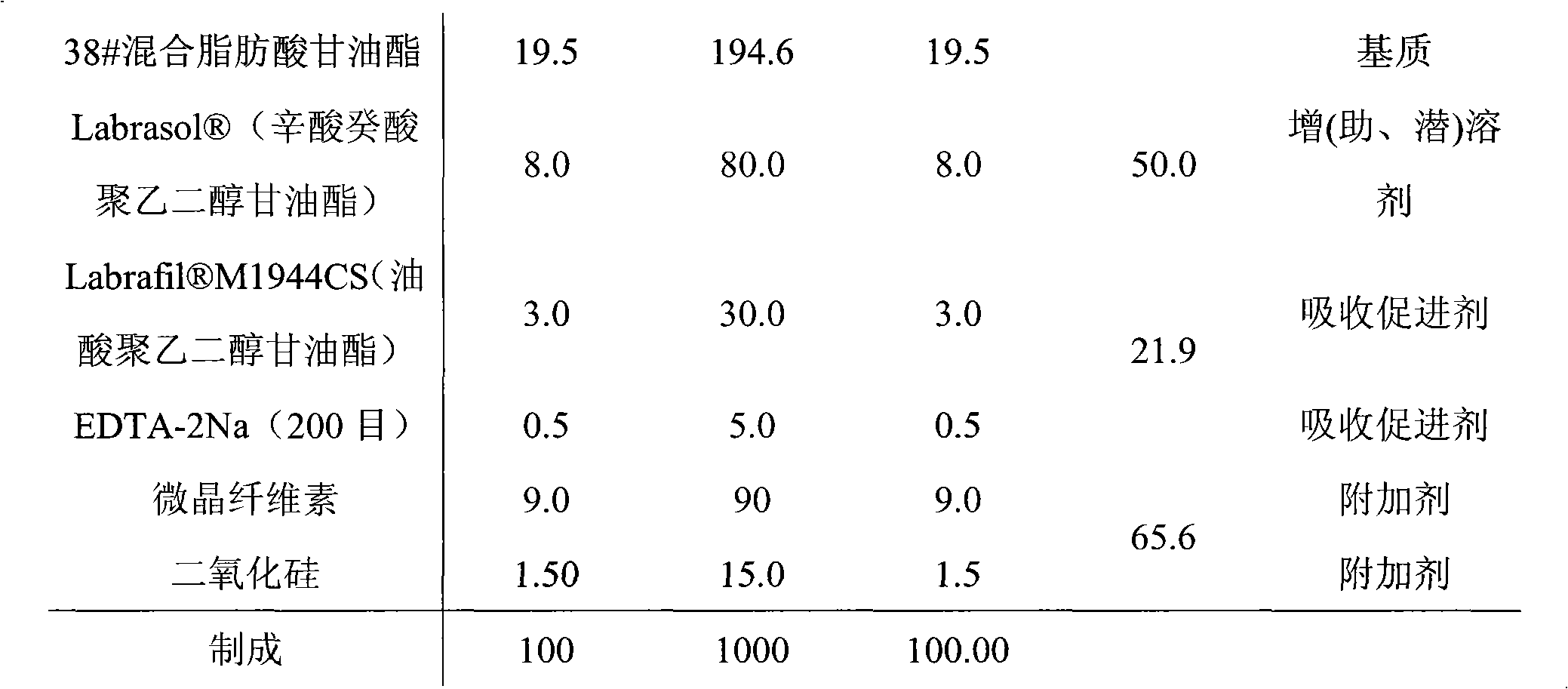Patents
Literature
190 results about "Rectal use" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rectal administration uses the rectum as a route of administration for medication and other fluids, which are absorbed by the rectum's blood vessels, and flow into the body's circulatory system, which distributes the drug to the body's organs and bodily systems.
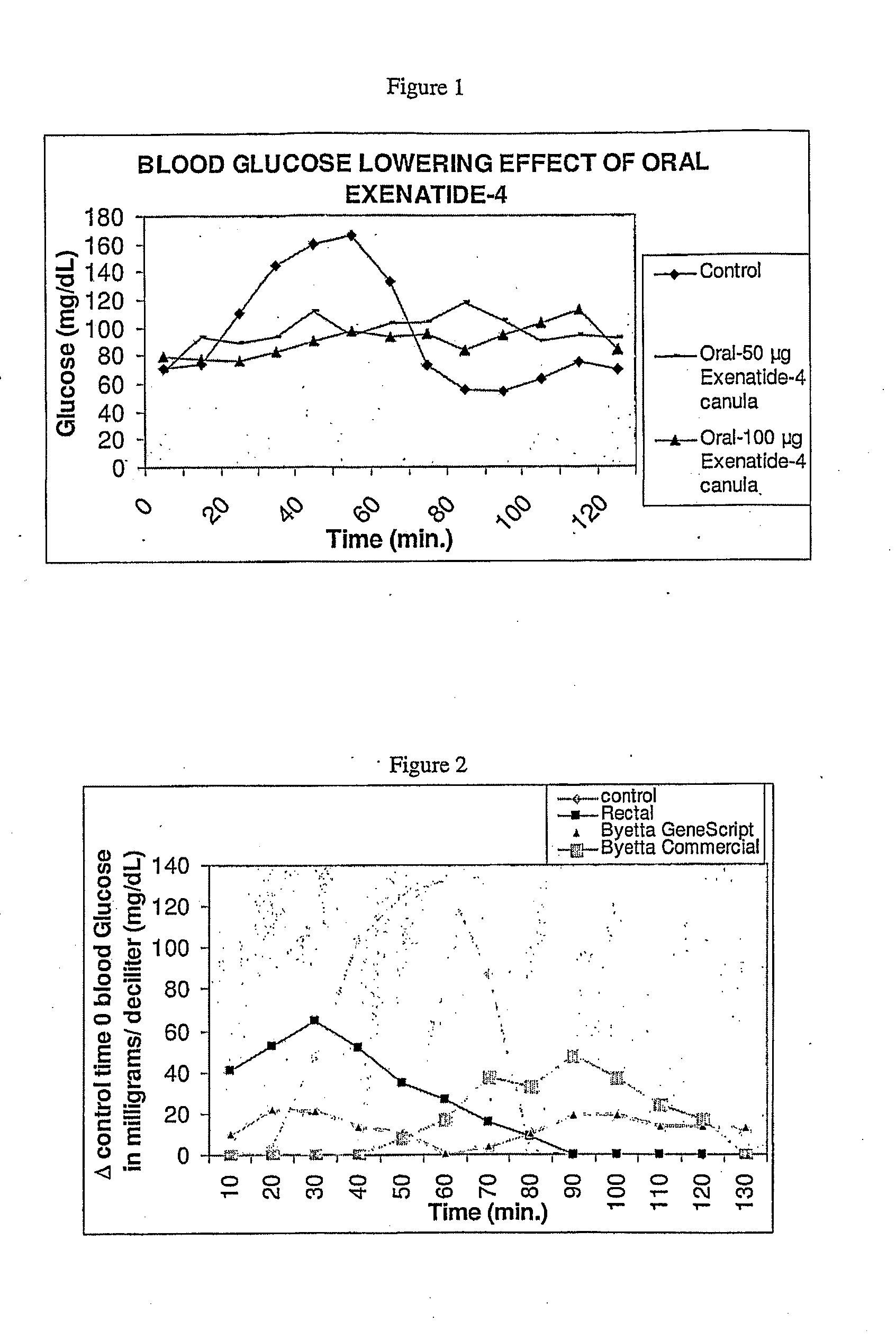
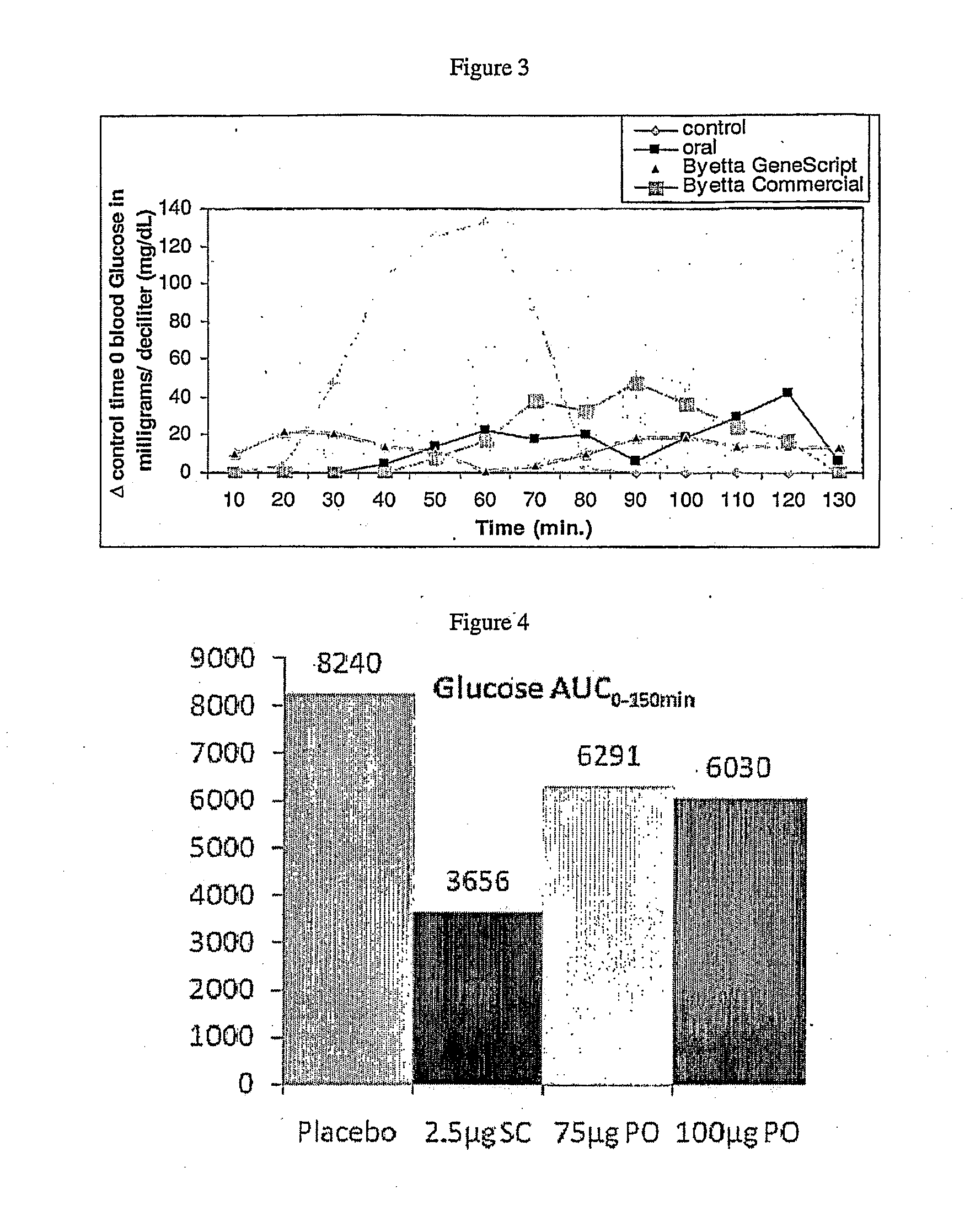
Methods and compositions for oral administration of exenatide
InactiveUS20110046053A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsMetabolism disorderDiabetes mellitusOral medication
This invention provides compositions comprising a byetta, fish oil, and a protease inhibitor, method for treating diabetes mellitus, comprising administering same, and methods for oral or rectal administration of a byetta.
Owner:ORAMED
Rapid acting drug delivery compositions
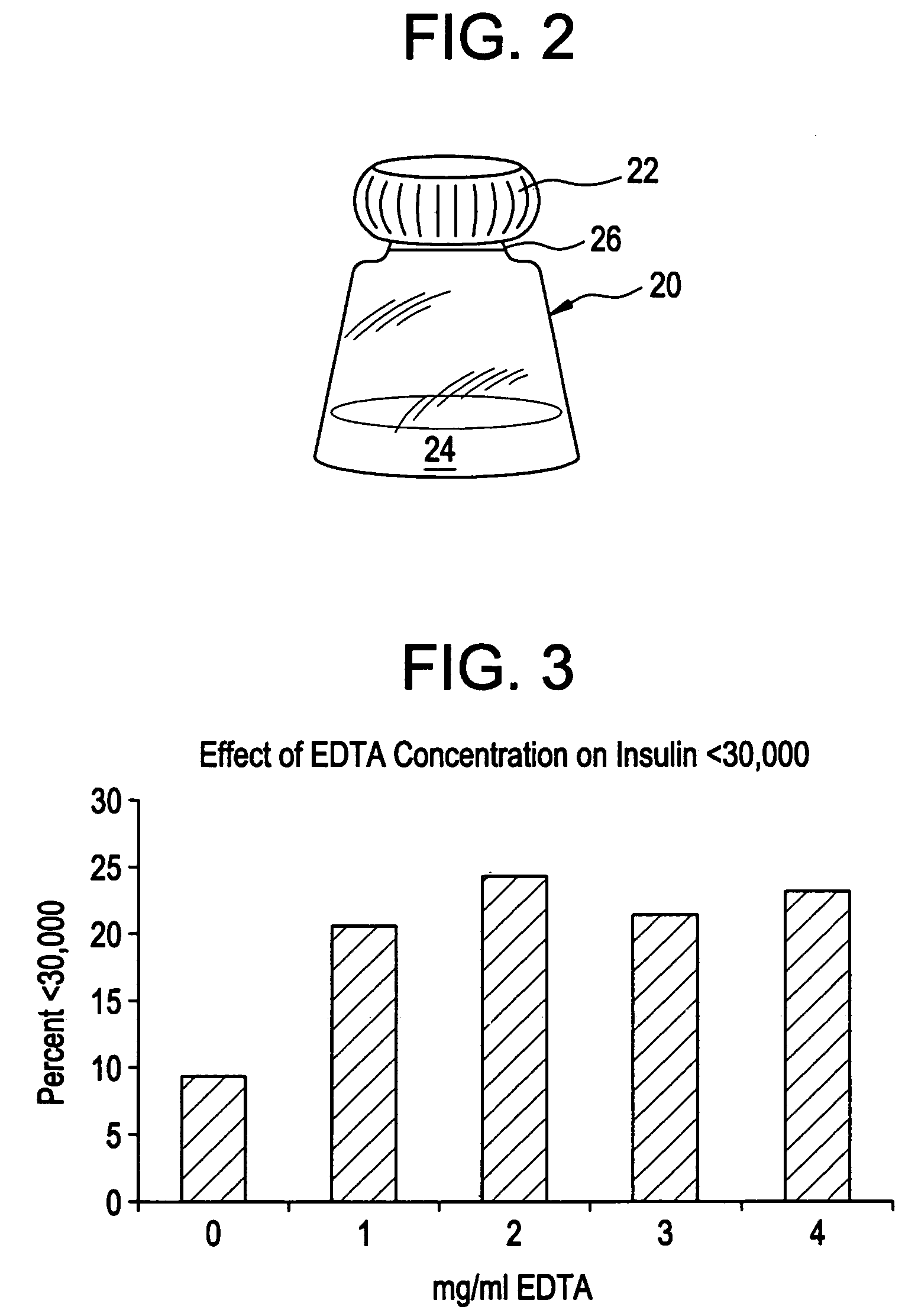
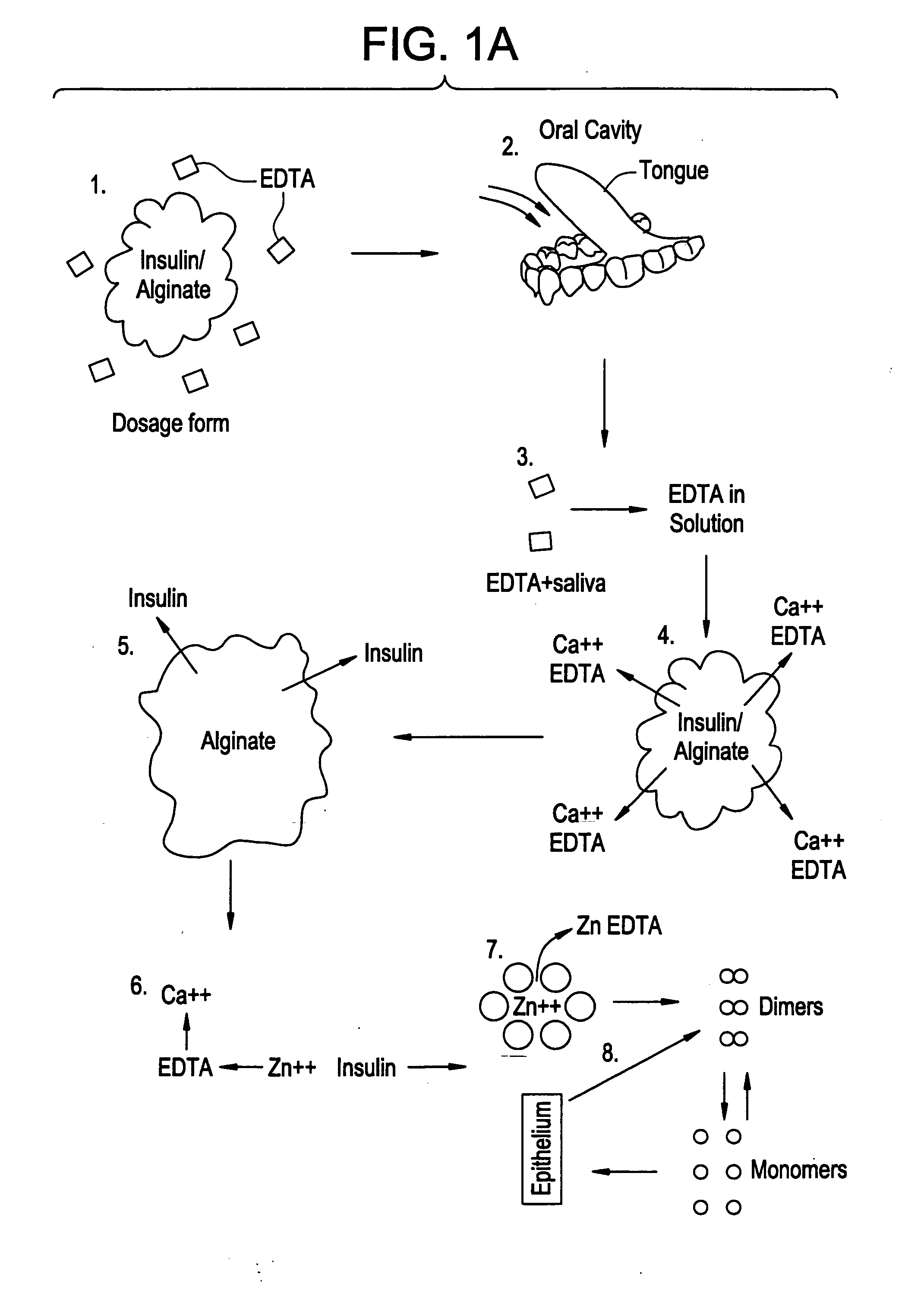
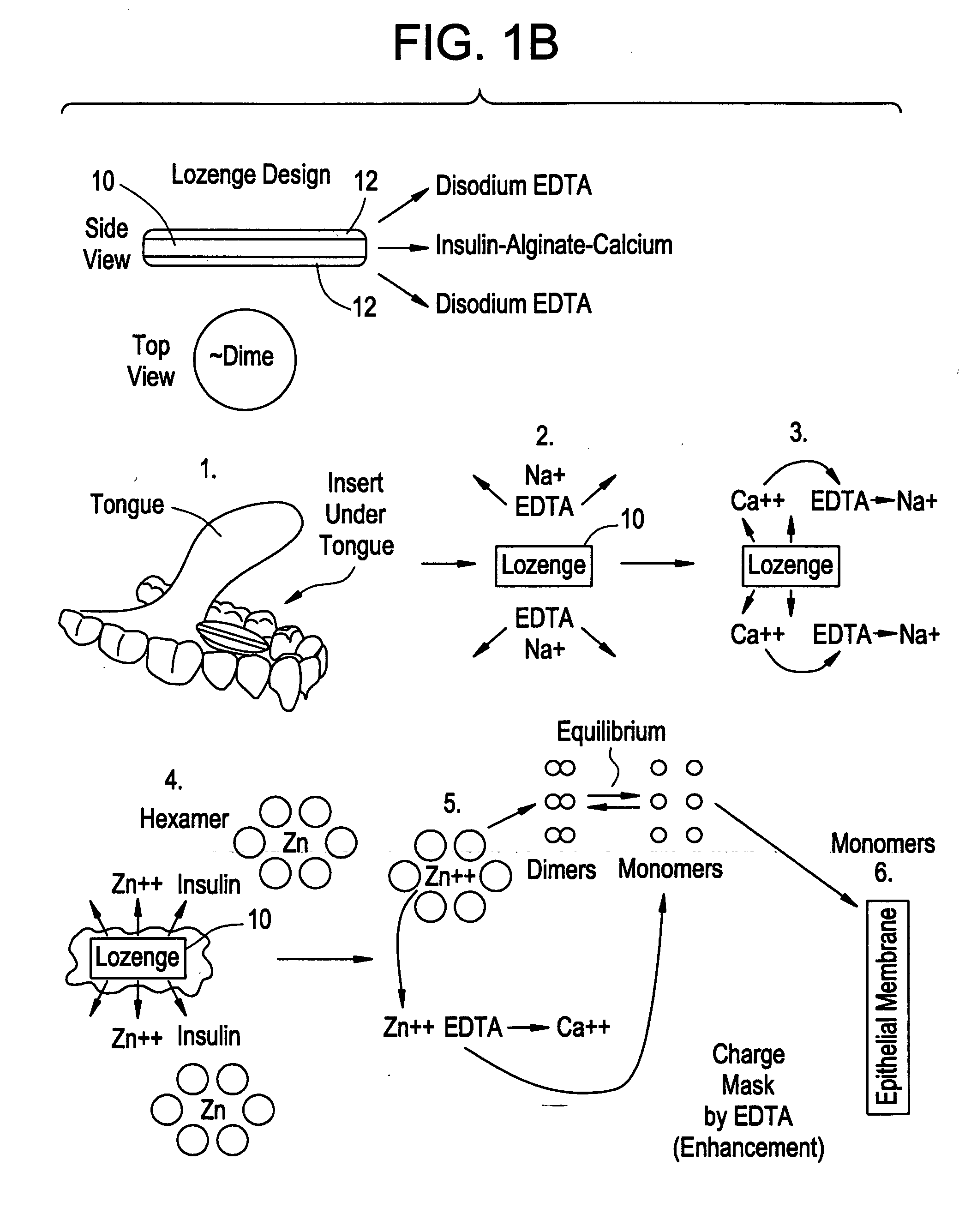
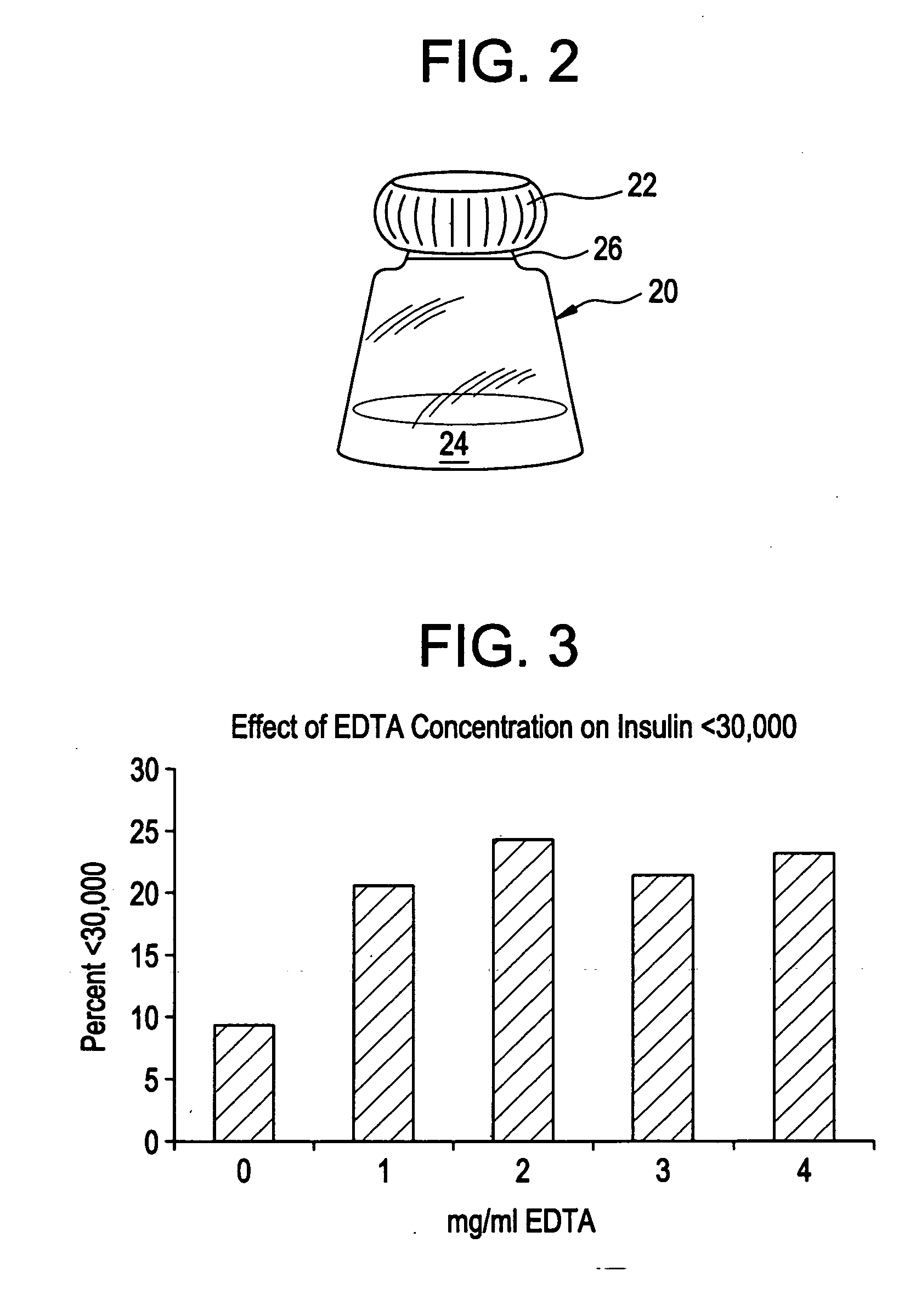
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Rapid acting drug delivery compositions
ActiveUS20050214251A1Improve stabilityQuick effectPowder deliveryPeptide/protein ingredientsNasal cavityBuccal use
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:ELI LILLY & CO
Composition and method for inhibiting platelet aggregation
The present invention provides novel compounds of dinucleotide polyphosphates and the method of preventing or treating diseases or conditions associated with platelet aggregation. The method comprises administering systemically to a patient a pharmaceutical comprising a purinergic P2τ receptor antagonist, in an amount effective to elevate its extracellular concentration to bind to P2τ receptors and inhibit P2τ receptor-mediated platelet aggregation. Methods of systemic administration include injection by intravenous, intramuscular, intrasternal and intravitreal routes, infusion, transdermal administration, oral administration, rectal administration and intra-operative instillation.
Owner:INSPIRE PHARMA +1
Self-expanding device for the gastrointestinal or urogenital area
InactiveUS20060142794A1Lower the volumeLose weightSurgeryDilatorsIntestinal structureReproductive tract
Devices for treatments of diseases and disorders associated with the gastrointestinal tract, especially the stomach, or urinogenital tract are described herein. Initially, the device is in a temporary form which is suitable for oral or rectal administration. After exposure to a stimulus, such as a temperature or pH change, the device changes shape to a permanent form, which allows it to become mechanically fixed in the stomach, esophagus or intestine. In one embodiment, the device is used to reduce the volume of the stomach, esophagus or intestine without interfering with the flow of the food through the gastrointestinal tract. The device may be used to help overweight patients lose weight and to deliver drugs to treat disorders and diseases in the in the stomach or intestine. The devices are manufactured from a stimuli-sensitive polymeric material, which is biocompatible and primarily adapted to the mechanical properties and geometry in the area to which it is applied. In the preferred embodiment, the material is a shape memory polymer. Depending on the desired application, the polymer may be either biodegradable or non-degradable.
Owner:MINEMOSCIENCE GMBH
Treatment for joint inflammation
The invention provides the use of a glucocorticoid substance which has a minimal systemic effect in the manufacture of a medicament for oral or rectal administration for non-topical use in the treatment of joint inflammation.
Owner:ASTRAZENECA AB
Rapid Acting Drug Delivery Compositions
Drug formulations for systemic drug delivery with improved stability and rapid onset of action are described herein. The formulations may be administered via buccal administration, sublingual administration, pulmonary delivery, nasal administration, subcutaneous administration, rectal administration, vaginal administration, or ocular administration. In the preferred embodiments, the formulations are administered sublingually or via subcutaneous injection. The formulations contain an active agent and one or more excipients, selected to increase the rate of dissolution. In the preferred embodiment, the drug is insulin, and the excipients include a metal chelator such as EDTA and an acid such as citric acid. Following administration, these formulations are rapidly absorbed by the oral mucosa when administered sublingually and are rapidly absorbed into the blood stream when administered by subcutaneous injection. In one embodiment, the composition is in the form of a dry powder. In another embodiment, the composition is in the form of a film, wafer, lozenge, capsule, or tablet. In a third embodiment, a dry powdered insulin is mixed with a diluent containing a pharmaceutically acceptable carrier, such as water or saline, a metal chelator such as EDTA and an acid such as citric acid. Devices for storing and mixing these formulations are also described.
Owner:BIODEL INC
Treatment of Meconium Aspiration Syndrome with Estrogens
ActiveUS20100184736A1Reduce incidenceReduce morbidityOrganic active ingredientsPharmaceutical delivery mechanismSuppositoryNon invasive
One aspect of the present invention relates to the use of an estrogen in the treatment of Meconium Aspiration Syndrome (MAS) in a newborn infant, said treatment comprising administering an effective amount of estrogen to said newborn infant within 7 days after birth. The present treatment offers the advantage that estrogens can be administered using non-invasive modes of administration, e.g. oral or rectal administration. Other aspects of the present invention relate to a suppository for use in newborn infants comprising at least 1 μg of estrogen and to an oral applicator comprising a container holding an aqueous liquid containing micronised estetrol and a metering dispenser for metering the liquid into the oral cavity of a newborn infant.
Owner:ESTETRA SRL
Nasal administration
InactiveUS20140144443A1Reduce deliveryGood effectRespiratorsOrganic active ingredientsNasal cavityCns effects
A delivery device for and method of providing for delivery of substance to the central nervous system (CNS) of a subject, the delivery device comprising: a nosepiece unit for insertion into a nasal airway of a subject and comprising an outlet unit which includes a nozzle for delivering substance into the nasal airway of the subject; and a substance supply unit which is operable to deliver a dose of substance to the nozzle; wherein the delivery device is configured such that at least 30% of the dose as initially deposited in the nasal airway is deposited in an upper posterior region of the nasal airway, thereby providing a CNS concentration of the substance, and hence CNS effect, which is significantly greater than that which would be predicted from a counterpart blood plasma concentration of the substance.
Owner:OPTINOSE AS
Nasal administration
InactiveUS20140144442A1Reduce deliveryGreat CNS effectRespiratorsOrganic active ingredientsNasal cavityCns effects
A delivery device for and method of providing for delivery of substance to the central nervous system (CNS) of a subject, the delivery device comprising: a nosepiece unit for insertion into a nasal airway of a subject and comprising an outlet unit which includes a nozzle for delivering substance into the nasal airway of the subject; and a substance supply unit which is operable to deliver a dose of substance to the nozzle; wherein the delivery device is configured such that at least 30% of the dose as initially deposited in the nasal airway is deposited in an upper posterior region of the nasal airway, thereby providing a CNS concentration of the substance, and hence CNS effect, which is significantly greater than that which would be predicted from a counterpart blood plasma concentration of the substance.
Owner:OPTINOSE AS
Methods and compositions for oral administration of exenatide
ActiveUS20130195939A1Reducing food intakeDecreased gastric motilityPeptide/protein ingredientsSkeletal disorderDiabetes mellitusOral medication
Owner:ORAMED
Compound of losartan compound or its medical salt and calcium channel blocker or its medical salt
InactiveCN101347427ALittle side effectsGood curative effectOrganic active ingredientsCardiovascular disorderCarboxylic acidLosartan
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Extracting method of high-purity cobratoxin and pharmaceutical composition containing high-purity cobratoxin
ActiveCN104327176AOvercoming Transgender DisadvantagesOvercoming the possibility of transgenderNervous disorderPeptide/protein ingredientsTherapeutic effectControllability
In consideration of physical and chemical properties that the cobratoxin is complex in component and cobratide is basic polypeptide, has molecular weight of about 7000 Dalton ad is relatively resistant to acid and alkali, and by considering the separating mechanism, the application characteristic, the long-term use stability, the cost controllability and the achievement of an extracting goal of each chromatographic column packing, the invention provides an extracting method of high-purity cobratoxin. The extracting method disclosed by the invention is stable in process, easily available in chromatography packing, low in comprehensive cost, good in operation condition adaptability and high in yield of neurotoxin. The invention further provides high-purity cobratoxin obtained by the method provided by the invention. An injection cobratide preparation prepared by the high-purity cobratoxin is less in impurities irrelevant with the treatment effect, quick to become effective in treatment effect and stable in curative effect. The invention further provides several pharmaceutical compositions taking the high-purity cobratoxin as an effective constituent formulated with preparation auxiliary materials needed for preparing a preparation as well as cobratide preparations with different dosage forms, which are suitable for being orally taken, absorbed by an oral cavity or rectal administration.
Owner:张庆宇
Cobratide extraction method, cobratide extracted thereby and formulation containing cobratide
ActiveCN101381408AHigh puritySimple methodNervous disorderPeptide/protein ingredientsFreeze-dryingUltrafiltration
The invention relates to a method for extracting cobratide, a medicine used to treat chronic pain, the cobratide extracted by the method and a preparation containing the cobratide. The method for extracting the cobratide comprises the following steps: firstly, a stock solution of snake poison of a cobra is pretreated with ammonium sulphate to remove partial other compositions in the snake poison,is subjected to desalination and concentration through ultrafiltration, dialysis or gel chromatography, and after the concentration, is purified by a cation column, and finally is subject to freeze-drying preservation after the concentration. The cobratide preparation comprises an enteric preparation, a rectal administration preparation, a colon-specific administration preparation and so on. Withcobratide extraction and purification processes provided by the method, the purity of the extracted cobratide is more than 95 percent, and the yield reaches 70 percent; and the method is simple and reliable, and saves cost and time.
Owner:BEIJING SAISHENG PHARMA
Compositions and methods for the treatment of multiple sclerosis
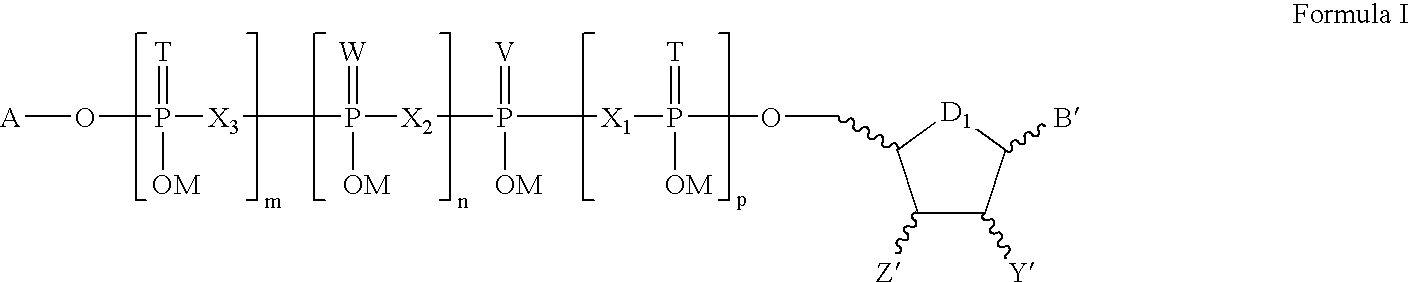
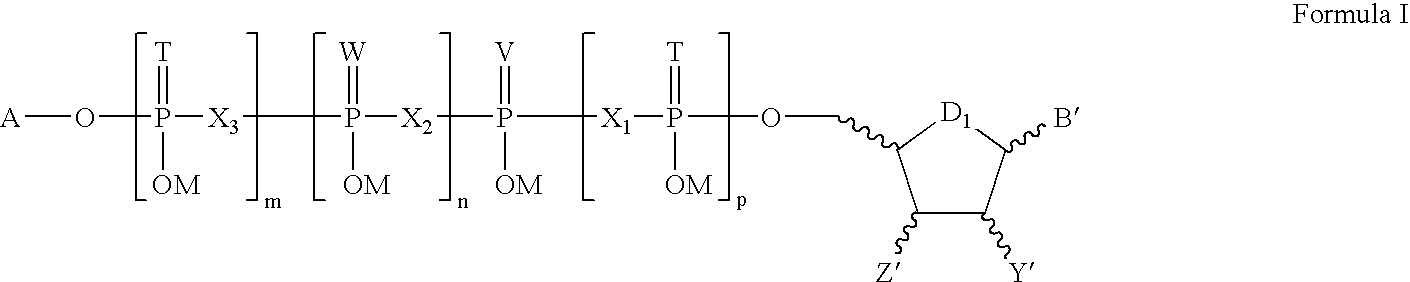
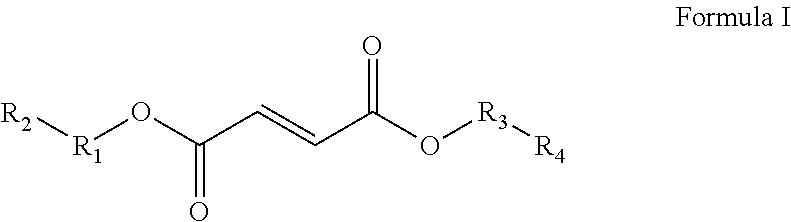
The present invention relates to compounds of Formula I and Formula II or its pharmaceutical acceptable salts, as well as polymorphs, solvates, enantiomers, stereoisomers and hydrates thereof. The pharmaceutical compositions comprising an effective amount of compounds of Formula I and Formula II; and formulated to treat an underlying etiology by oral administration, delayed release or sustained release, transmucosal, syrup, topical, parenteral administration, injection, subdermal, oral solution, rectal administration, buccal administration or transdermal administration.
Owner:CELLIXBIO PTE LTD
Pharmaceutical compositions for rectal administration
InactiveUS20140256661A1Avoiding unwanted side effectSymptoms improvedBiocideTetracycline active ingredientsRectal useBuccal administration
The present invention relates to a pharmaceutical composition, in particular a composition formulated for enema administration, wherein the composition comprises metronidazole or a pharmacologically acceptable derivative thereof in an amount to effectively treat both acute and chronic pouchitis and / or proctitis.
Owner:ARMSTRONG DAVID NIGEL
A rectal in-situ gel for antipyretic and preparation method thereof
The invention discloses a rectal in-situ gel for antipyretic and a preparation method thereof, which comprises the following components in terms of mass fraction: 1-20% of antipyretic drug, and 5-30% of in-situ gel matrix , 0.1-10% of tackifier, 0.1-10% of lubricant, 0.1-10% of preservative, 0.1-10% of transdermal absorption regulator, and the balance is water. The rectal in-situ gel for antipyretic provided by the present invention not only has no drug dead angle, but also enhances the adsorption capacity and prolongs the drug effect by adding bioadhesive substances, and reduces the excretion of the drug caused by the stool caused by the solid preparation; it has good Inclusiveness, it can be either a specific medicinal ingredient or an active ingredient of a traditional Chinese medicine extract.
Owner:陕西医药控股集团新药技术开发有限公司
Carrying suppository for cavity/canal drug administration
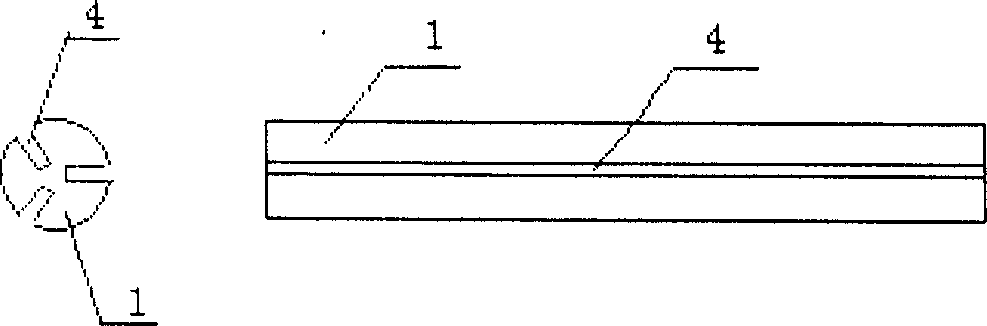
the invention disclose a carrying suppository for cavity / canal drug administration, and includes suppository with cartilage used for vagina, rectum, nasal cavity drug administration, and novel suppository fit for rectum drug administration and special mold caddy for molding, packing, vivo imbedding. Wherein the suppository with cartilage is comprised by suppository core 1, pull wire 2, drug-containing carrier stroma and adhesive agent and / or developing agent; The rectum drug administration suppository is comprised by drug-containing carrier stroma, isolated layer, and adhesion coat; The special mold caddy is comprised by hollow suppository push rod and mainbody mold, wherein the mainbody mold includes case cap 5, upper cavity mold 6 in the case, lower mold 7 connected together with the case, wherein the upper mold can be integrated or two-body, i.e. a central mold 8 is added. By using the provided suppository, focus can fully contact with the drug, and the drug will not flow, and has longer active time and good positioning property, so that the suppository can realize drug administration against focus local.
Owner:丛繁滋
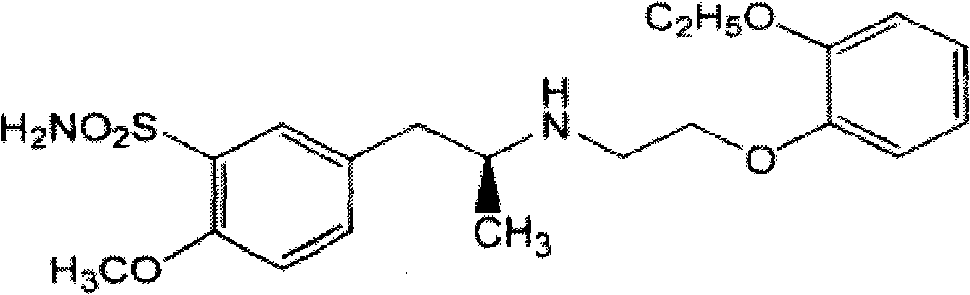
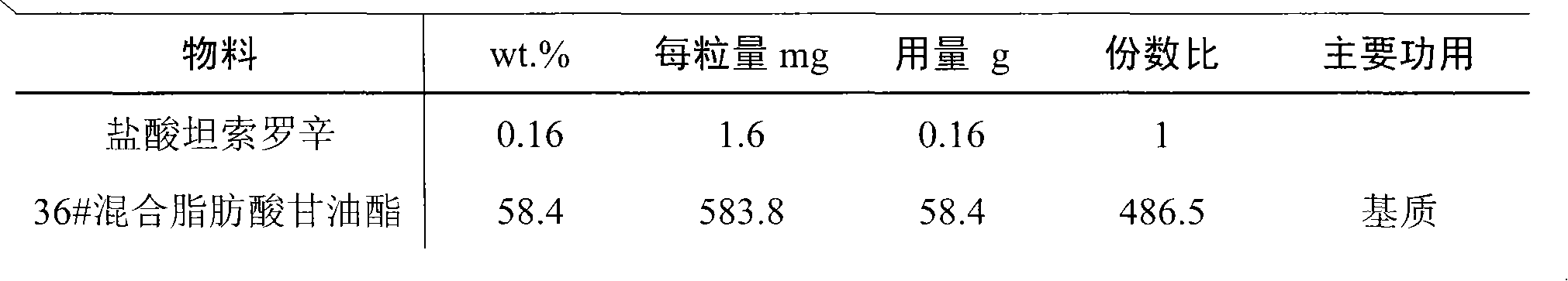
Rectal administration composition containing tamsulosin
InactiveCN101889996AImprove solubilityGood curative effectSuppositories deliveryOrganic non-active ingredientsSide effectWhole body
The invention relates to a rectal administration composition containing tamsulosin, which comprises effective quantities of tamsulosin or pharmaceutically acceptable salts or derivatives thereof, solubilizer and sorbefacient, wherein the effective quantity of tamsulosin or pharmaceutically acceptable salts or derivatives thereof is metered according to the equivalence tamsulosin hydrochloride; the content of the tamsulosin or pharmaceutically acceptable salts or derivatives thereof is equivalent to 0.025-1.6 mg of tamsulosin hydrochloride; and the preparation per unit weighs 0.8-4 g. The rectal administration composition containing tamsulosin can overcome the defects of poor curative effect and great toxic or side effect in oral administration and systemic injection administration, and the defects of great side effect and poor patient dependence in partial injection administration, and can prolong the duration of drug actions, thereby providing a better treatment means for medical care personnel and patients. The production technology is simple and suitable for industrial mass production.
Owner:张立英 +1
Therapeutic antisense oligonucleotide composition for the treatment of inflammatory bowel disease
InactiveUS20090275631A1Improve responseMinimal systemic exposureOrganic active ingredientsPeptide/protein ingredientsTolerabilityUlcerative colitis
Disclosed herein is a method for the sustained amelioration and / or treatment of ulcerative colitis comprising rectal administration of a compound comprising an antisense oligonucleotide having the sequence 5′-GCCCAAGCTGGCATCCGTCA-3′, ISIS 2302. The method results in a decrease in the indications of ulcerative colitis for an extended period (greater than 90 days) after the conclusion of the administration of the composition. The composition is well tolerated and systemic exposure is minimal.
Owner:ATLANTIC TECH VENTURES
Application of dragon blood and dragon-blood extract
ActiveCN101559149APrevent or mitigate damageGood radiation protectionDermatological disorderPlant ingredientsRectal useOral solutions
The invention relates to application of dragon blood and dragon blood extract, which belongs to the field of Chinese medicaments. In particular, the invention relates to the application of dragon blood raw medicinal materials, dragon blood total phenols and dragon blood total flavonoids in preparing medicaments for preventing or treating radiation damage. In order to achieve the application of the invention, the application of the dragon blood or the dragon blood extract via or not via gastrointestinal tract includes but is not limited to oral, subcutaneous, intramuscular, intravenous, transdermal, nasal and rectal administration. Pharmaceutical compositions prepared from the dragon blood or the dragon blood extract and medically acceptable pharmaceutical excipient include but are not limited to various types of tablets, capsules, dropping pills, soft capsules, oral solution, pills, powder, granules, lozenges, decoction paste, syrup, liquid extract agent, extract agents, injections, as well as other clinically appropriate dosage forms. A preparation contains necessary additives, and the additives include but are not limited to fillers, wetting agents, adhesives, disintegrants, lubricants or pH regulators.
Owner:北京理工亘舒科技有限公司
Rectal drug delivery device with electric regulation function in gastroenterology department
InactiveCN107802948AEasy Control of InjectionEasy to switchMedical devicesElectric controlRectal use
The invention discloses a rectal drug delivery device for gastroenterology with an electric adjustment function, which comprises a pressure pad, a pressure rod, a pressure rod positioning sleeve, a handle, a drug cartridge, an inner cavity, a liquid separator, an injection needle, and an enema tube , a rectal drug delivery device for gastroenterology with electric adjustment function of the present invention, when in use, the worm gear of the ring motor drives the rack to move back and forth, so that the pull bar connected to the rack pulls the ball valve to rotate along the ball valve shaft pin, and the two ends The return spring is tensioned to keep the through hole of the ball valve in an upward damping state. When the motor is started, the through hole of the ball valve becomes horizontal and the channel is connected; through the cooperation of the electric control valve and the liquid dispenser, the The drug delivery device can be easily controlled to inject with a needle or an enema tube, and the switch is convenient and quick, reducing the time for changing tools and working heads.
Owner:陈双
Therapeutic antisense oligonucleotide composition for the treatment of inflammatory bowel disease
Disclosed herein is a method for the sustained amelioration and / or treatment of ulcerative colitis comprising rectal administration of a compound comprising an antisense oligonucleotide having the sequence 5'-GCCCAAGCTGGCATCCGTCA-3', ISIS 2302. The method results in a decrease in the indications of ulcerative colitis for an extended period (greater than 90 days) after the conclusion of the administration of the composition. The composition is well tolerated and systemic exposure is minimal.
Owner:IONIS PHARMA INC
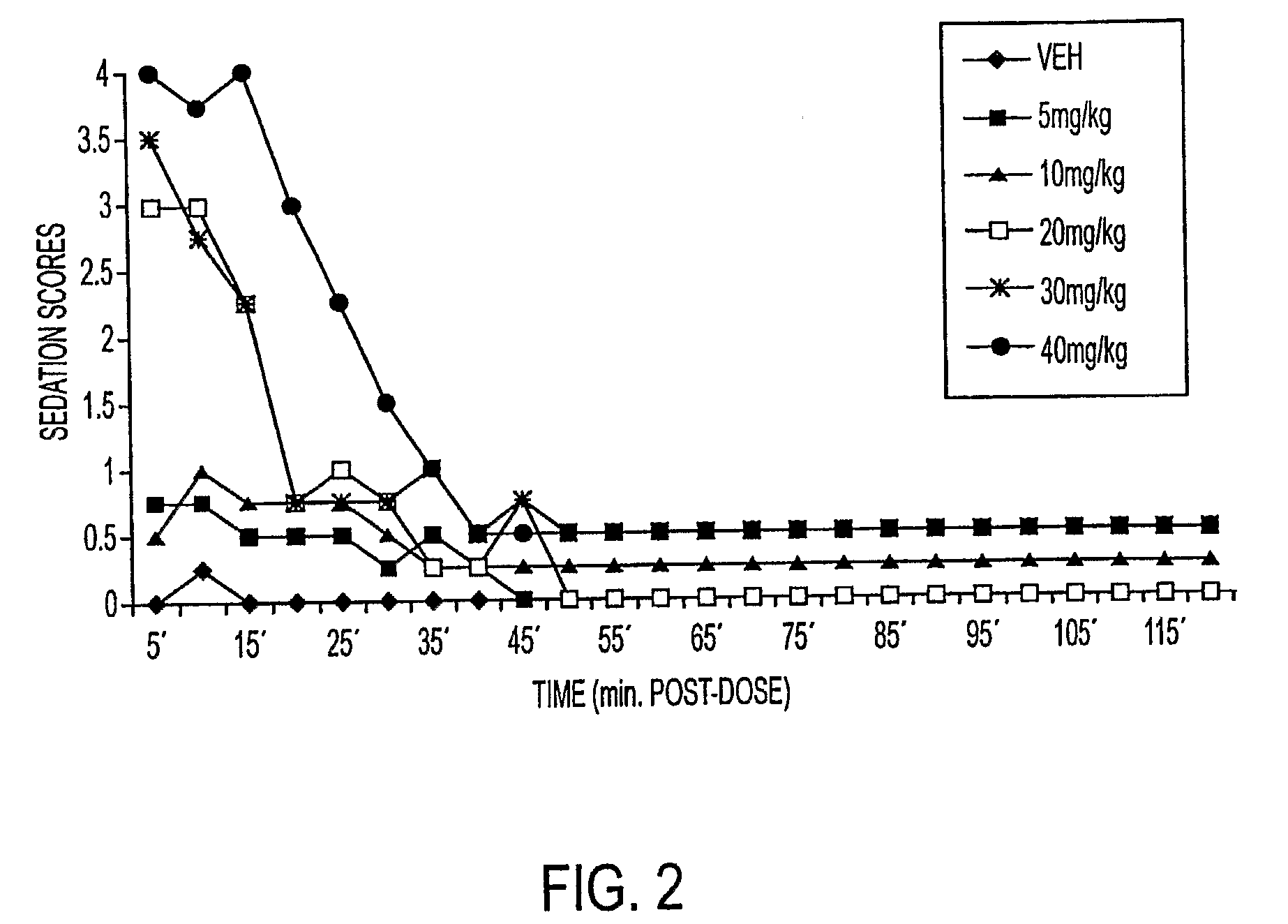
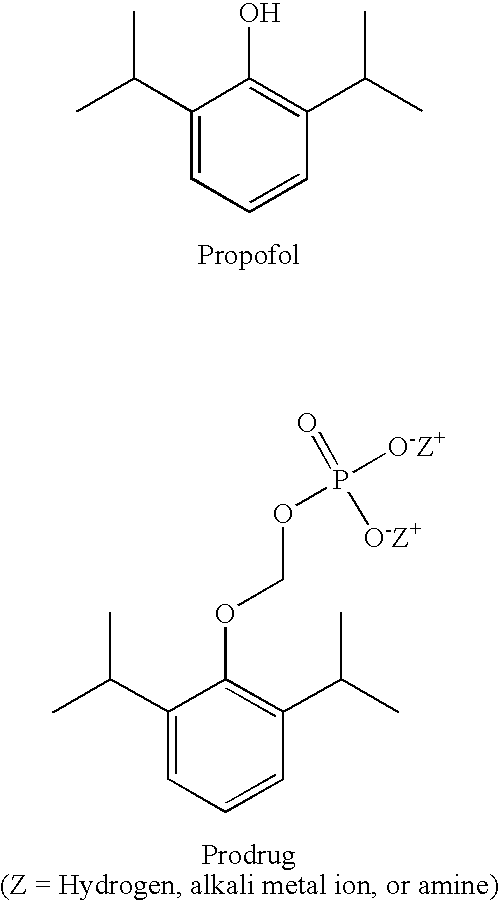
Methods of Administering Water-Soluble Prodrugs of Propofol
A method of administering a prodrug of propofol, preferably O-phosphonooxymethyl propofol disodium salt, comprises the subcutaneous or rectal administration of the prodrug in amounts sufficient to induce or maintain a generalized anesthetized state, a conscious sedated state, or to treat insomnia, anxiety, nausea, vomiting, pruritus, epilepsy, and a range of pain syndromes, including migraine pain, and other medical conditions.
Owner:EISAI INC
Preparation for vaginal and rectal use and relative production method
InactiveUS20110305744A1Inhibition formationShorten speedBiocideOrganic active ingredientsMicrometerRectal use
A preparation for vaginal and rectal use comprising hyaluronic acid, with an average particle size comprised between about 50 micrometers and about 200 micrometers and a molecular weight comprised between about 1,000,000 Da and about 1,800,000 Da.
Owner:FARMA DERMA
Application of Shanhaidan chrysanthemum indicum injection in aerosol inhalation, spray bottle aerial fog and rectal administration and method
InactiveCN105311081AMeet the requirements of physical and chemical propertiesNon-irritatingAntibacterial agentsAerosol deliveryDiseaseIrritation
The invention discloses application of Shanhaidan chrysanthemum indicum injection in aerosol inhalation, spray bottle aerial fog and rectal administration and a method. The method comprises the following steps: adding an osmotic pressure regulator with mass fraction of 0.2-0.3% in chrysanthemum indicum injection; regulating a pH value to be 6.3-6.8 by using a pH regulator; preparing an obtained solution into an aerosol inhalation preparation so that the aerosol inhalation preparation is directly inhaled into a respiratory system in an atomization mode; or placing the obtained solution in a spray bottle to obtain a spray bottle aerial fog preparation which is used for covering the respiratory system in a local spraying mode; or adding a rectal absorption enhancer in the chrysanthemum indicum injection and preparing into a rectal administration preparation with a pH regulator, wherein the rectal administration preparation enters blood circulation through haemorrhoidal veins. PH values and osmotic pressures of the three preparations in the invention meet requirements of physicochemical properties of the respiratory system and an intestinal environment, a respiratory tract and an intestinal tract are not irritated, and respiratory tract infection diseases caused by viruses, bacteria and drug-resistance bacteria can be clinically treated by the chrysanthemum indicum injection.
Owner:陕西医药控股集团山海丹药业股份有限公司
Traditional Chinese medicine compound preparation for treating ulcerative colitis and preparation method thereof
ActiveCN105250821AReduce stimulationInhibit inflammationDigestive systemMolluscs material medical ingredientsUlcerative colitisBud
The invention provides a traditional Chinese medicine compound preparation for treating ulcerative colitis. The traditional Chinese medicine compound preparation is prepared from, by weight, 20-40 parts of figwortflower picrorhiza rhizome, 10-20 parts of dahurian patrinia herbs, 9-15 parts of cortex phellodendri, 9-15 parts of sophora flavescens, 10-20 parts of hairyvein agrimonia herbs and buds, 15-20 parts of largehead atractylodes rhizome, 20-40 parts of coix seeds, 9-20 parts of common bletilla pseudobulb, 3-10 parts of panax notoginseng, 9-15 parts of thunberg fritillary bulbs, 9-15 parts of cuttlebone, 9-15 parts of garden burnet roots, 10-20 parts of radix paeoniae alba and 9-15 parts of licorice roots. The invention further provides a preparation method of the traditional Chinese medicine compound preparation for treating the ulcerative colitis, the traditional Chinese medicine compound preparation is prepared into enteric-coated drop pills (oral administration) or enteric-coated suppositories (rectal administration), the drug is dissolved and absorbed in intestinal juice, the drug can be guided to the illness station, and stimulation of a cold drug to the stomach is relieved; meanwhile, application of the enteric-coated suppositories offers help to part patients who can not be treated through oral administration.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
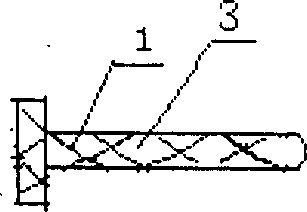
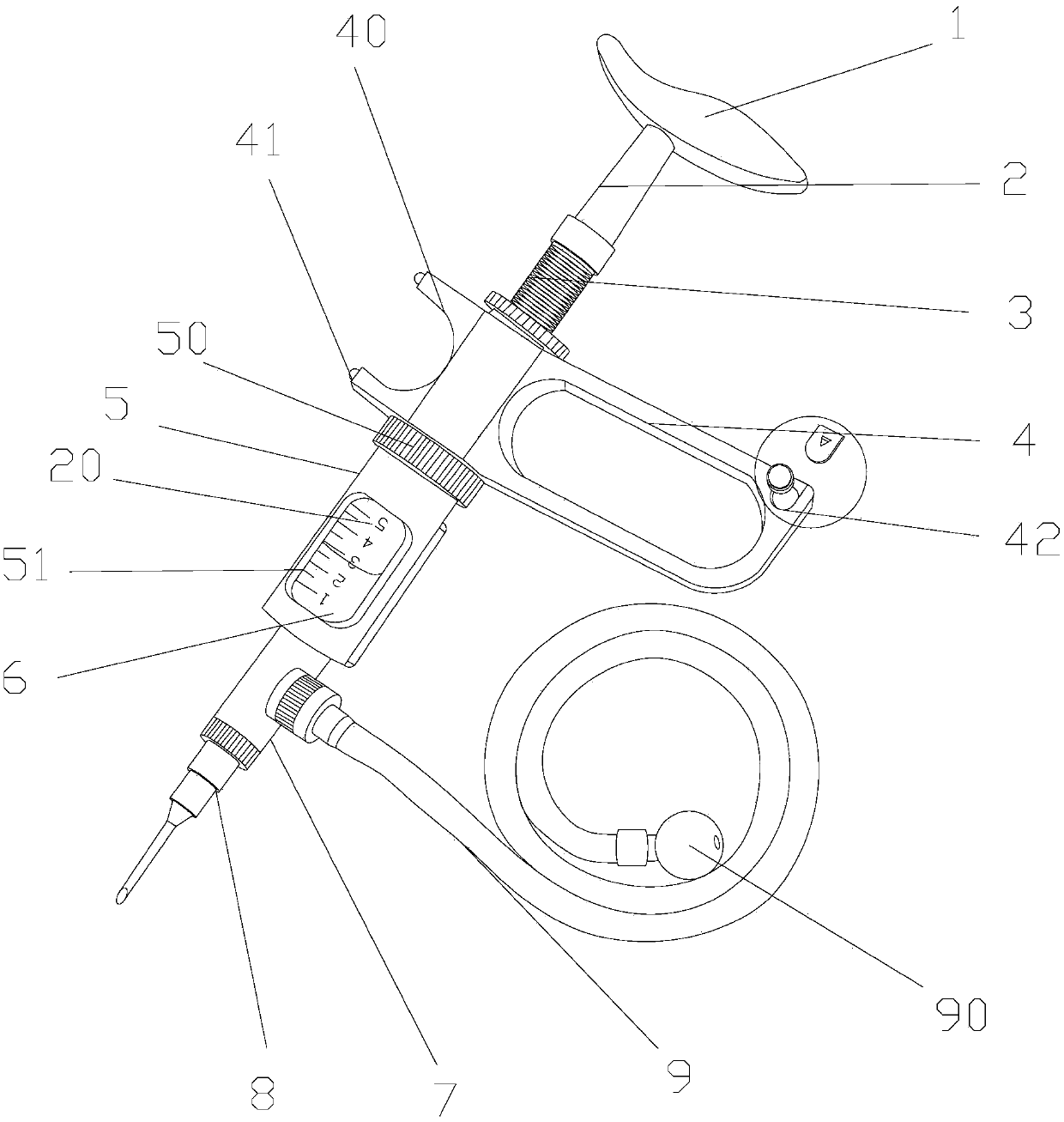
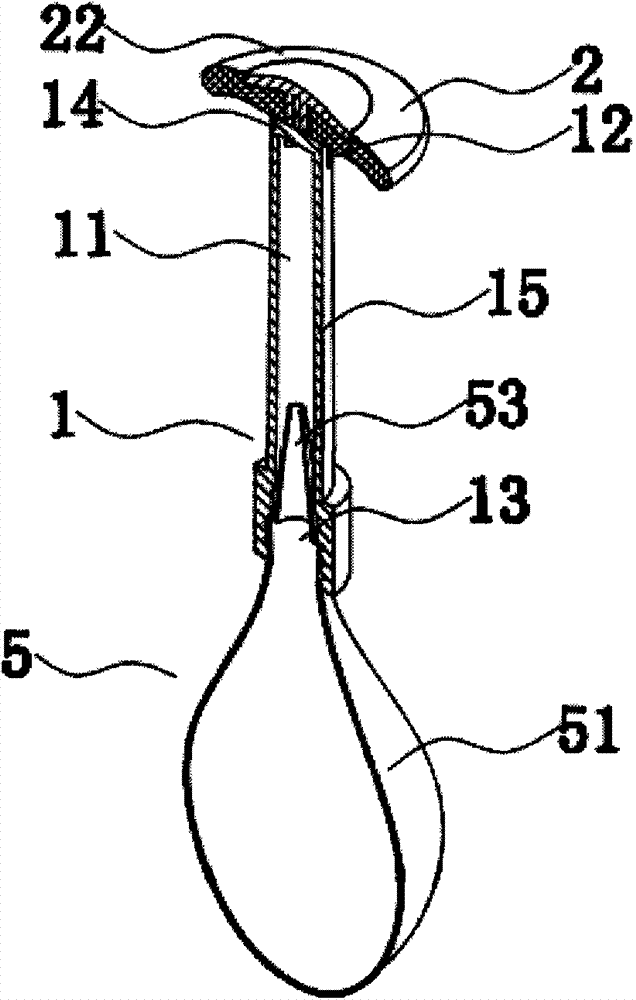
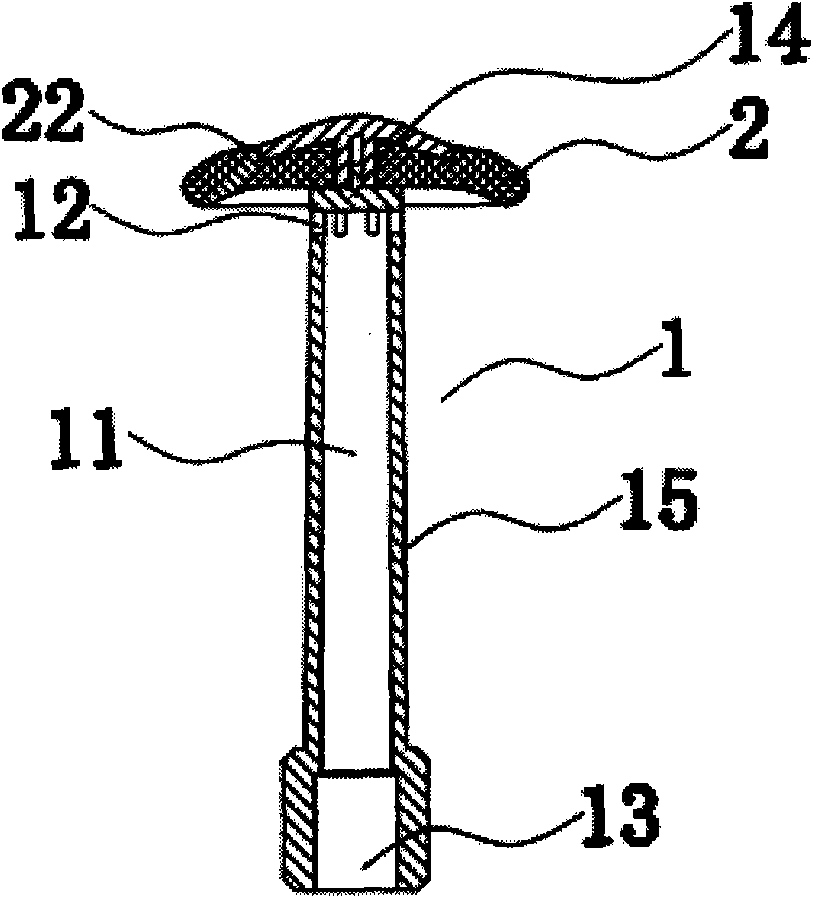
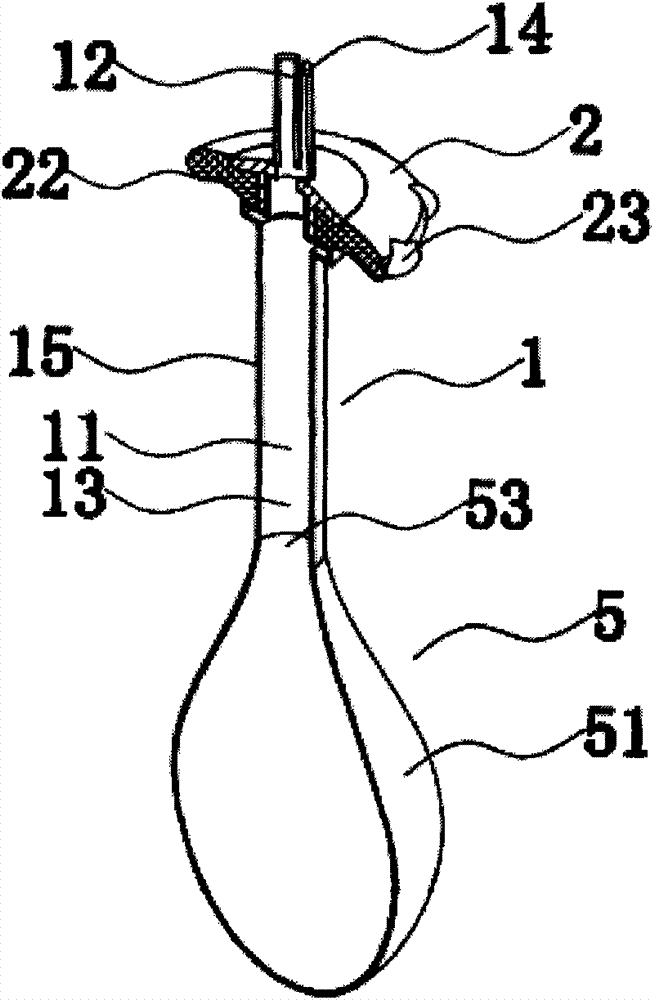
Rectal administration apparatus
The invention provides a rectal administration apparatus, comprising an injection part. The injection part is a hollow tubular part; the top end and / or the sidewall of the injection part are provided with liquid outlets; the other end of the injection part is provided with a liquid inlet. The rectal administration apparatus further comprises an auxiliary part; the auxiliary part easily deformable is connected with the injection part and partially protrudes out towards the outside of the sidewall of the injection part; the auxiliary part has the function of fully lubricating the rectal wall and the function of expanding the anorectum to stimulate bowel movement, thus promoting defecation. The defects of the traditional glycerol enema are overcome, the rectal administration apparatus is more convenient and quicker to use, and better treatment is provided for patients with constipation.
Owner:BEIJING WANSHENG RENHE TECH
Traditional Chinese medicine enema for treating exogenous fever of infants
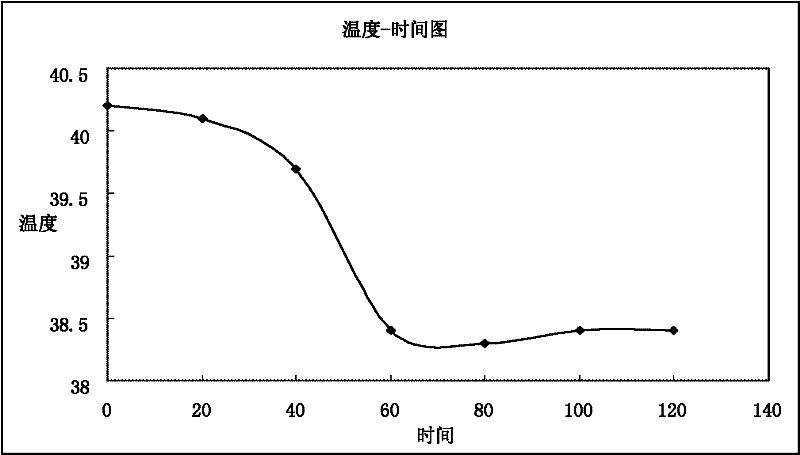
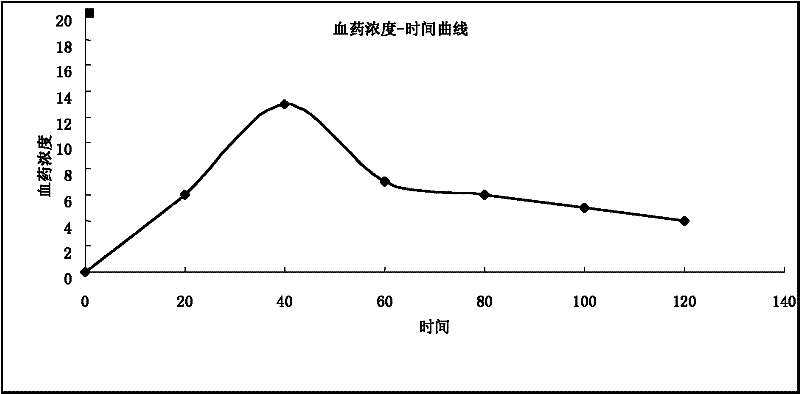
The invention relates to the technical field of a traditional Chinese medicine preparation, and discloses a traditional Chinese medicine enema for treating exogenous fever of infants. The enema is prepared by taking radix scutellariae, honeysuckle, forsythia, dandelion, gypsum and rhizoma anemarrhenae as raw materials through the steps of soaking, decocting and filtering. The invention is characterized in that each dose of enema is prepared from the following raw materials in proportioning by weight: 2-5g of radix scutellariae, 5-8g of honeysuckle, 2-5g of forsythia, 5-8g of dandelion, 9-12g of gypsum and 2-5g of rhizoma anemarrhenae. The enema has the functions of clearing away heat and toxic material, diminishing inflammation and allaying the fever, and is a preferred medicament for allaying the exogenous fever of infants. Compared with the existing treatment method, the enema disclosed by the invention can be quickly absorbed through rectal administration, has a good treatment effect, reduces the 'first pass effect' of general medicaments, has high bioavailability and low price, is convenient and easy to implement, does not have toxic side effects, has an obvious effect on allaying the fever, and also solves the problem that infants are afraid of needles and medicaments. Observed by 200 clinical cases, the antipyretic effective rate within 36 hours is 100%.
Owner:冯陆冰 +2
Composition for oral or rectal administration
InactiveUS20050181042A1Maintain good propertiesBiocideOrganic active ingredientsLipid formationFood supplement
A solid pharmaceutical or food supplement tablet or suppository composition has a melting point of 25° C. or higher and comprises a continuous lipid component comprising one or more polar lipids, one or more non-polar lipids, optionally one or several of water and mono- to trivalent alcohol in an amount of up to 15% by weight of the composition, and one or more agents selected from pharmacologically active agent and food supplement agent. Also disclosed is a corresponding tablet and a corresponding suppository, processes for production of the composition and the tablet and the suppository, and a method of preventing or treating conditions amenable to preventive or therapeutic treatment by administration of the tablet or suppository.
Owner:DSM IP ASSETS BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com