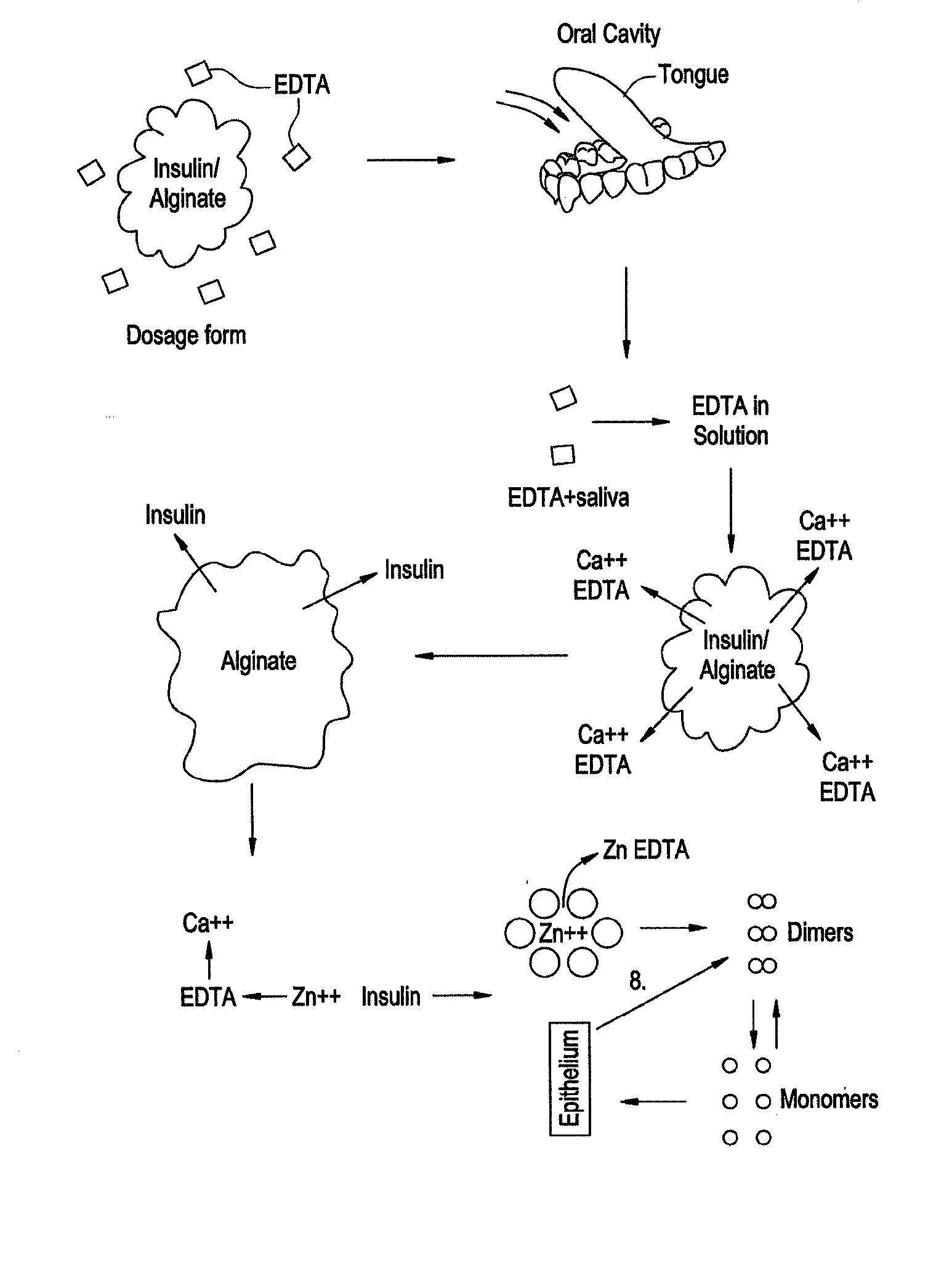
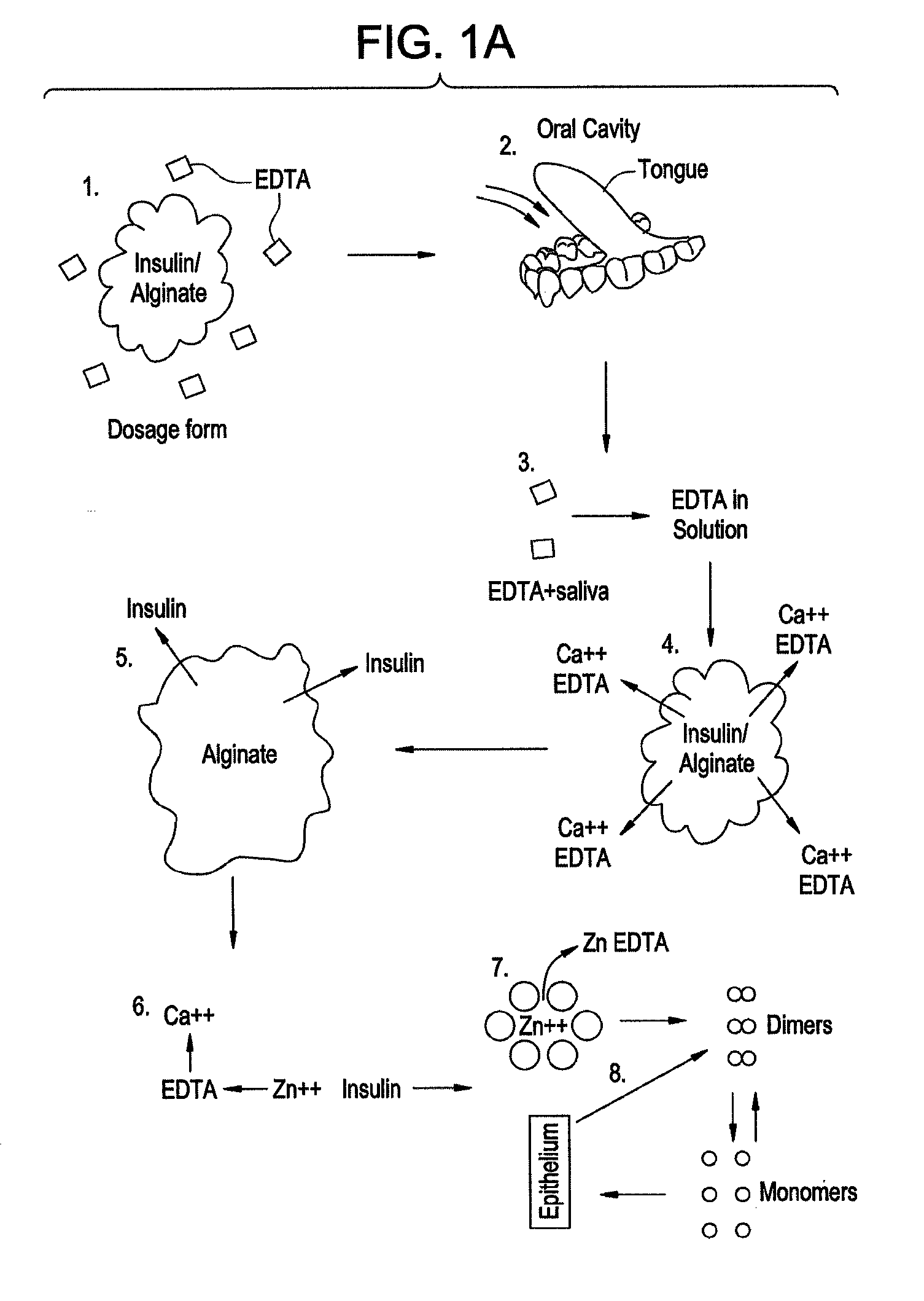
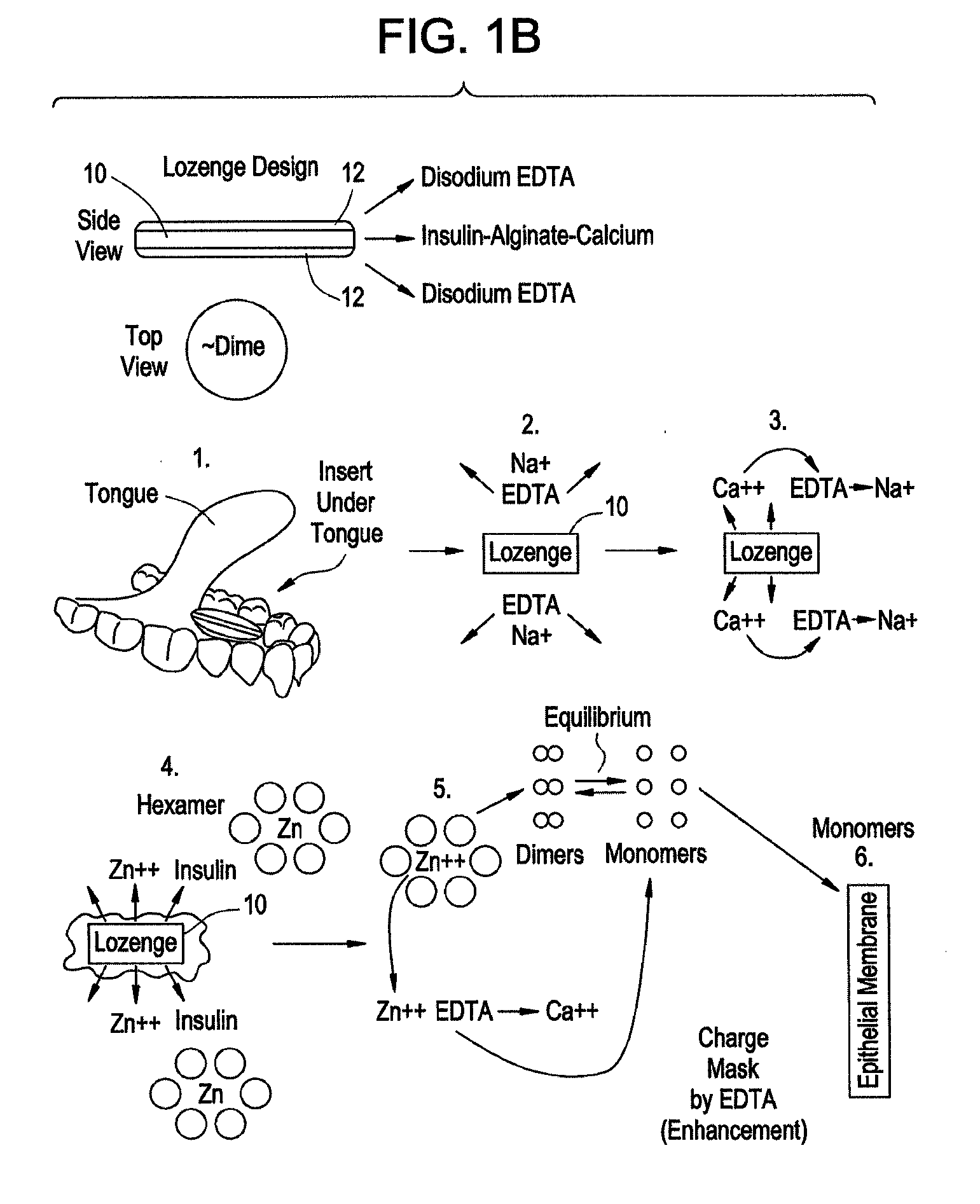
Rapid Acting Drug Delivery Compositions
a drug delivery and composition technology, applied in the direction of powder delivery, small article dispensing, rigid containers, etc., can solve the problems of inability to effectively deliver drugs, complex lack of effective oral delivery means, and inability to develop oral delivery systems for peptides, in general, and insulin, etc., to improve stability, rapid onset of action, and increase the effect of the ra
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Insulin Solutions Containing Different Concentrations of EDTA on Conversion of Insulin from a Hexameric Form to Monomer / Dimers
[0096] Materials
[0097] Human recombinant Insulin (Akzo-Nobel), Citric acid and disodium EDTA were used in this experiment in distilled water. Nanosep microtubes with 30,000 MW cutoff (Pall Scientific) were used to separate the hexamers (36,000 MW) from the dimers / monomers (6-12,000 MW) in the insulin solutions. Analysis was performed by HPLC using a waters 2695 separations module fitted with a symmetry 300™ C4 5 um column (Waters Corp, Milford, Mass., #186000285) and a photodiode array detector (at 220 nm absorbance). A gradient method (25-32% ACN in water, 0.05% TFA) was used to separate the insulin from the other ingredients.
[0098] Methods
[0099] Human recombinant Insulin was dissolved in 2 mg / mL Citric acid to from a 1 mg / ml solution and 1.5 mL of this solution was subsequently pipetted into test tubes. EDTA was added to each tube in order to ...
example 2
Effect of Administration of Sublingual Dry Powder Insulin Formulation on a Patient's Insulin and Glucose Levels
[0103] The insulin and blood glucose levels following a single sublingual administration of a dry powder insulin formulation were measured in one male, 35-year old, Type 1 diabetic patient. The dose administered to the patient contained 6 mg insulin, 4 mg citric acid and 4 mg EDTA. The insulin in the formulation was approximately 28 IU / mg.
[0104] The patient fasted overnight and arrived at the clinic in the early morning. An IV line with a saline drip was attached to the patient. The patient was instructed to open his mouth and touch his upper palette with his tongue and the dry powder formulation was sprinkled under his tongue. He was then instructed to lower his tongue, close his mouth and not swallow for one minute.
[0105] Blood glucose was measured at five minutes prior to the application of the sublingual insulin formulation. Following administration of the insulin fo...
example 3
Determination of Effect of EDTA and Various Acids on Insulin Particle Size
[0112] A study was conducted to determine the effect of EDTA, a chelator, in combination with various acids: acetic acid, hydrochloric acid, ascorbic acid and citric acid, on insulin particle size. Controls included insulin with EDTA and no acid, and insulin with acid and no EDTA.
[0113] Using the same techniques described in example 1, insulin (1 mg / ml) was dissolved in a food acid, EDTA added, and the mixture on top of a 30,000 mw cutoff filer in a microtube, and then spun for 10 minutes at 10,000 rpm. Each mixture was tested at pH 3 (without buffer) and pH 7.0 (with phosphate buffer). The results were calculated as a percent of insulin recovered in the filtrate, compared to the starting quantity (percent).
[0114] The results are depicted graphically in FIGS. 4 and 5a-d. The results show that the combination of EDTA and citric acid produces a significantly greater amount of lower weight (i.e., monomeric rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com