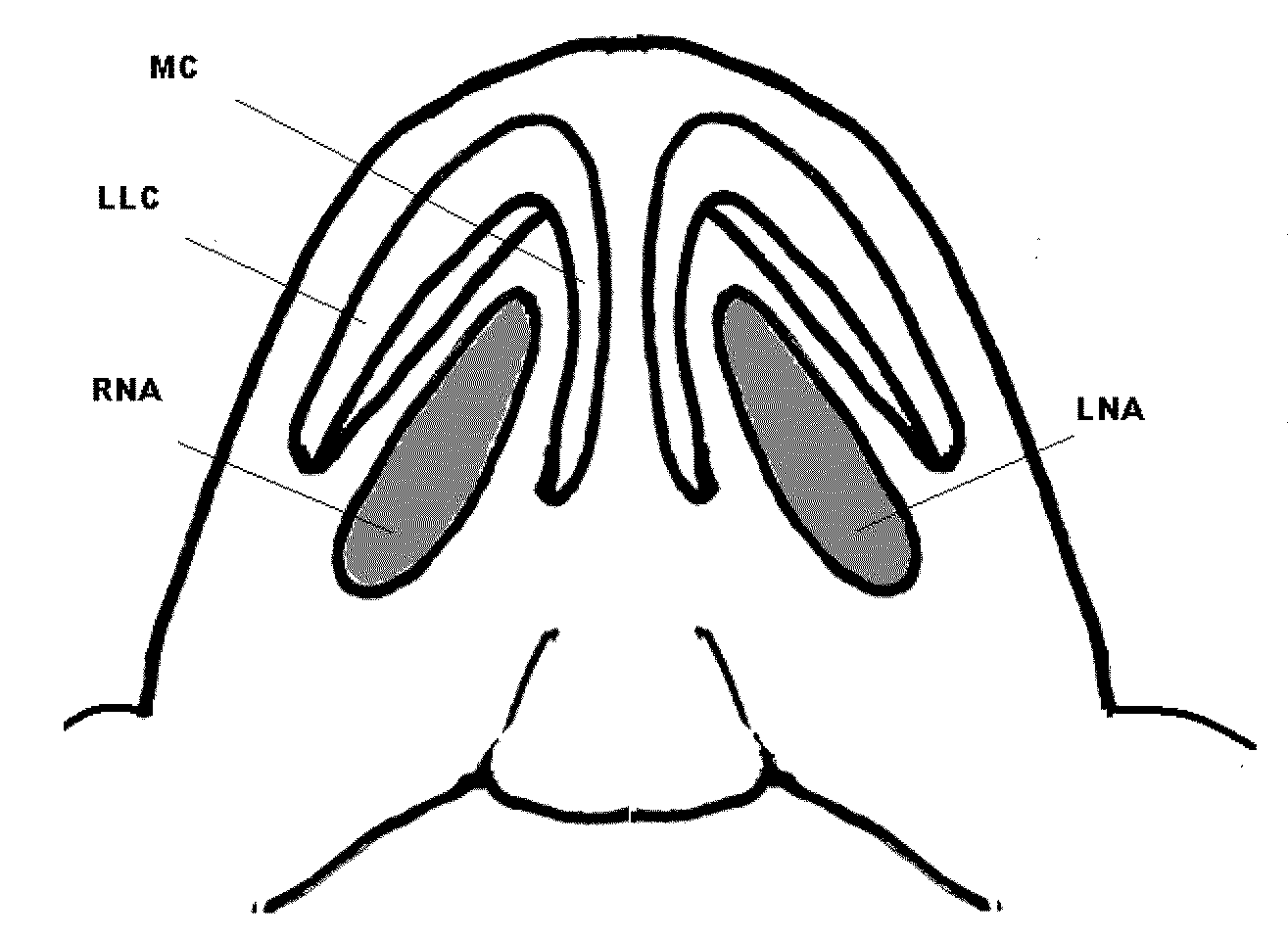
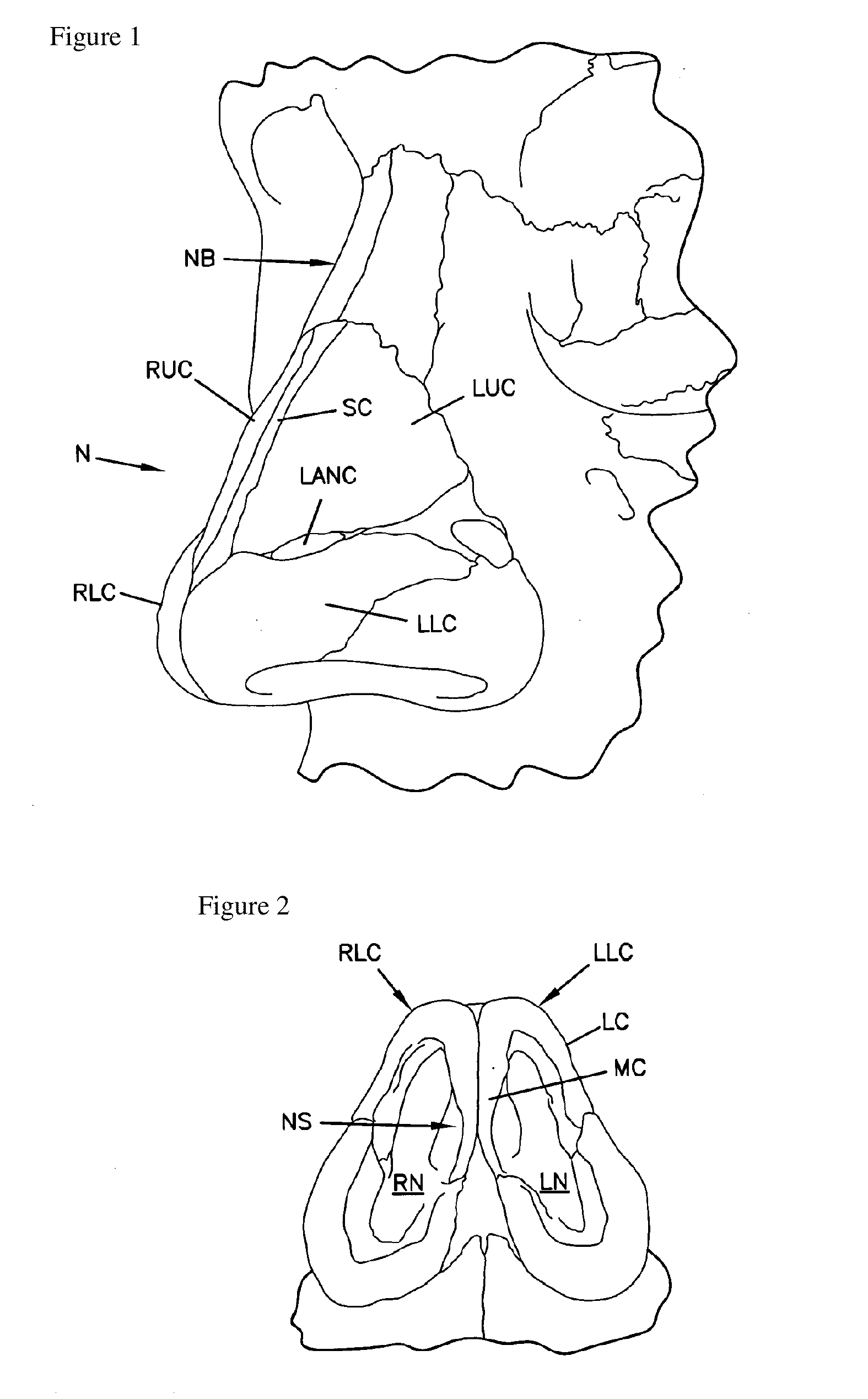
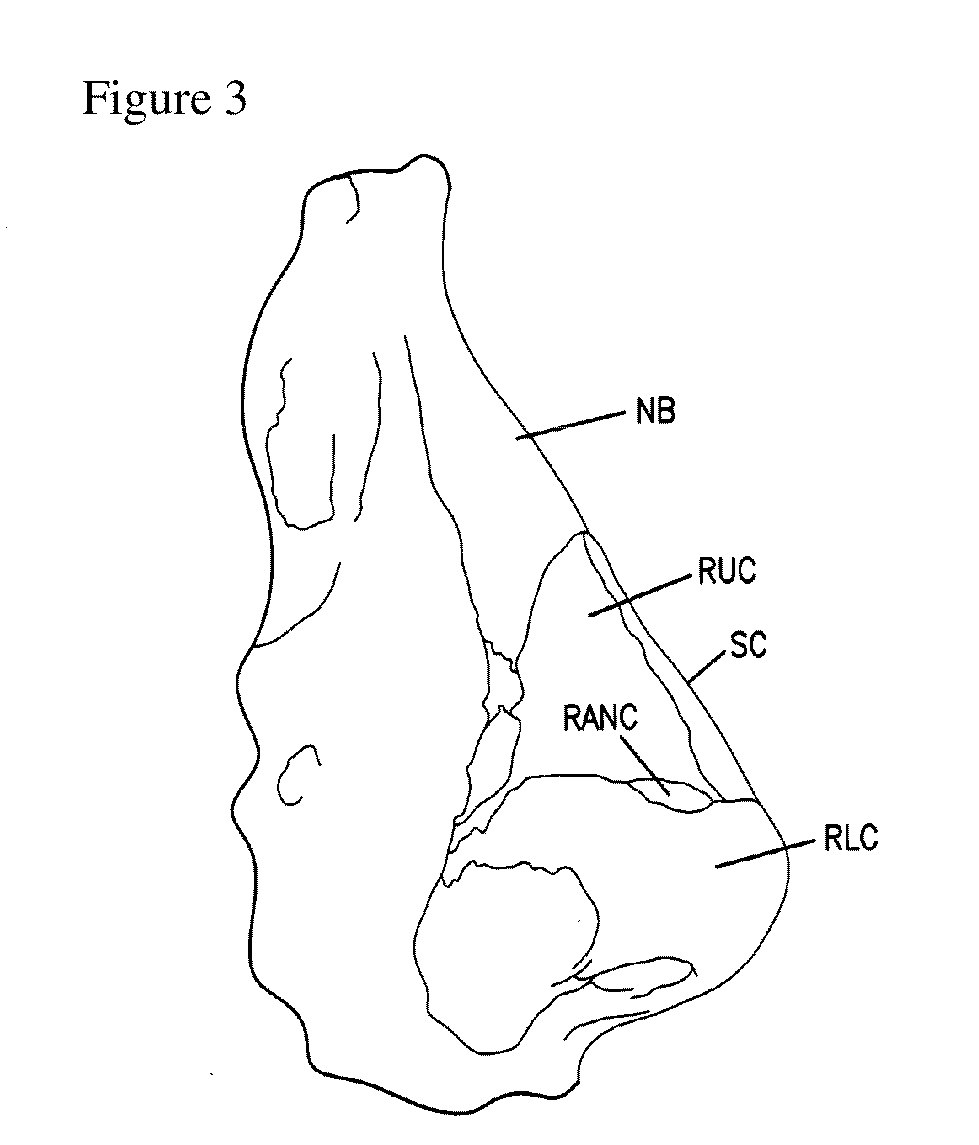
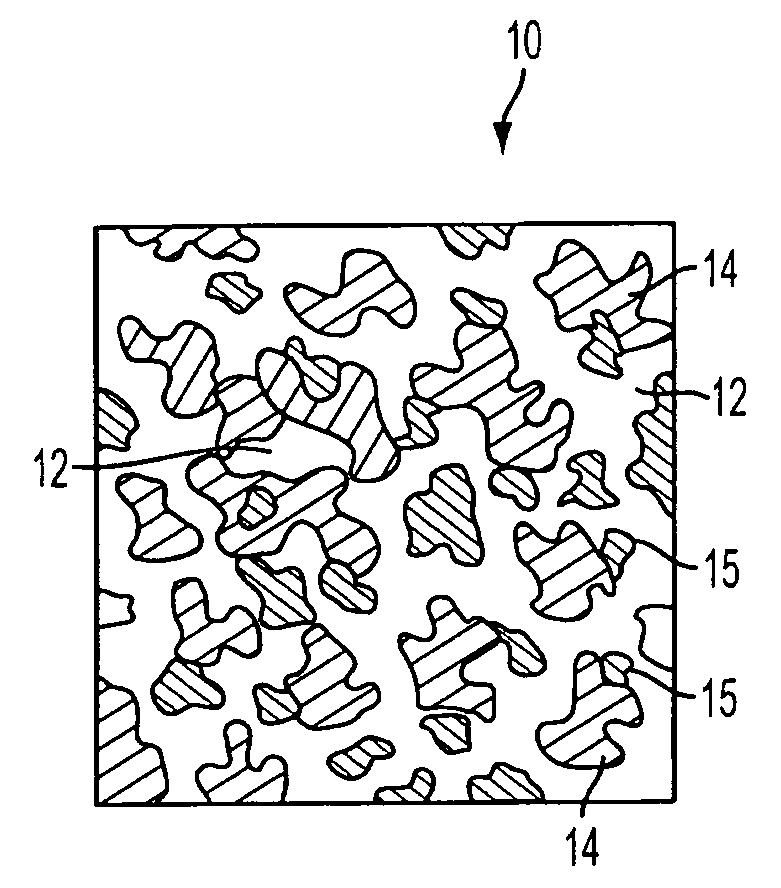
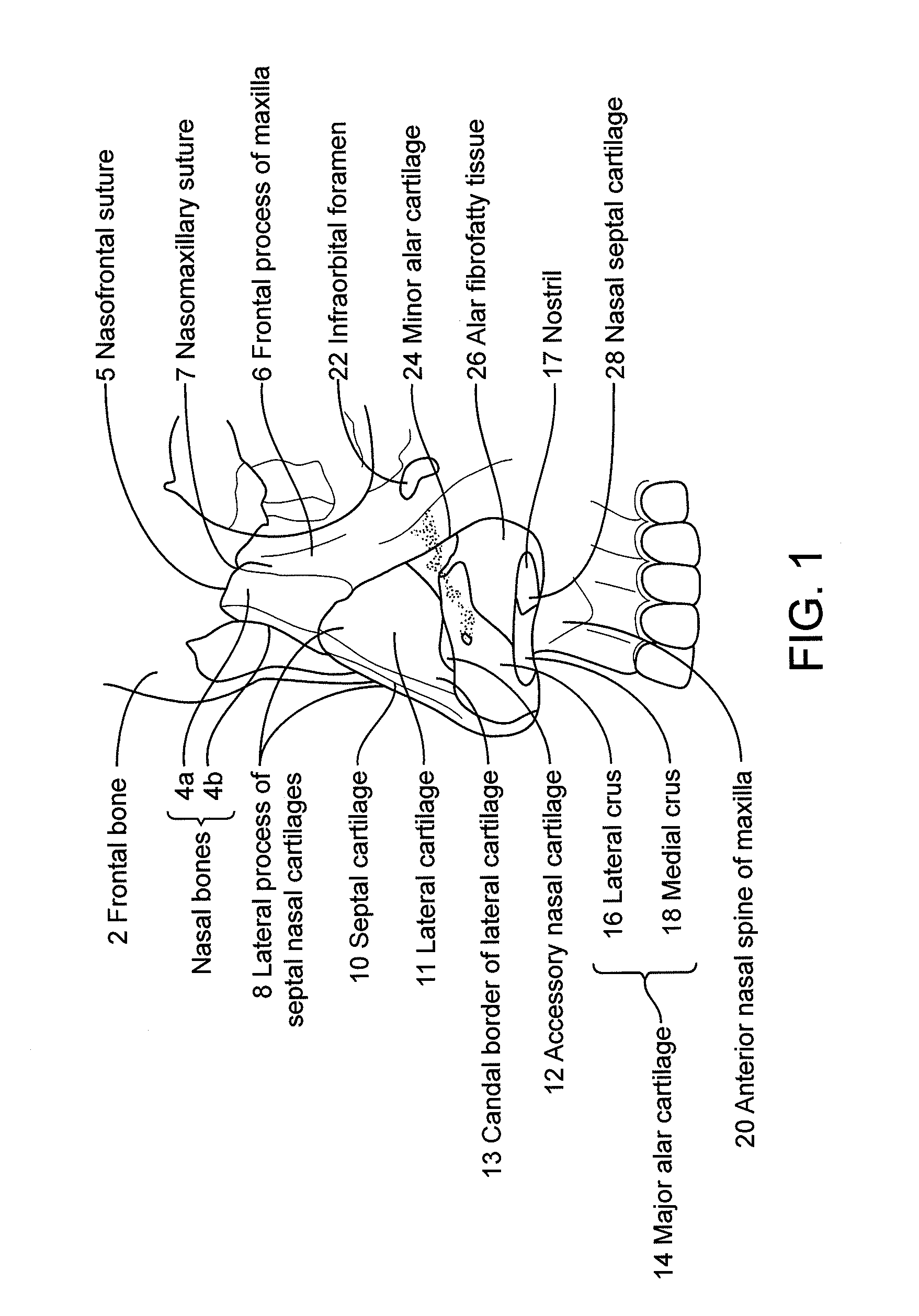
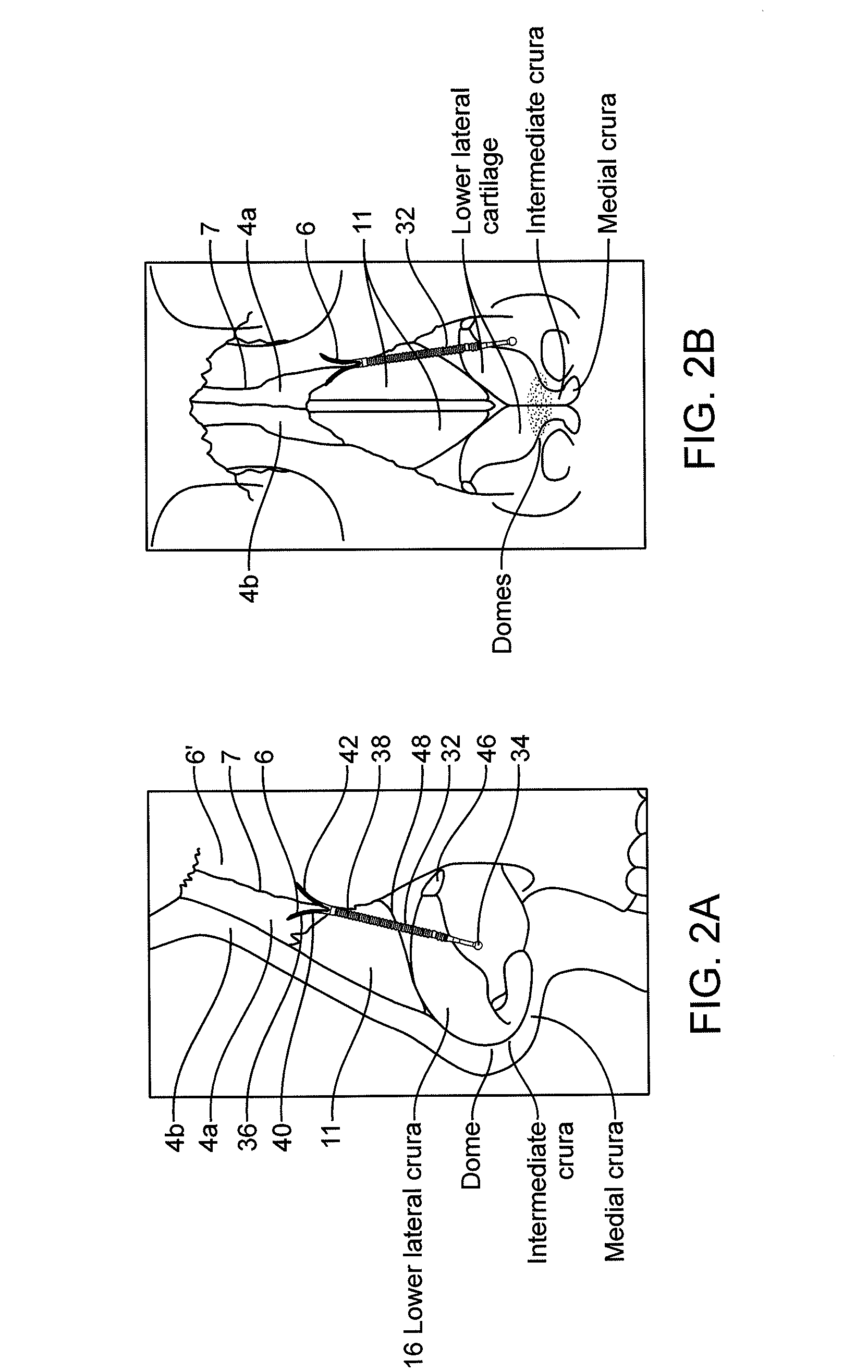
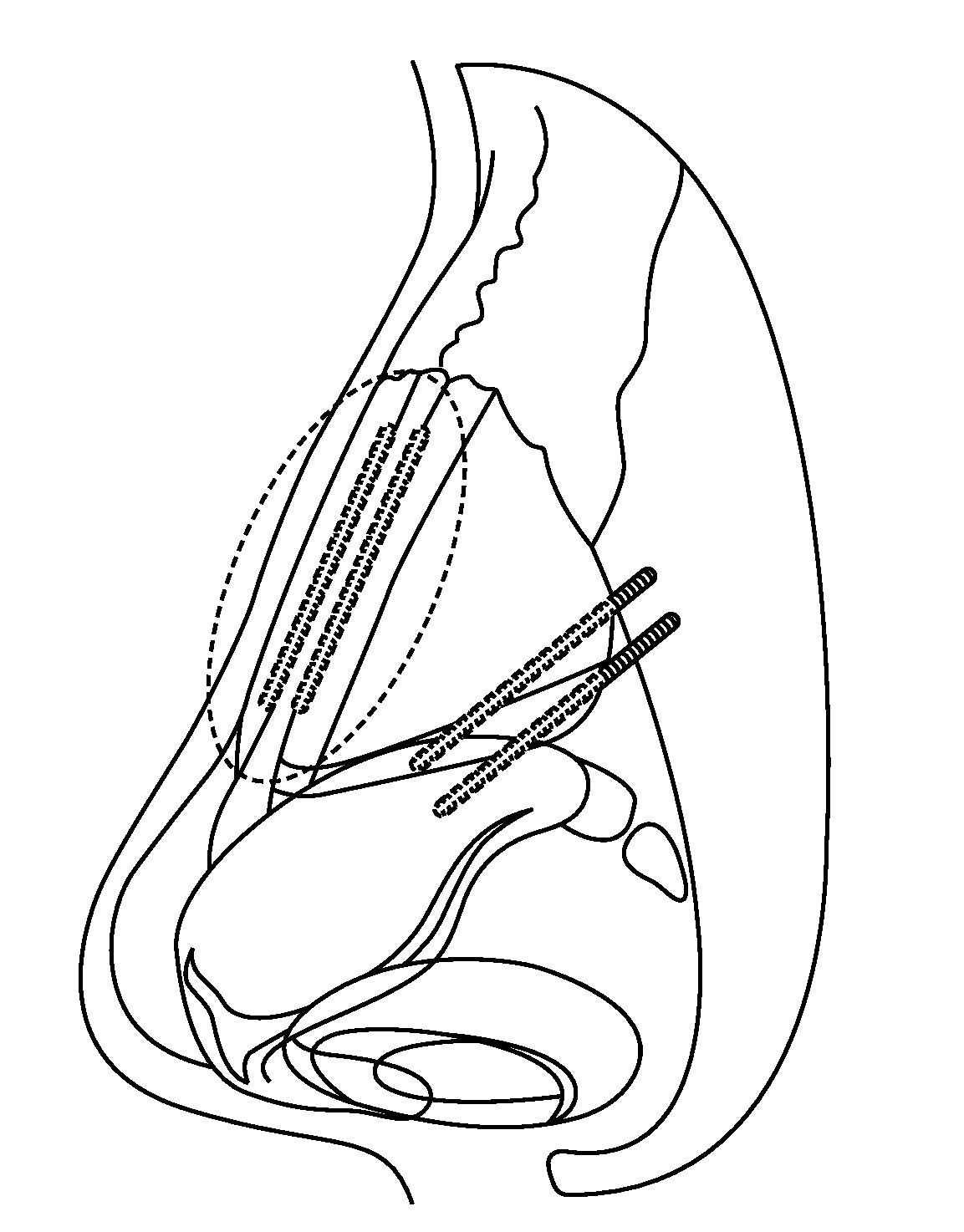
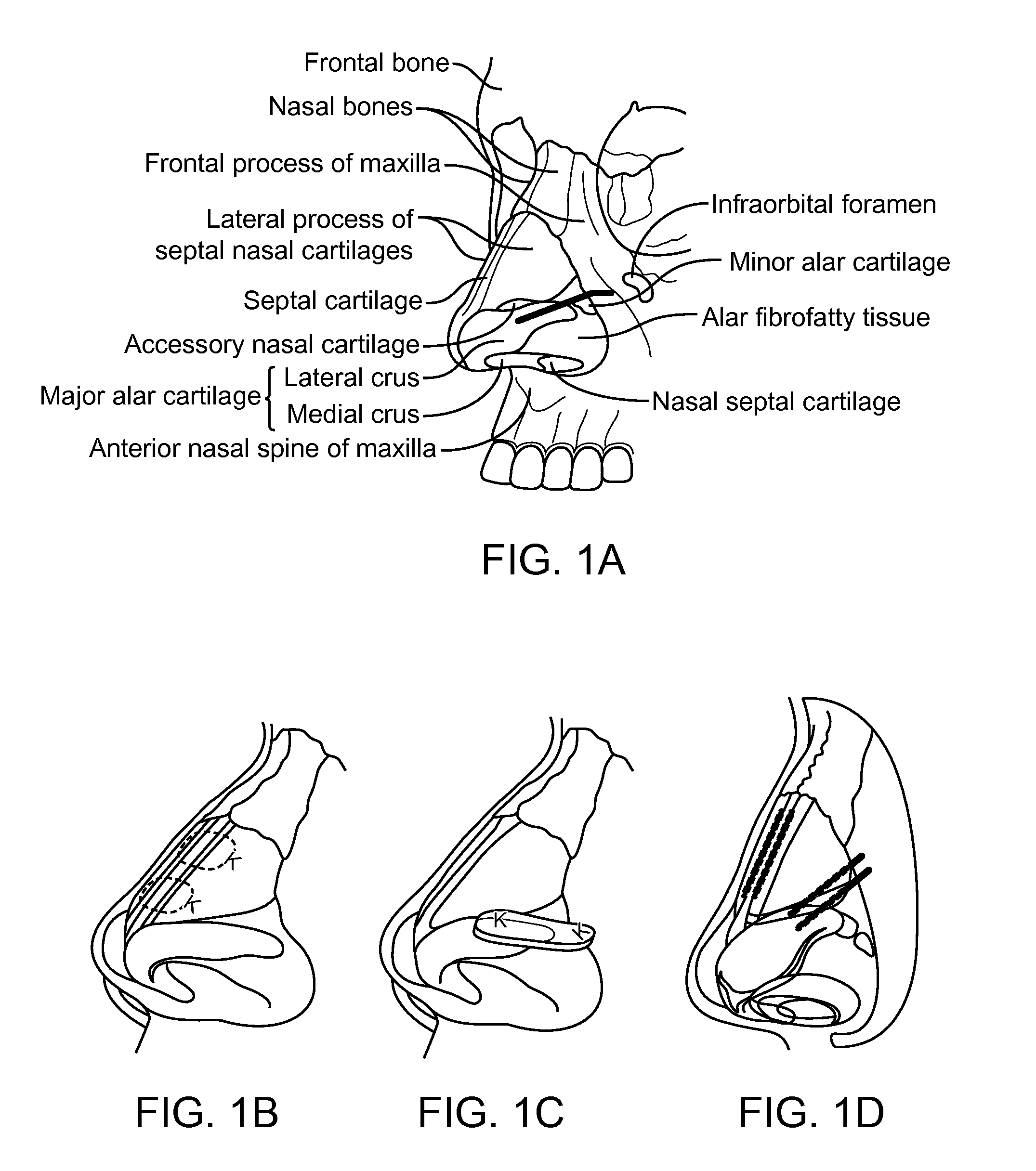
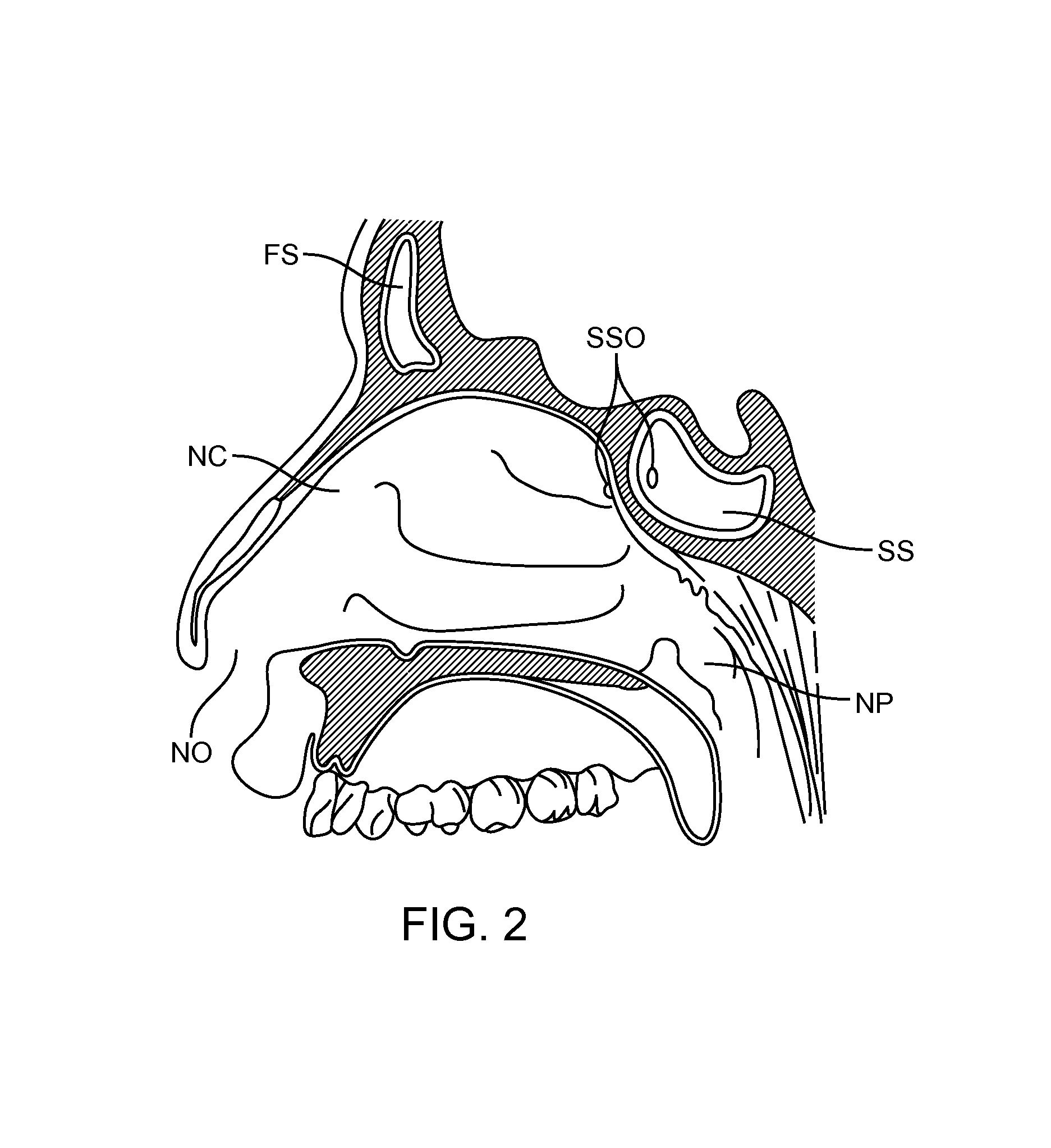
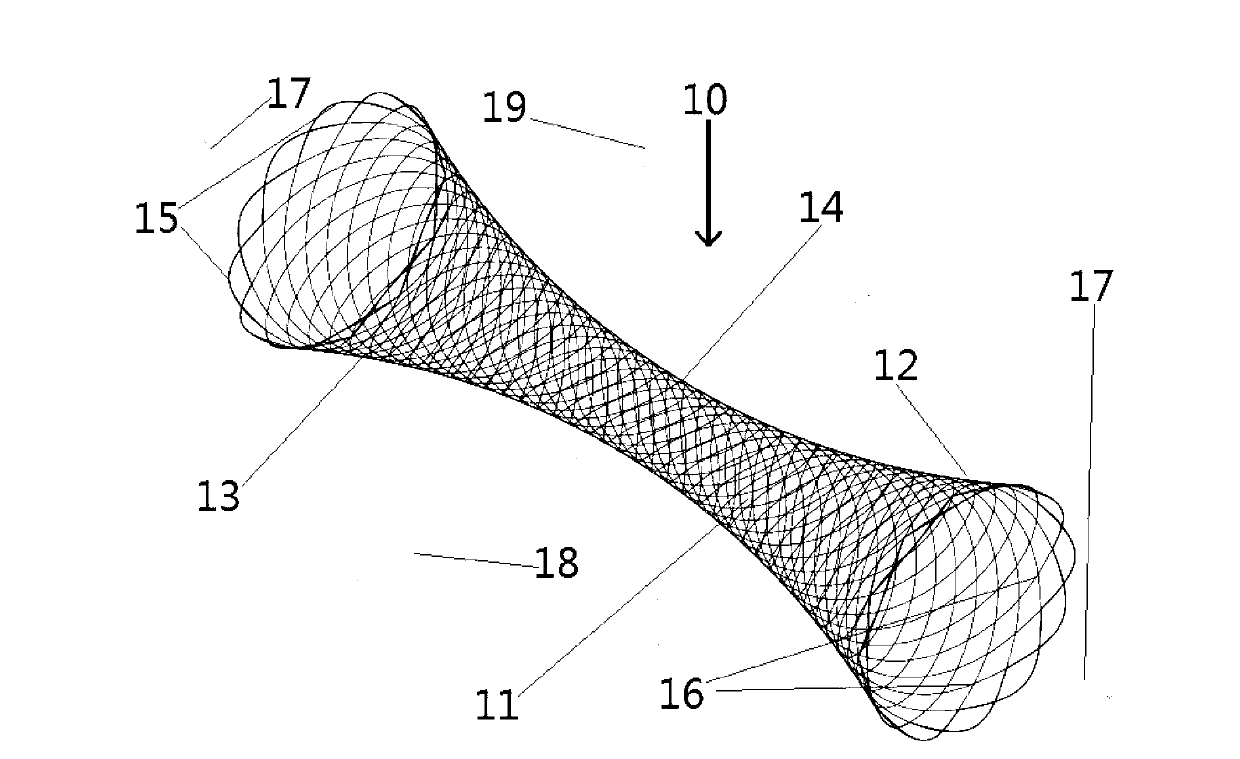
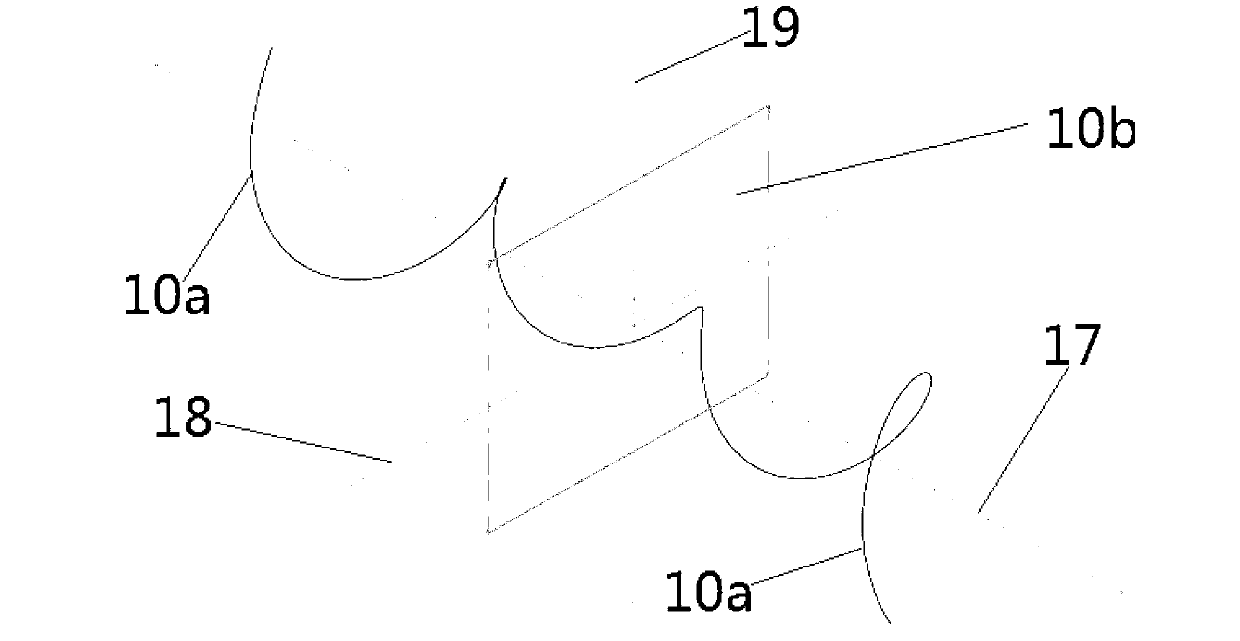
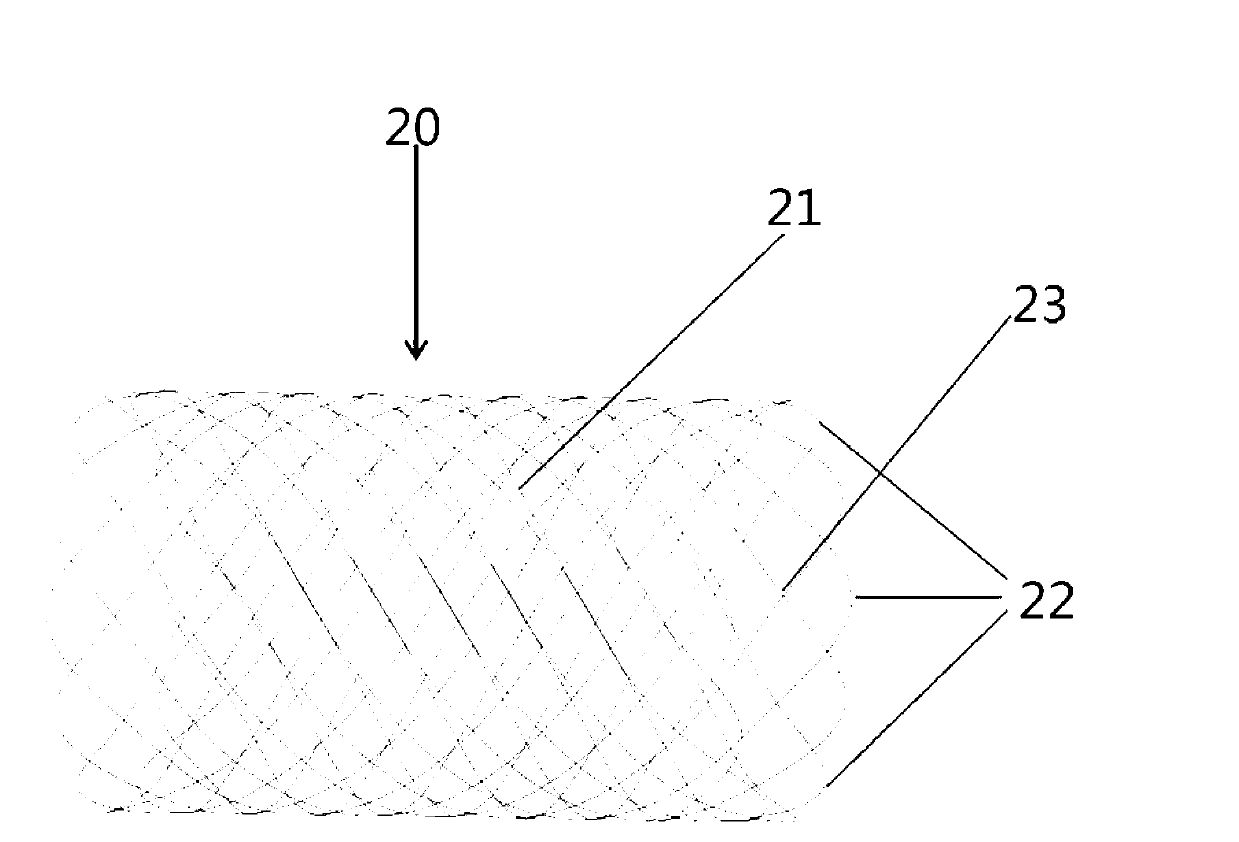
Patents
Literature
224results about "Nose implants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
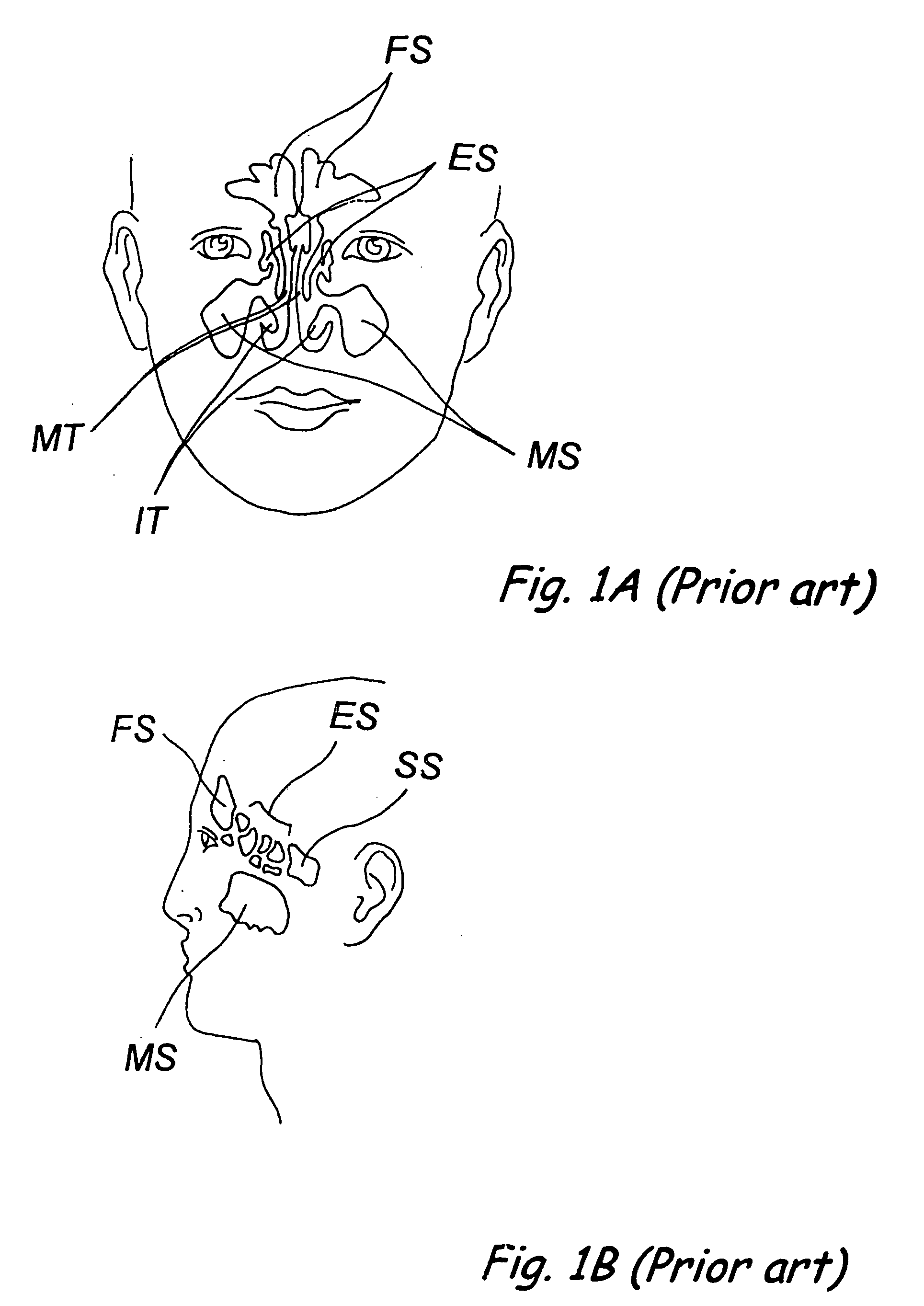
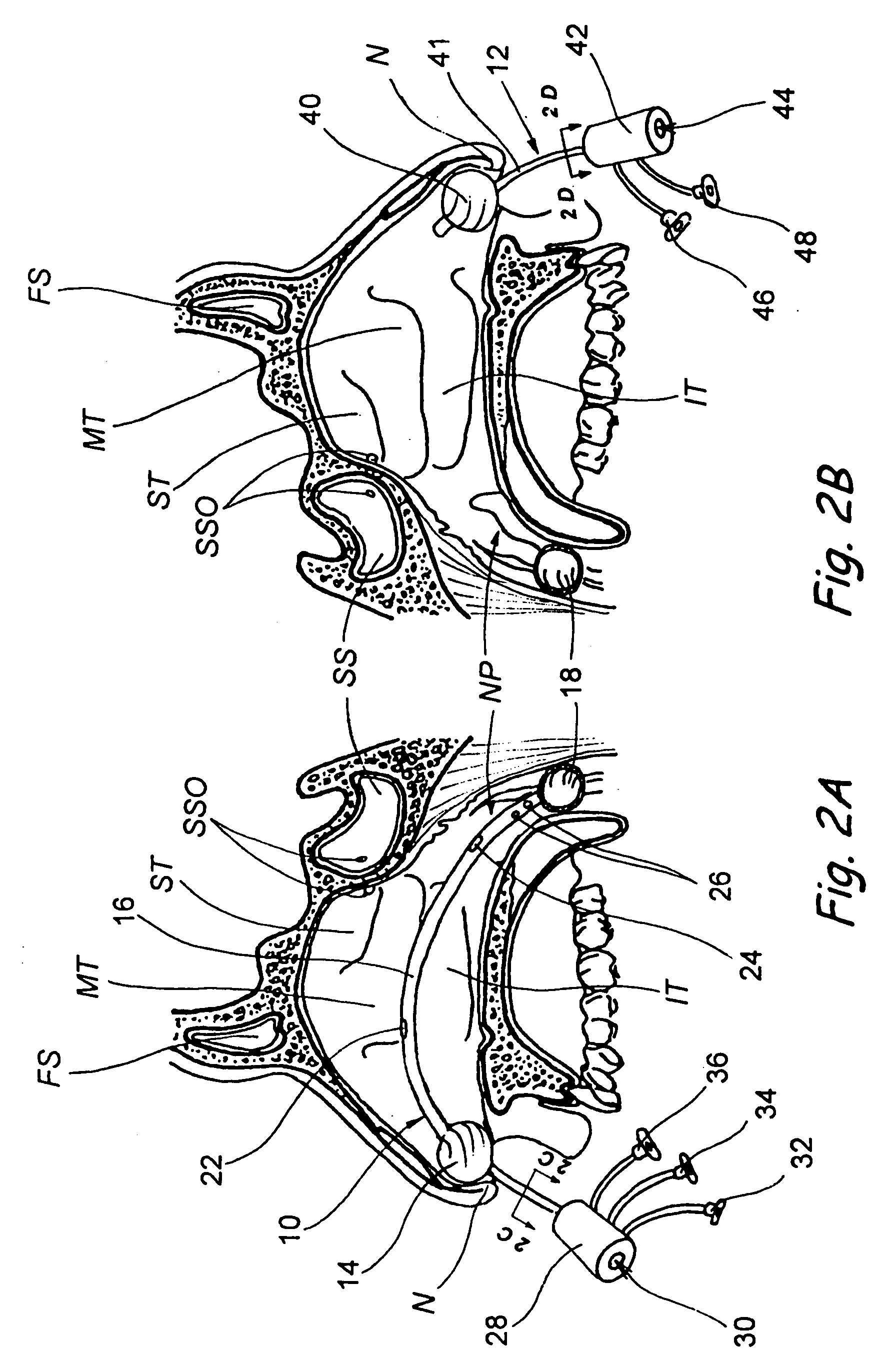
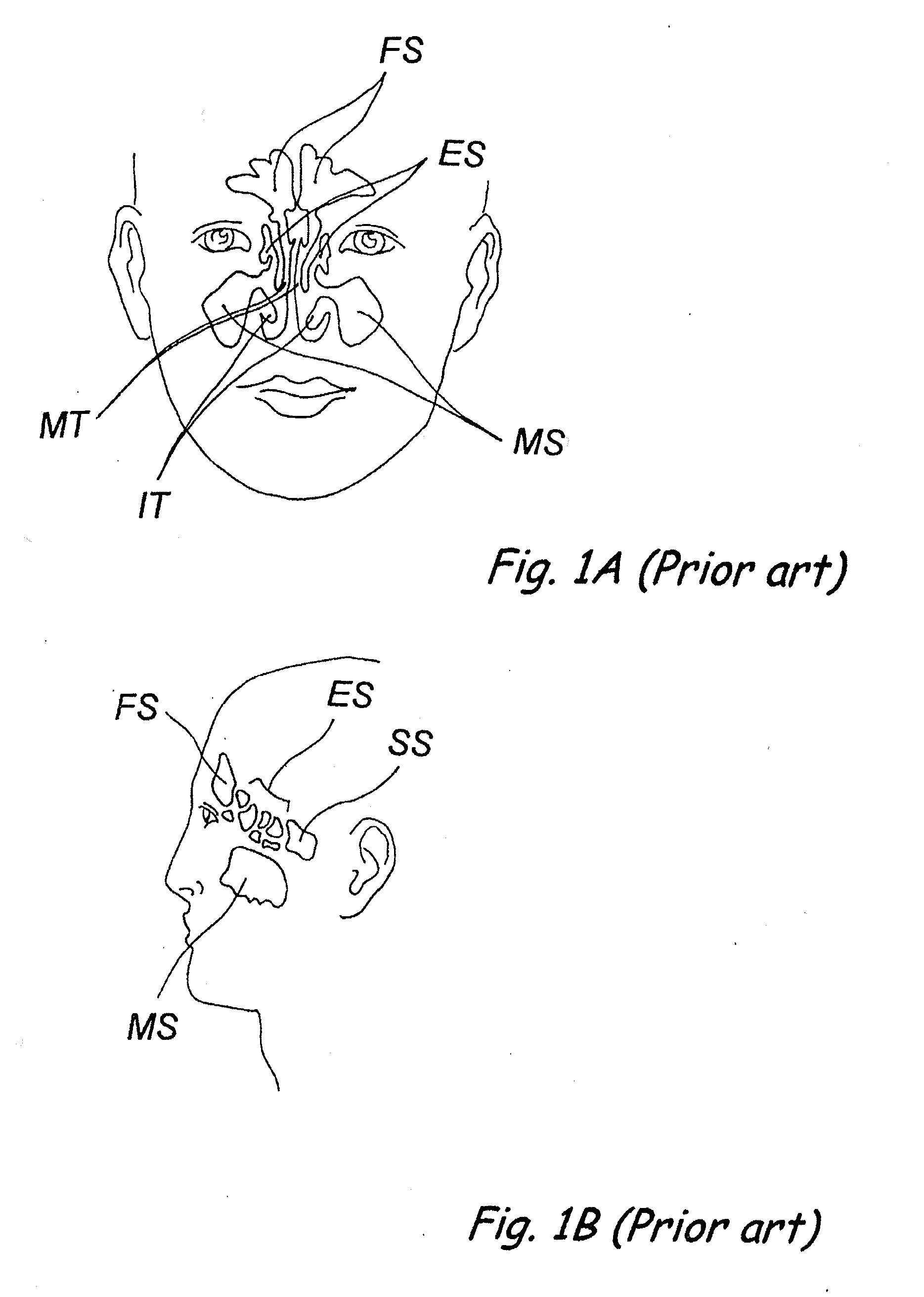
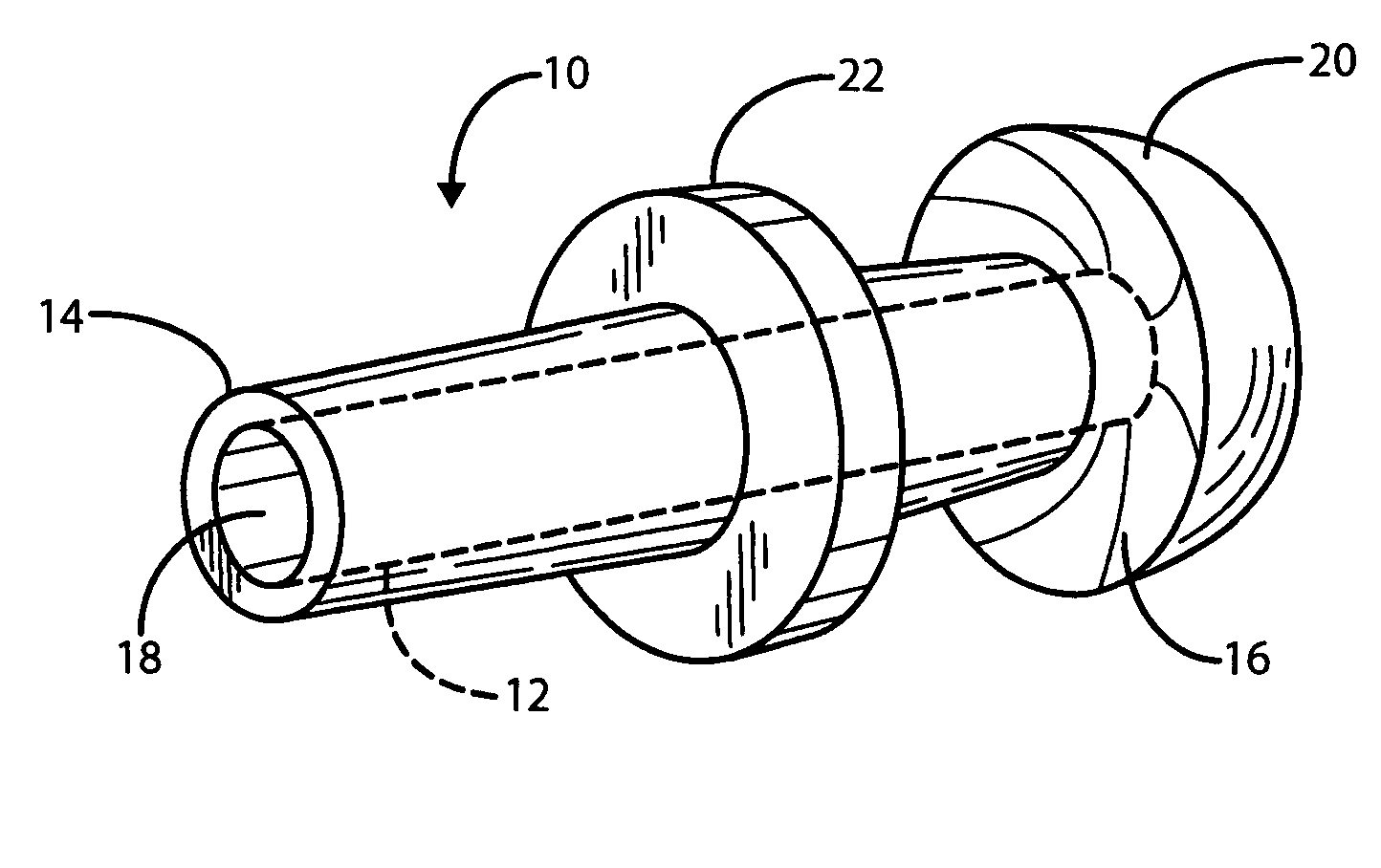
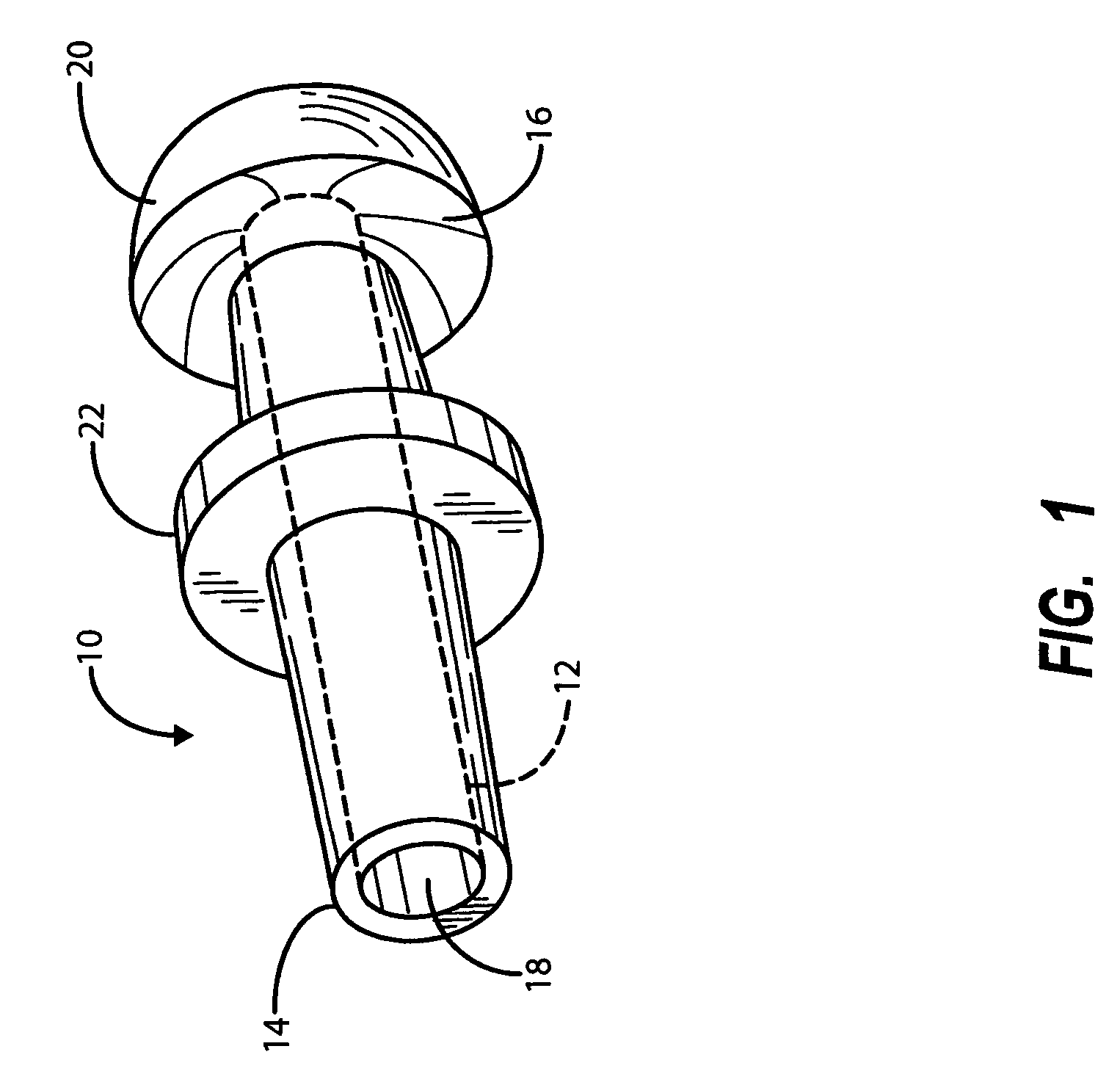
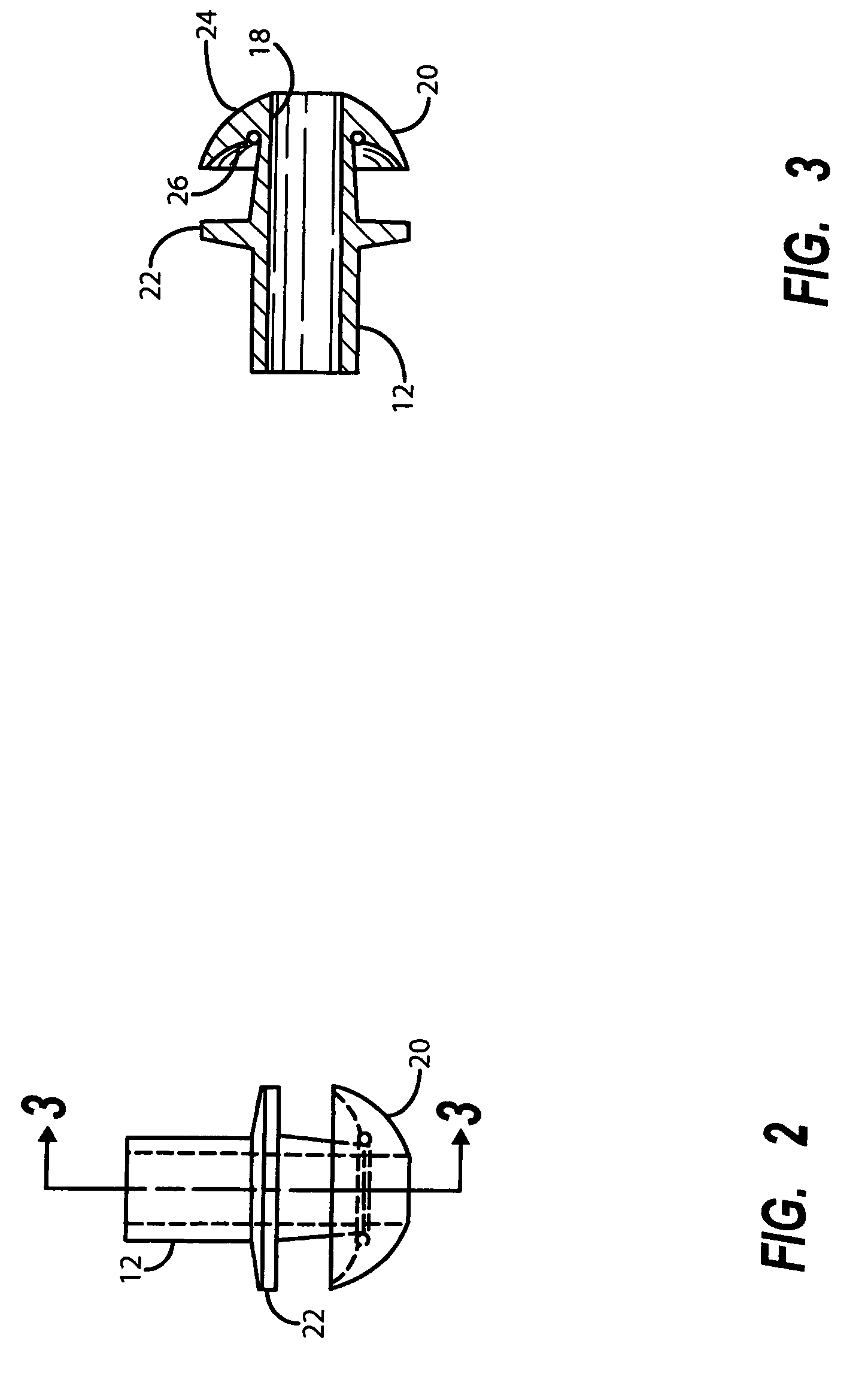
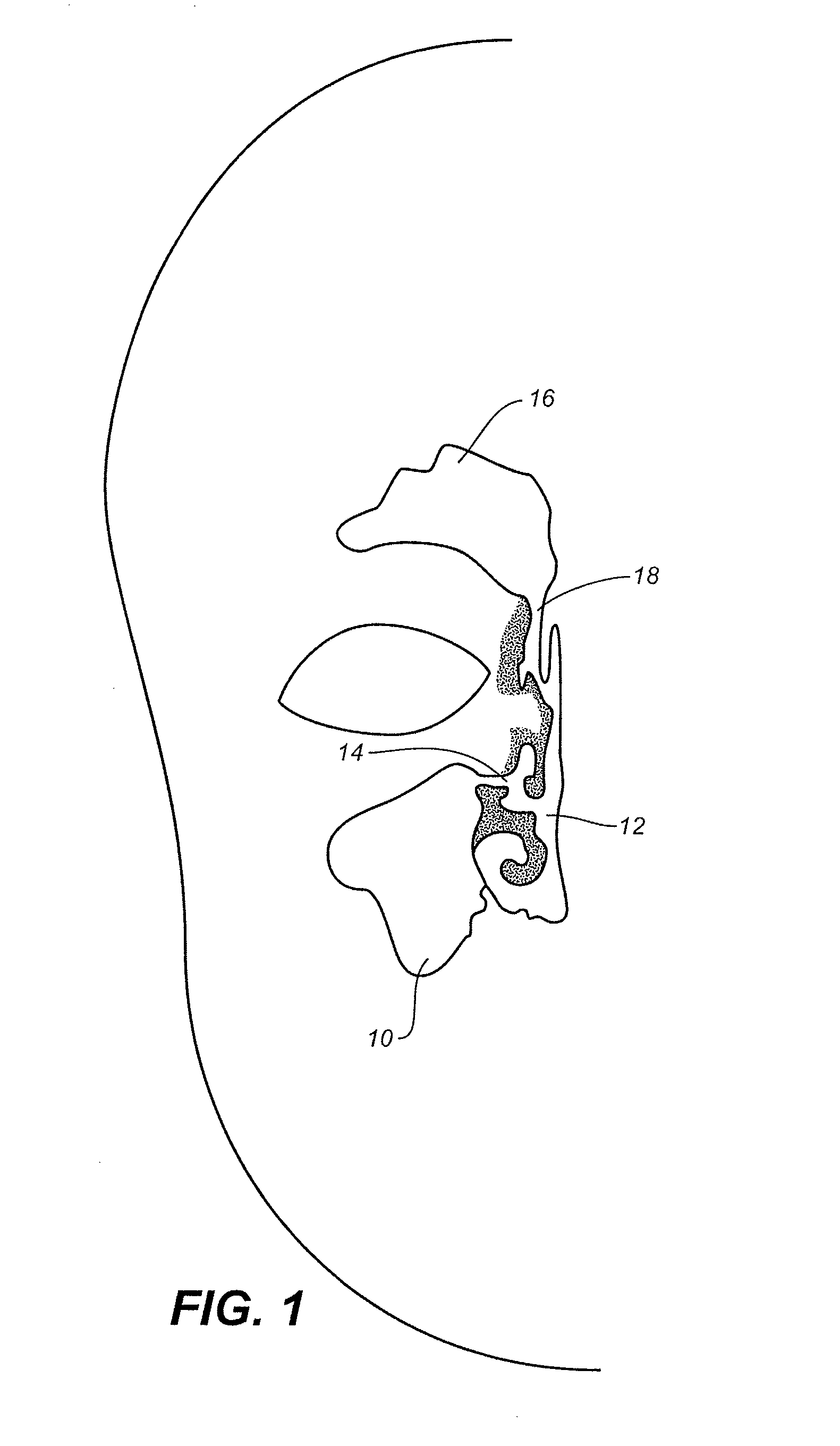
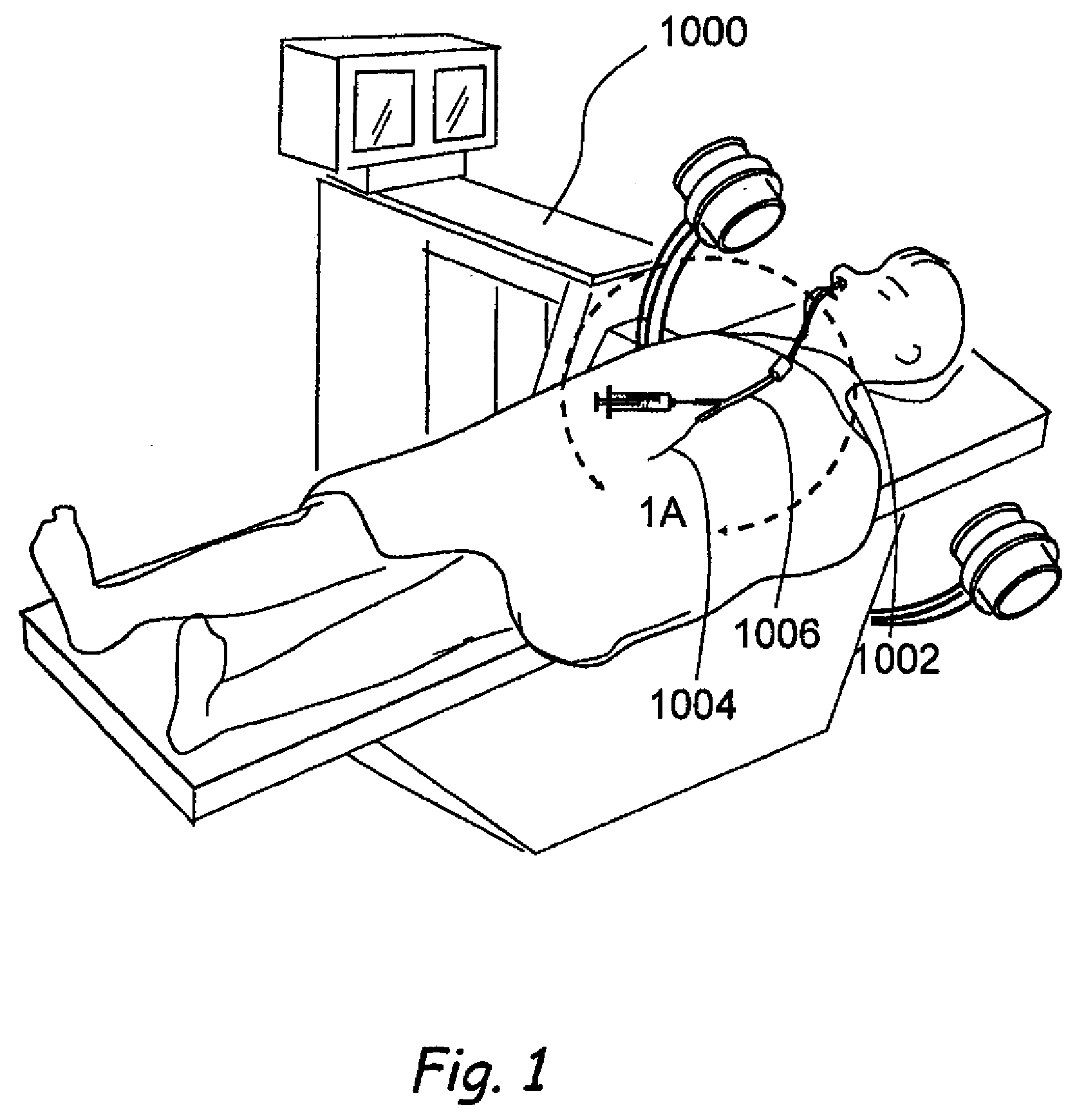
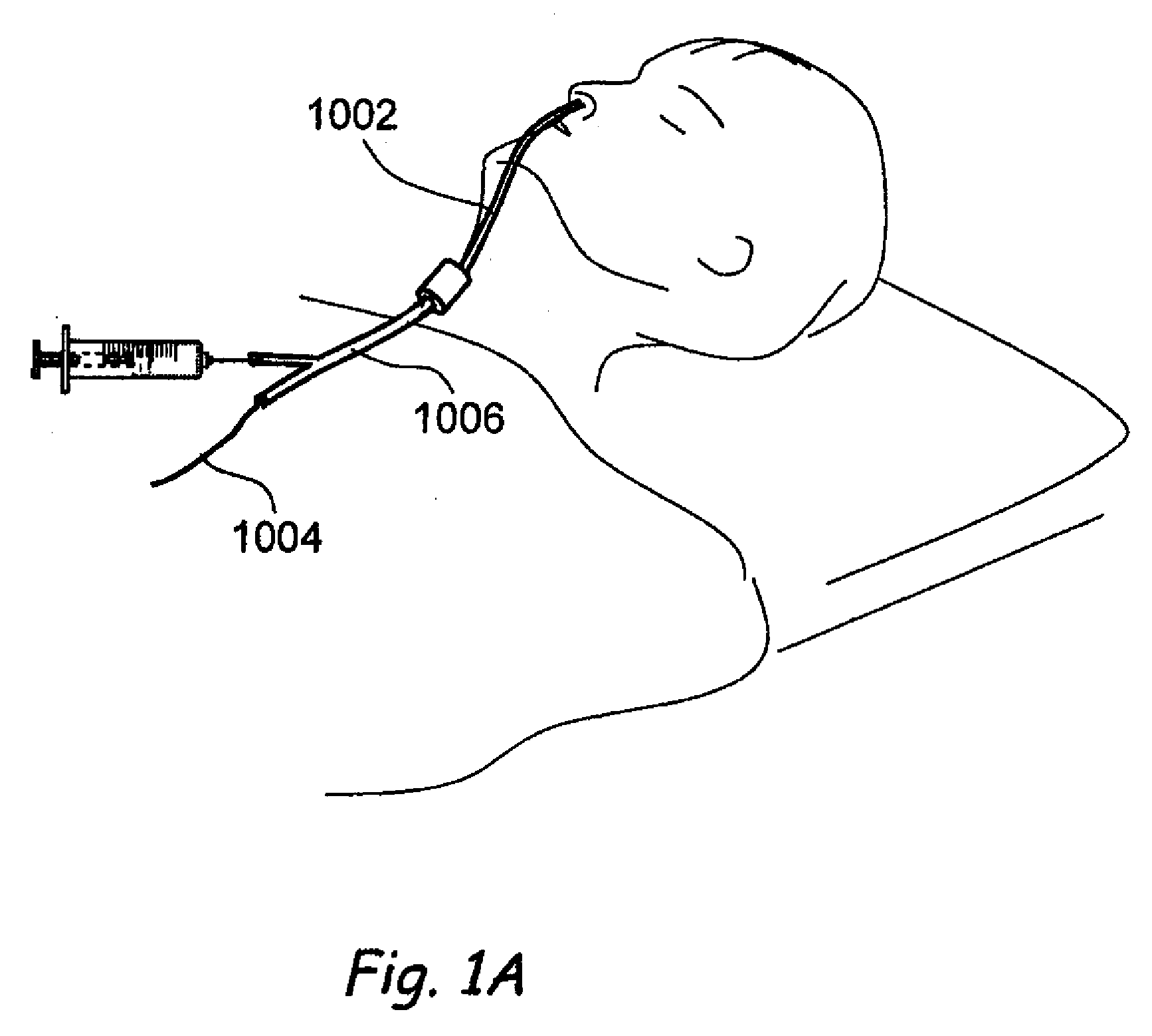
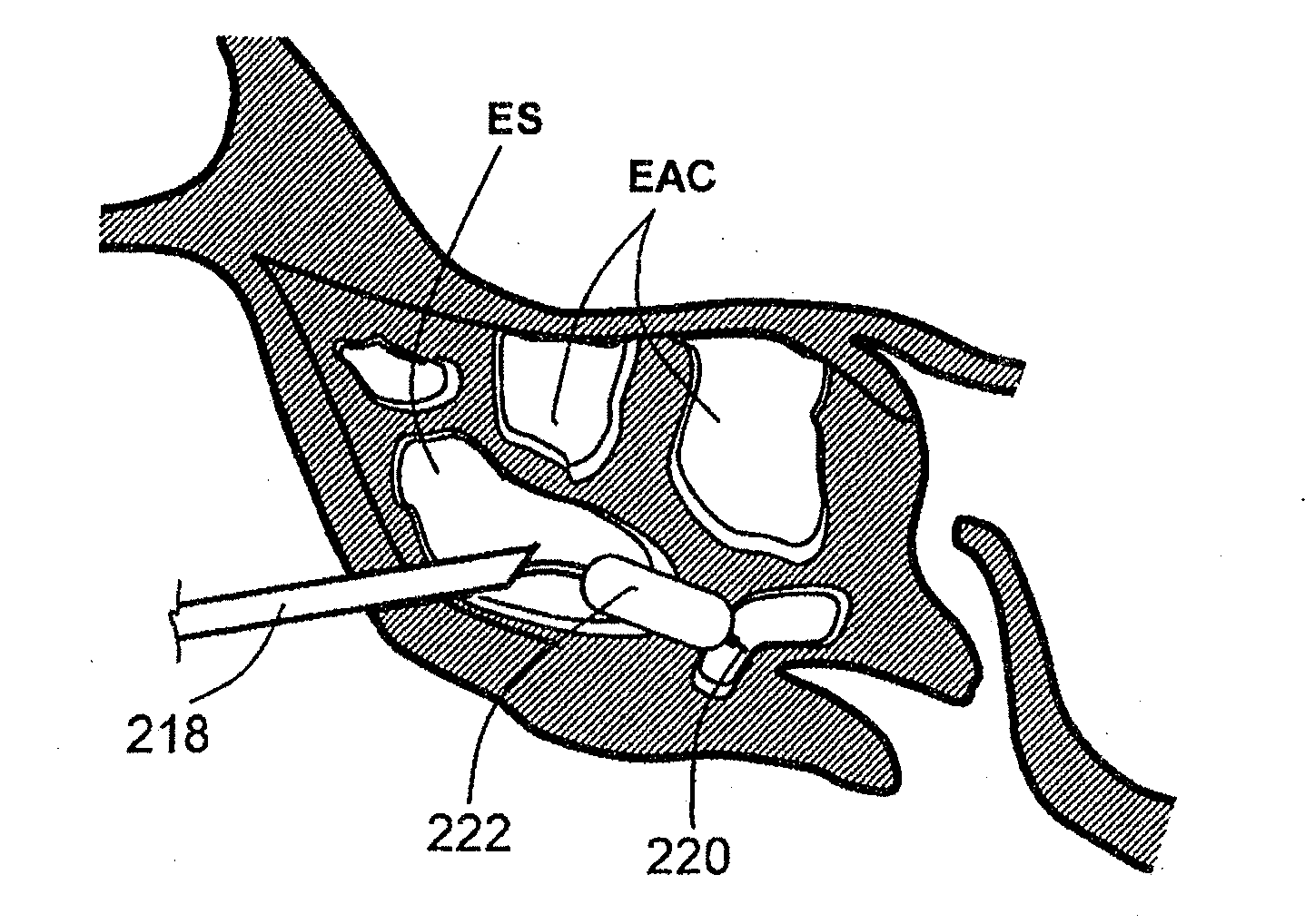
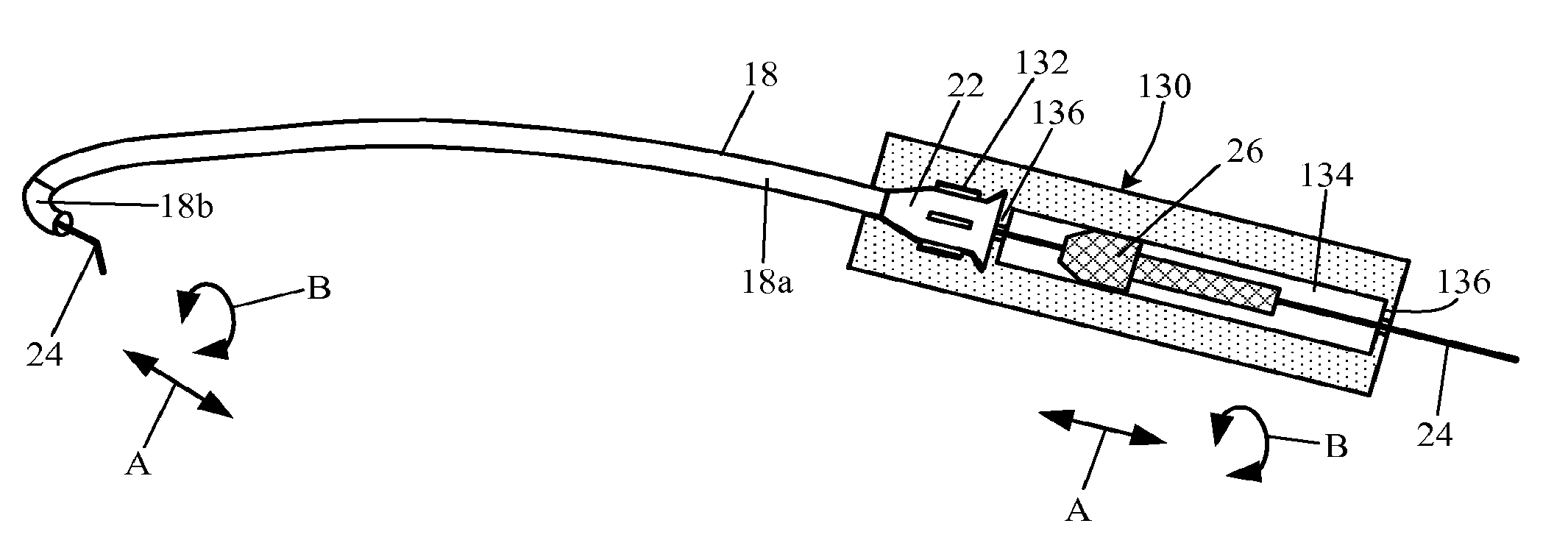
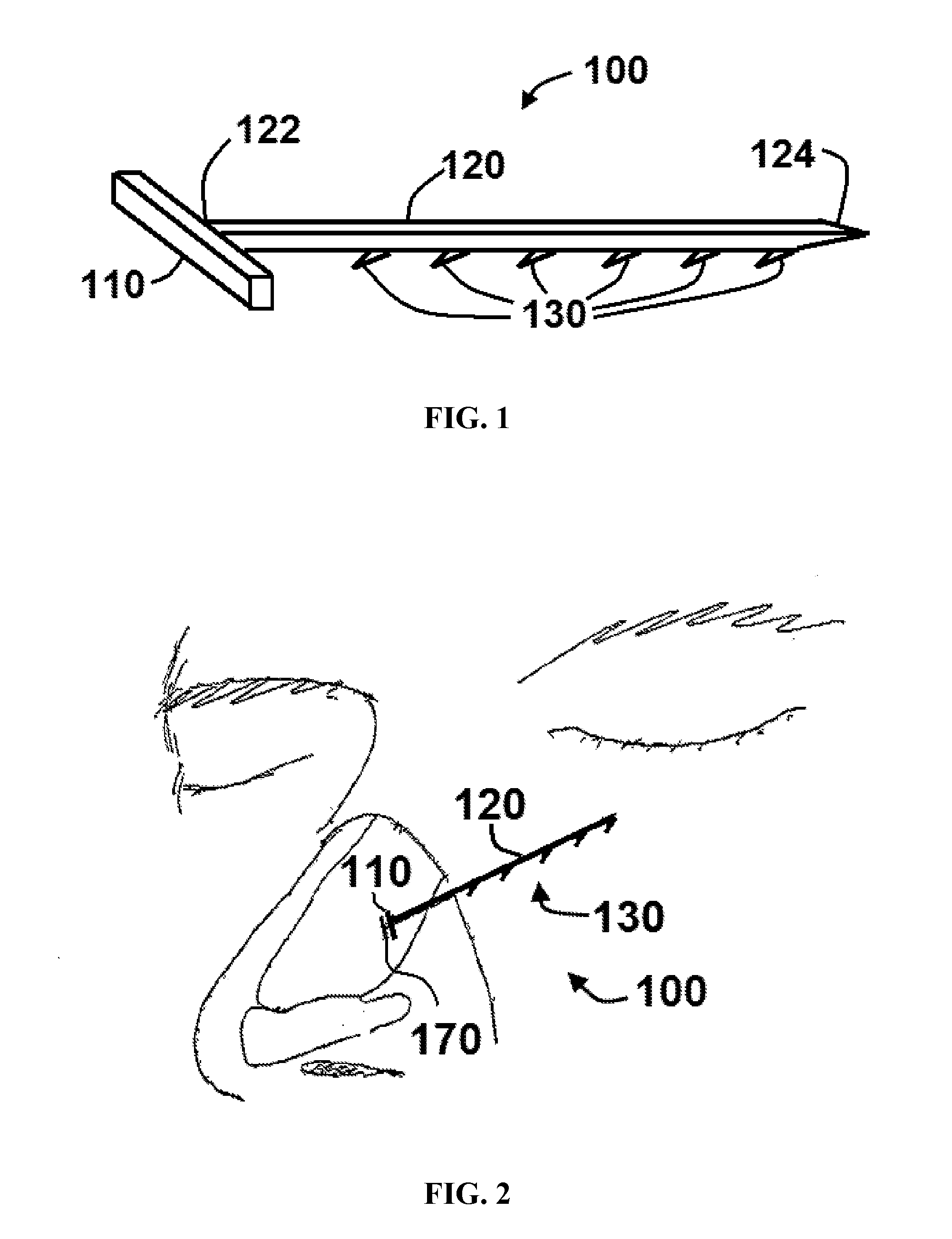
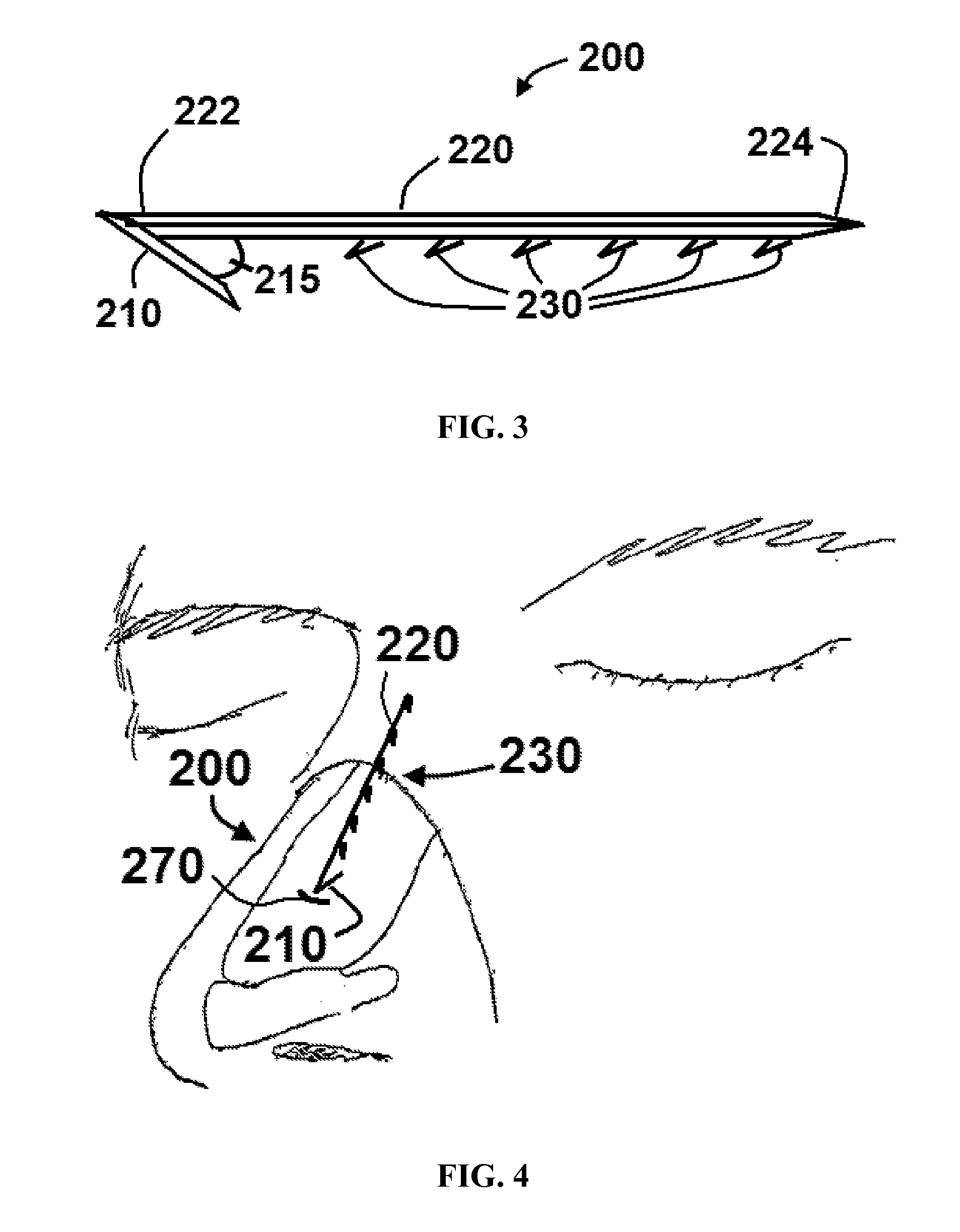
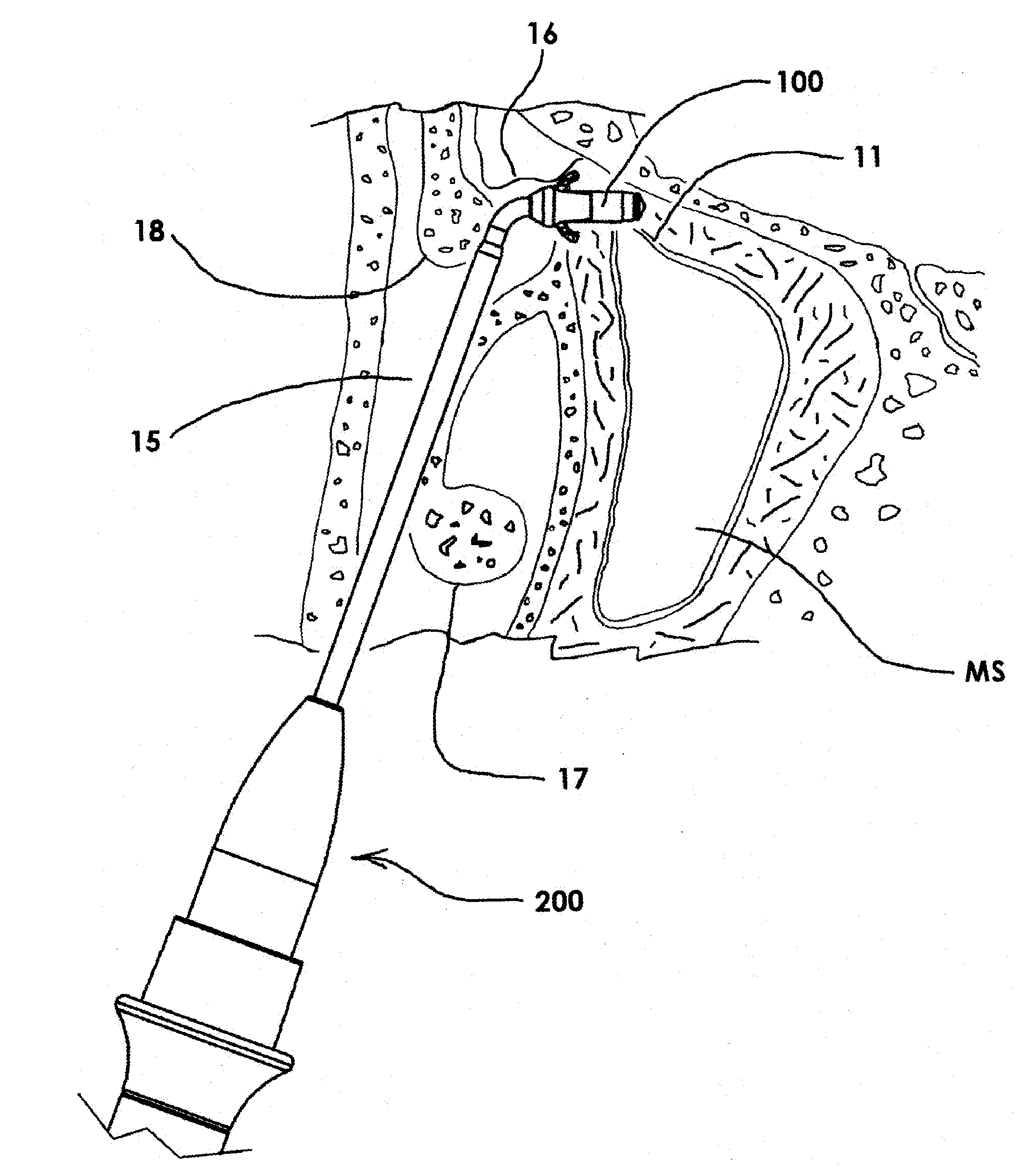
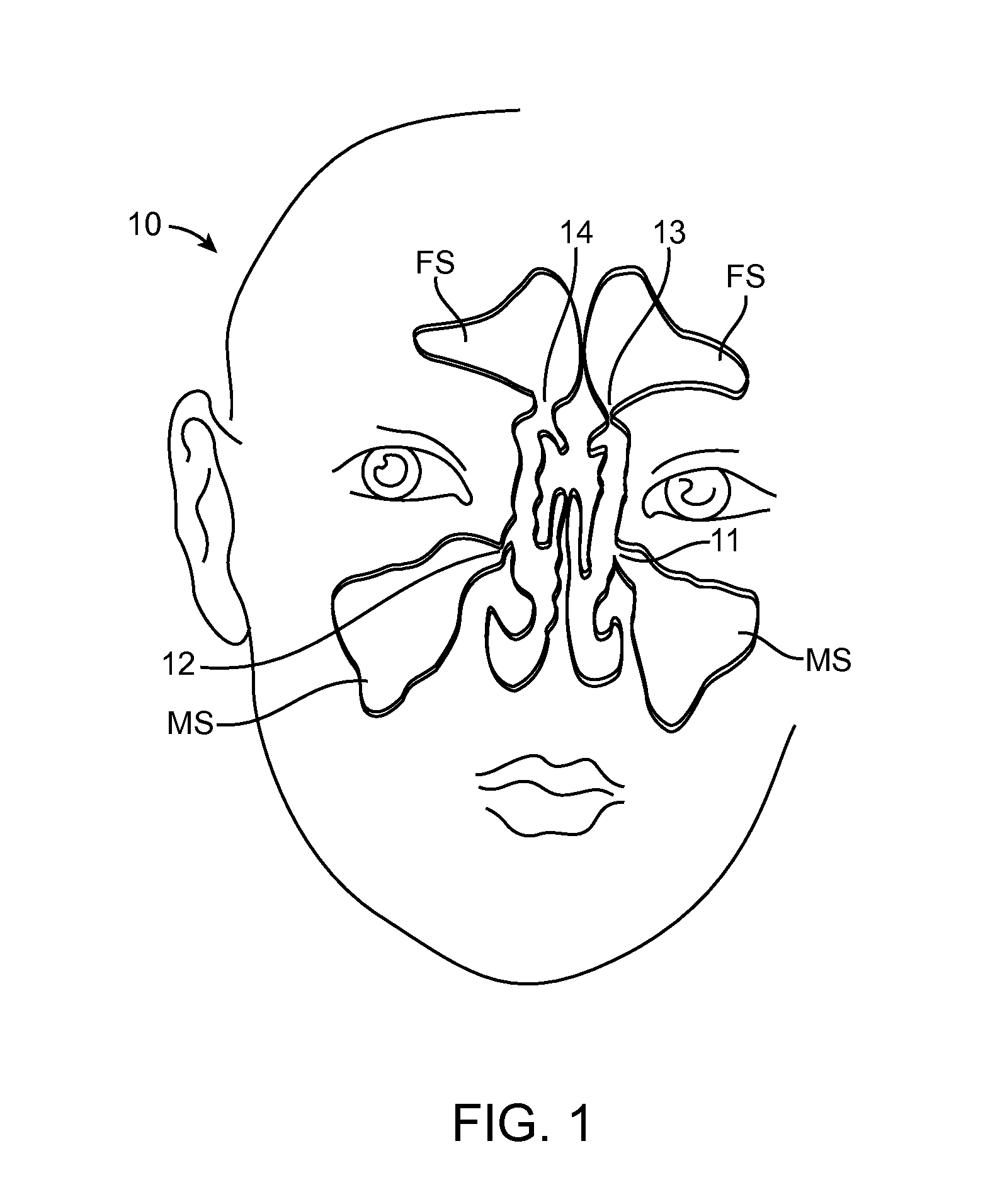
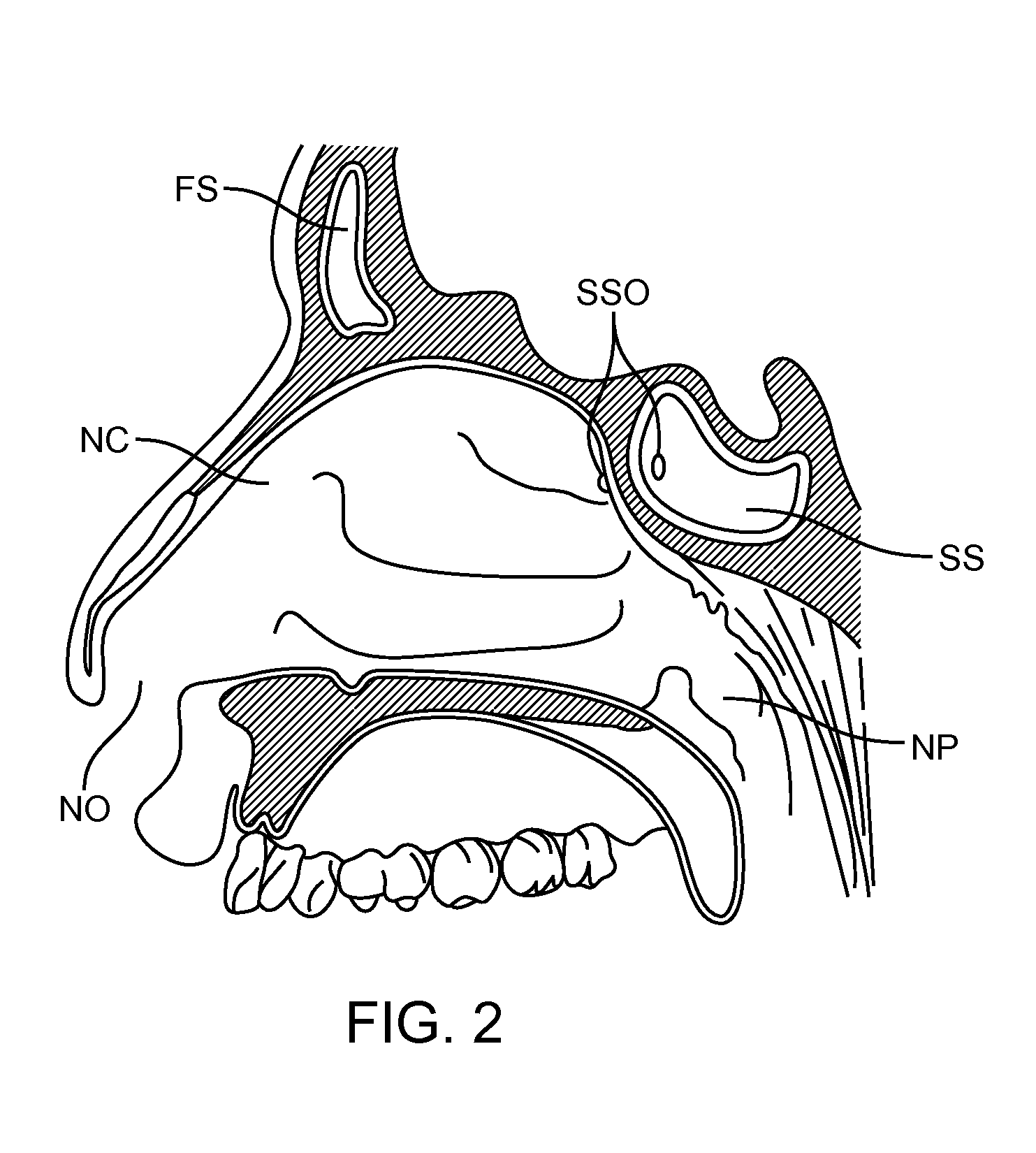
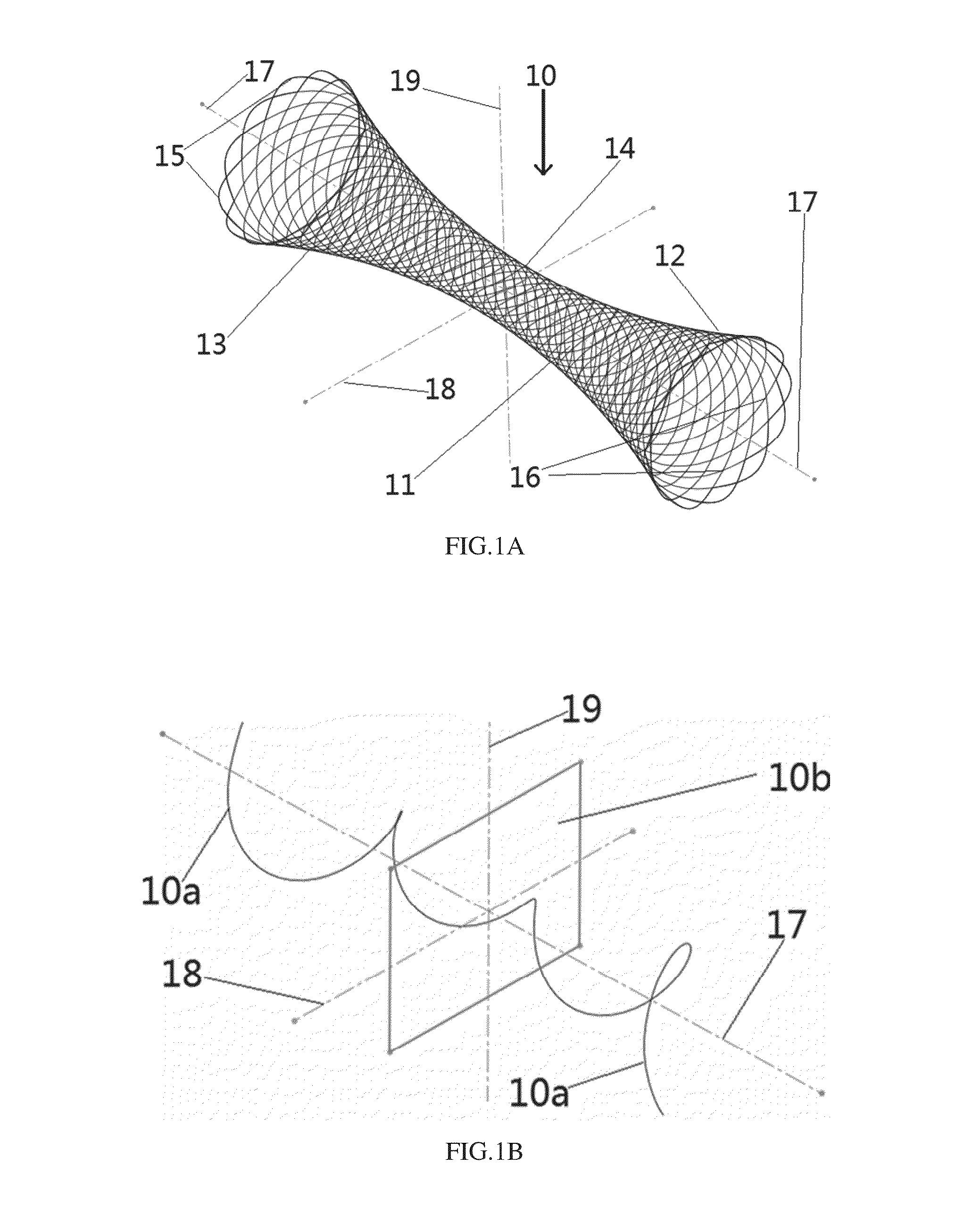
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
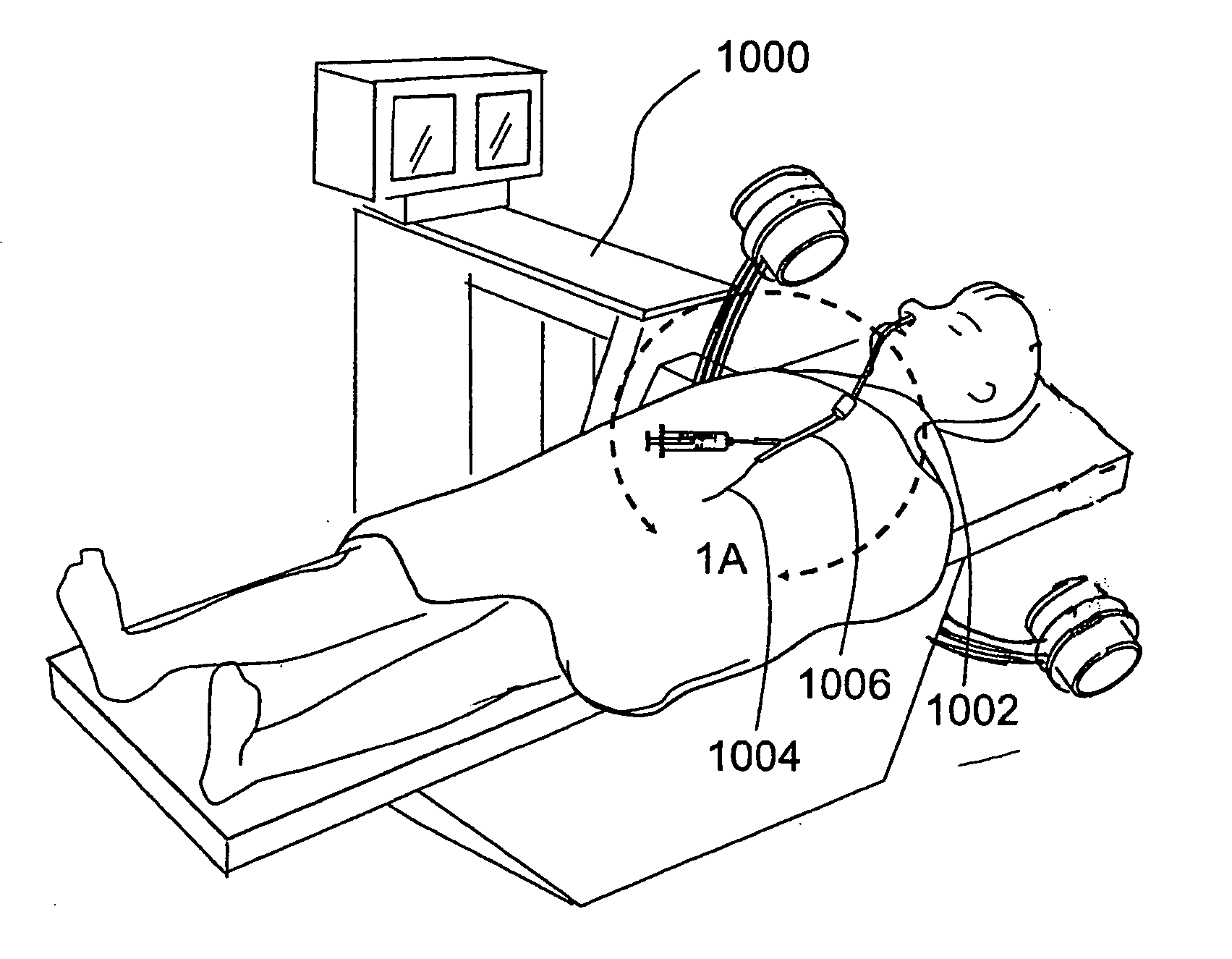
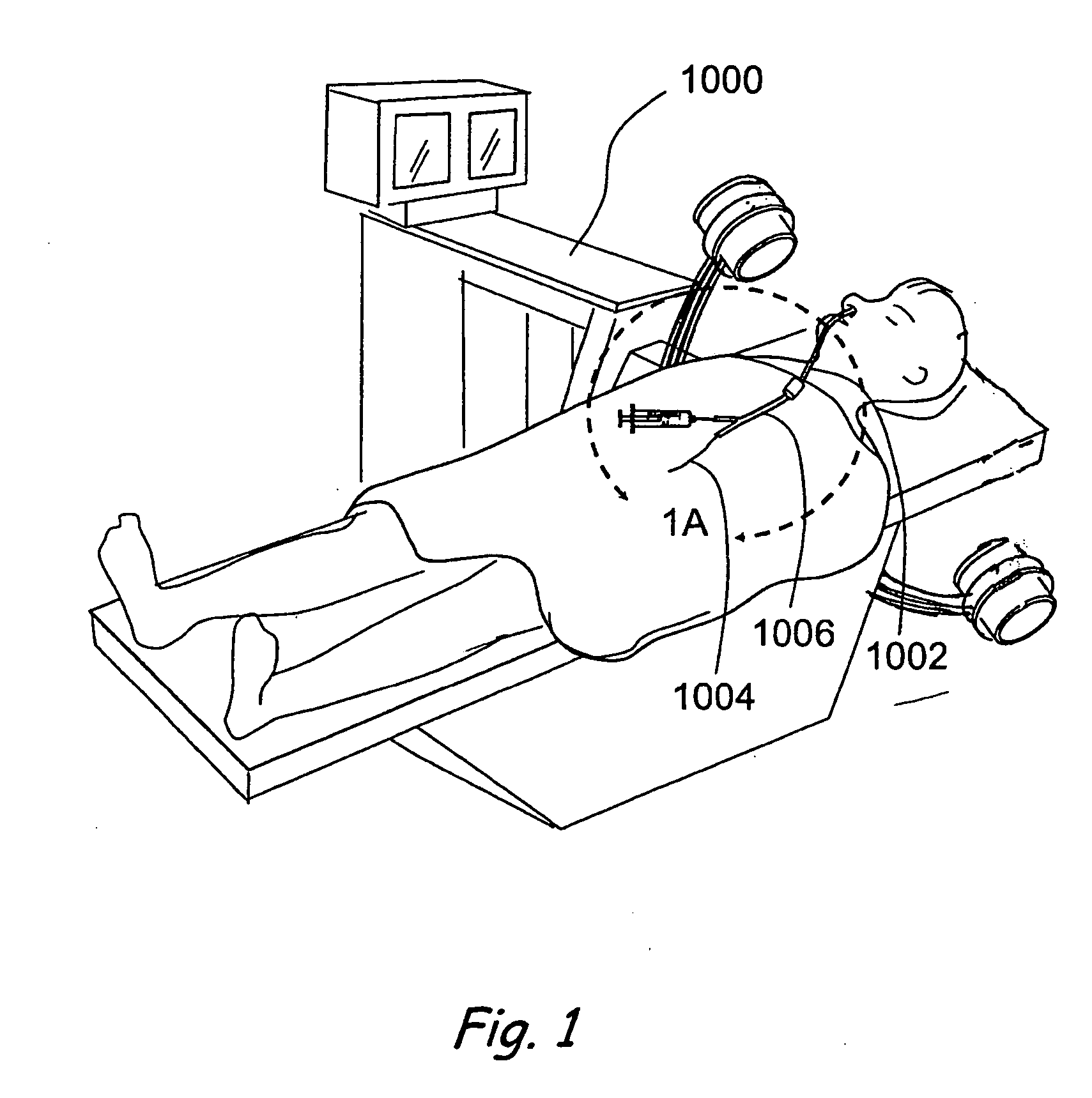
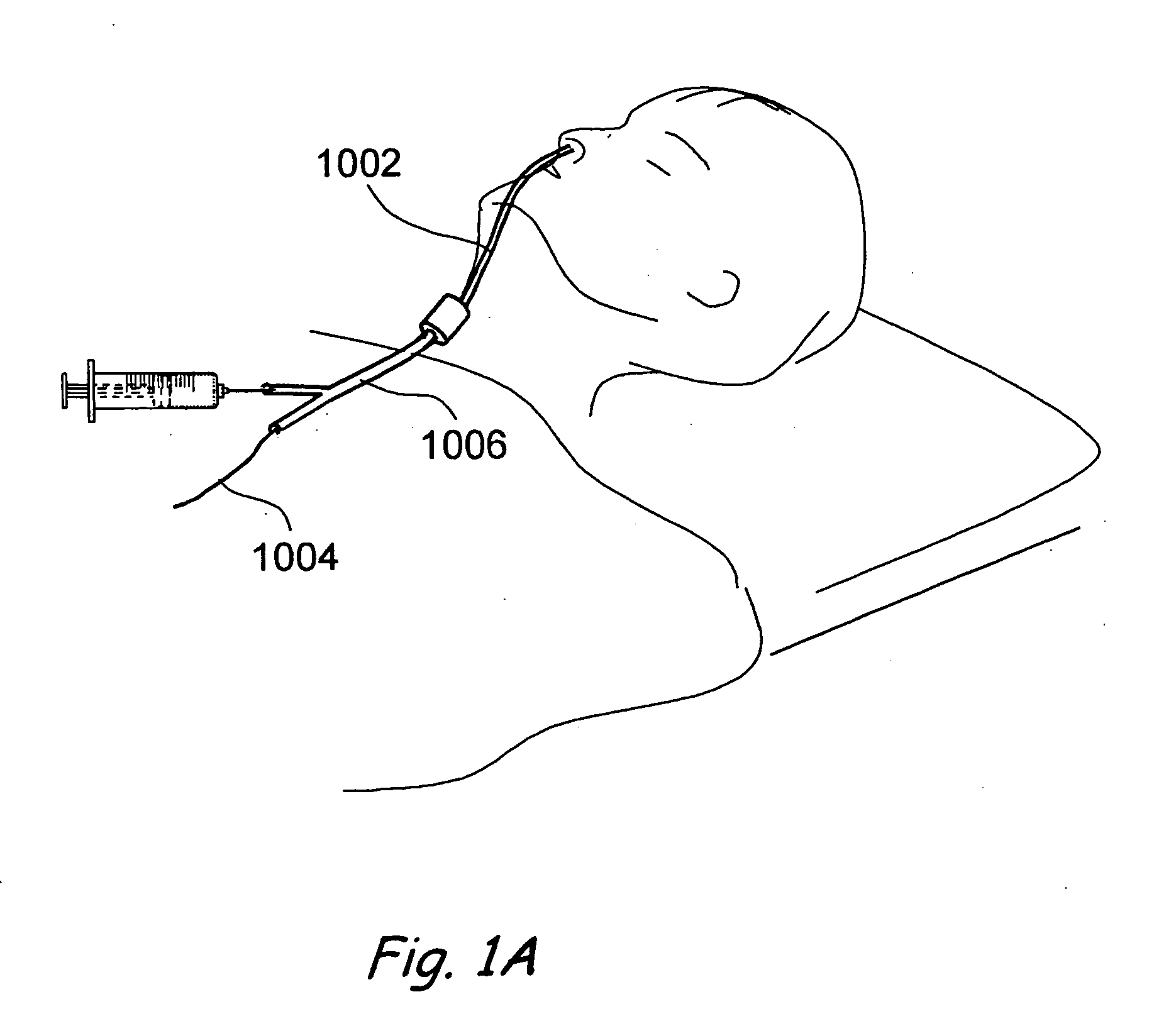
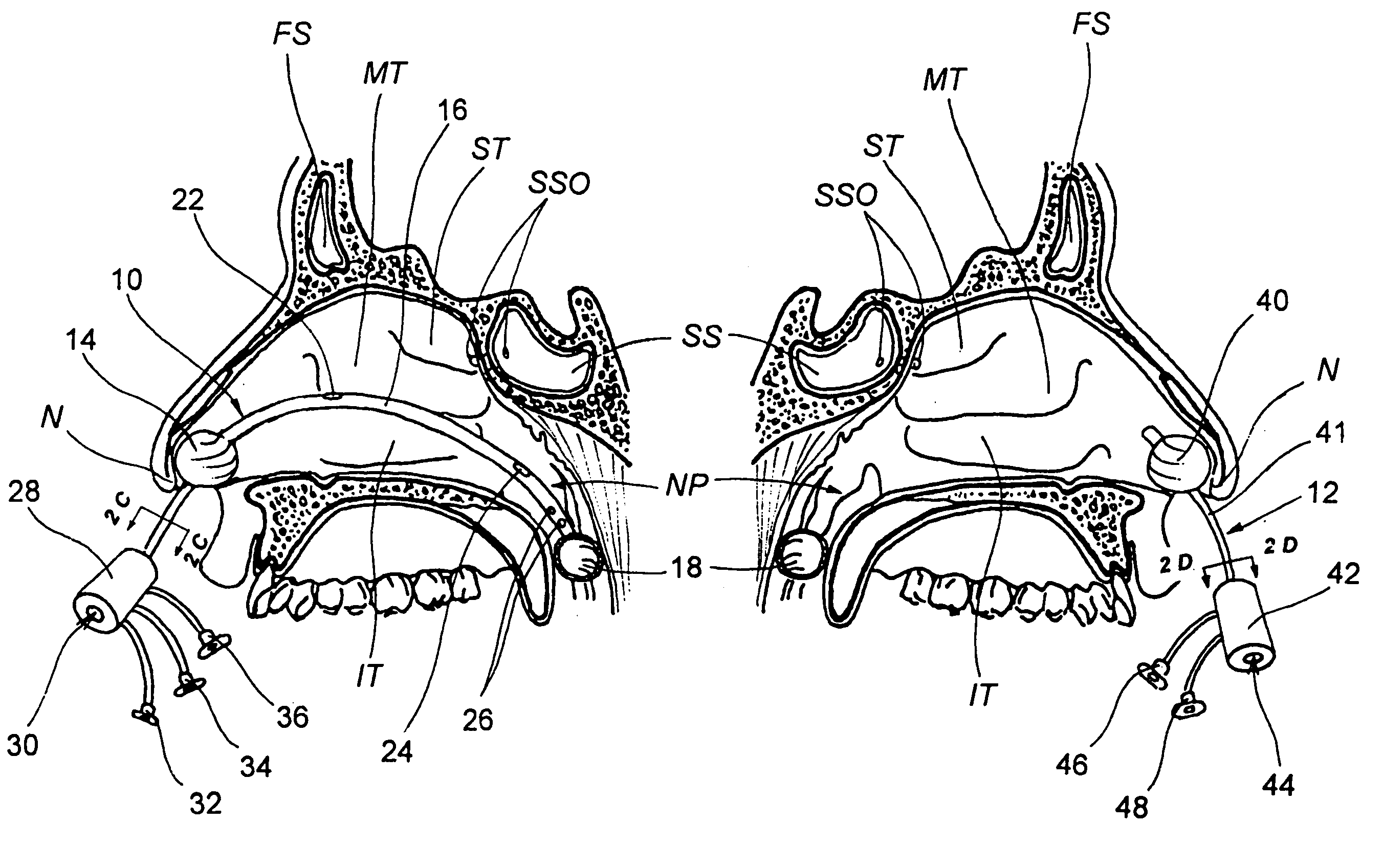
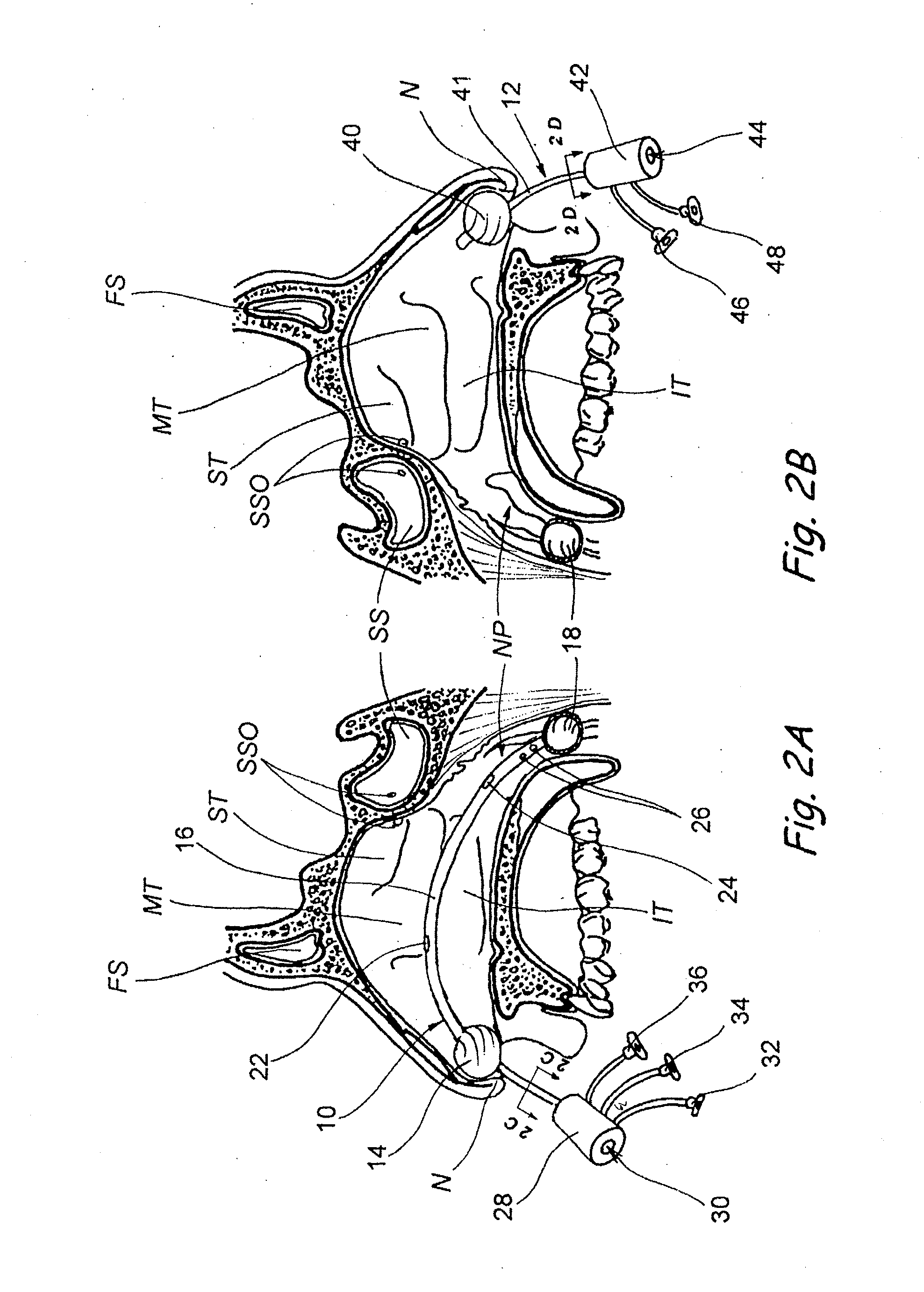
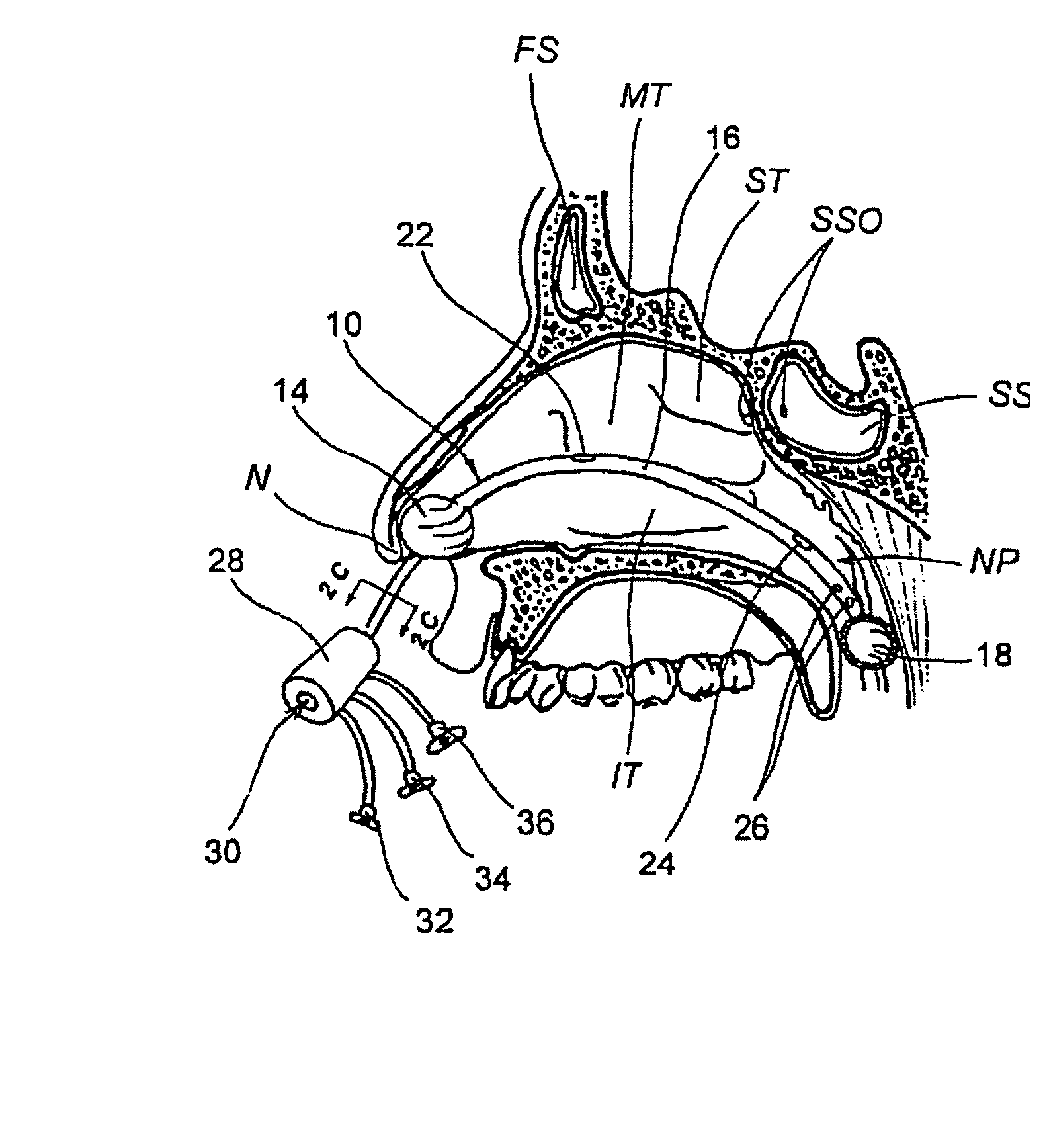
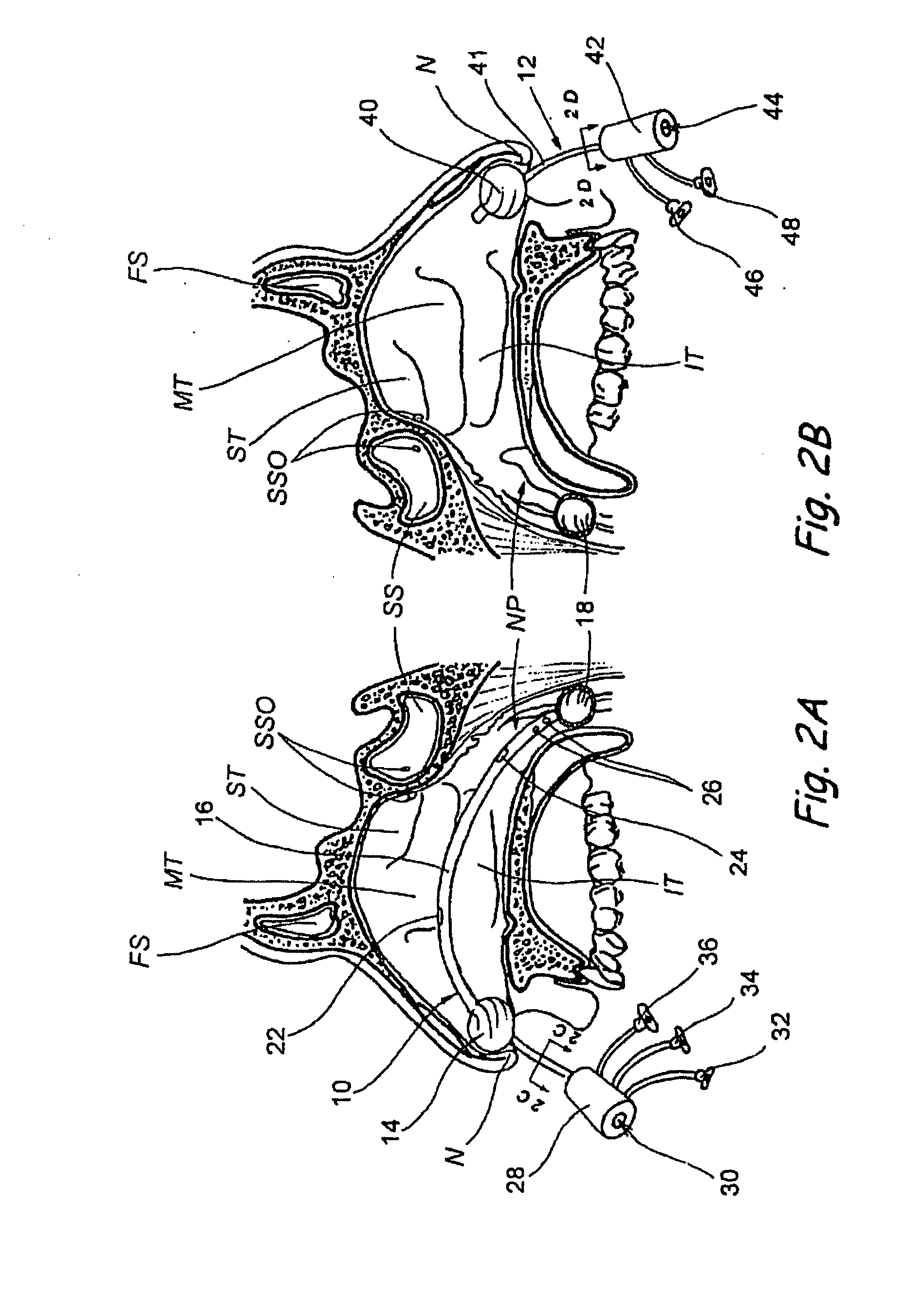
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
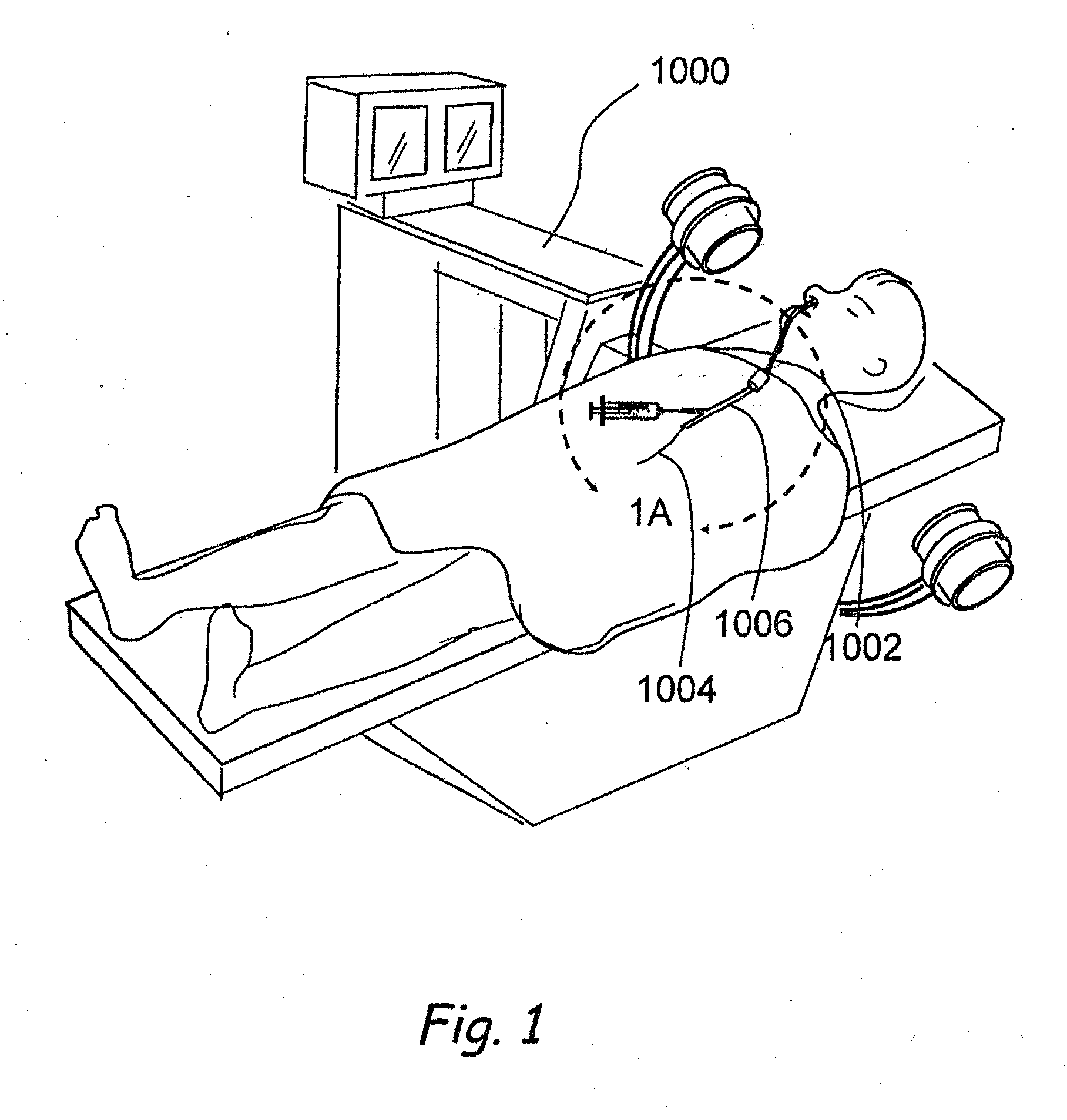
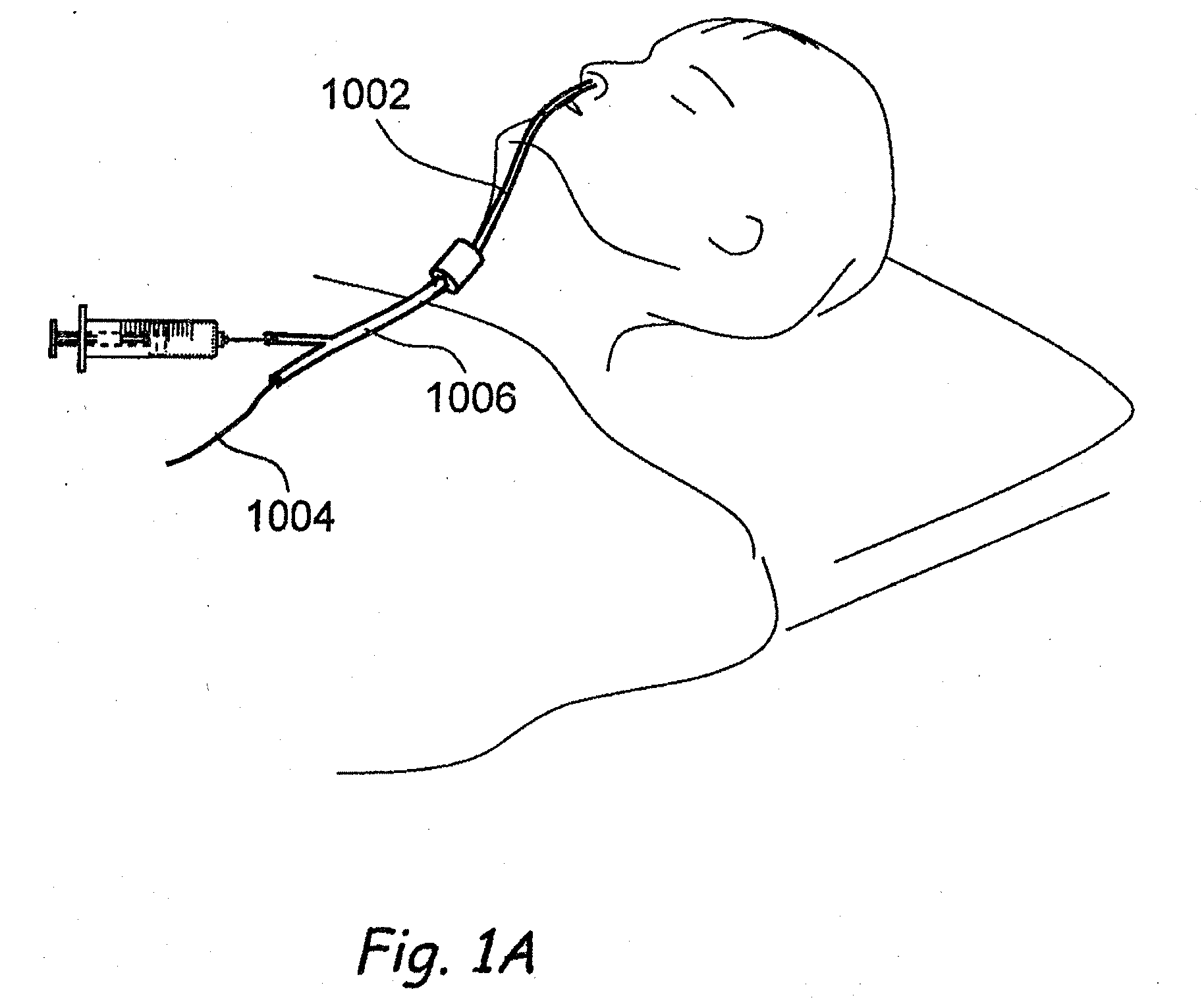
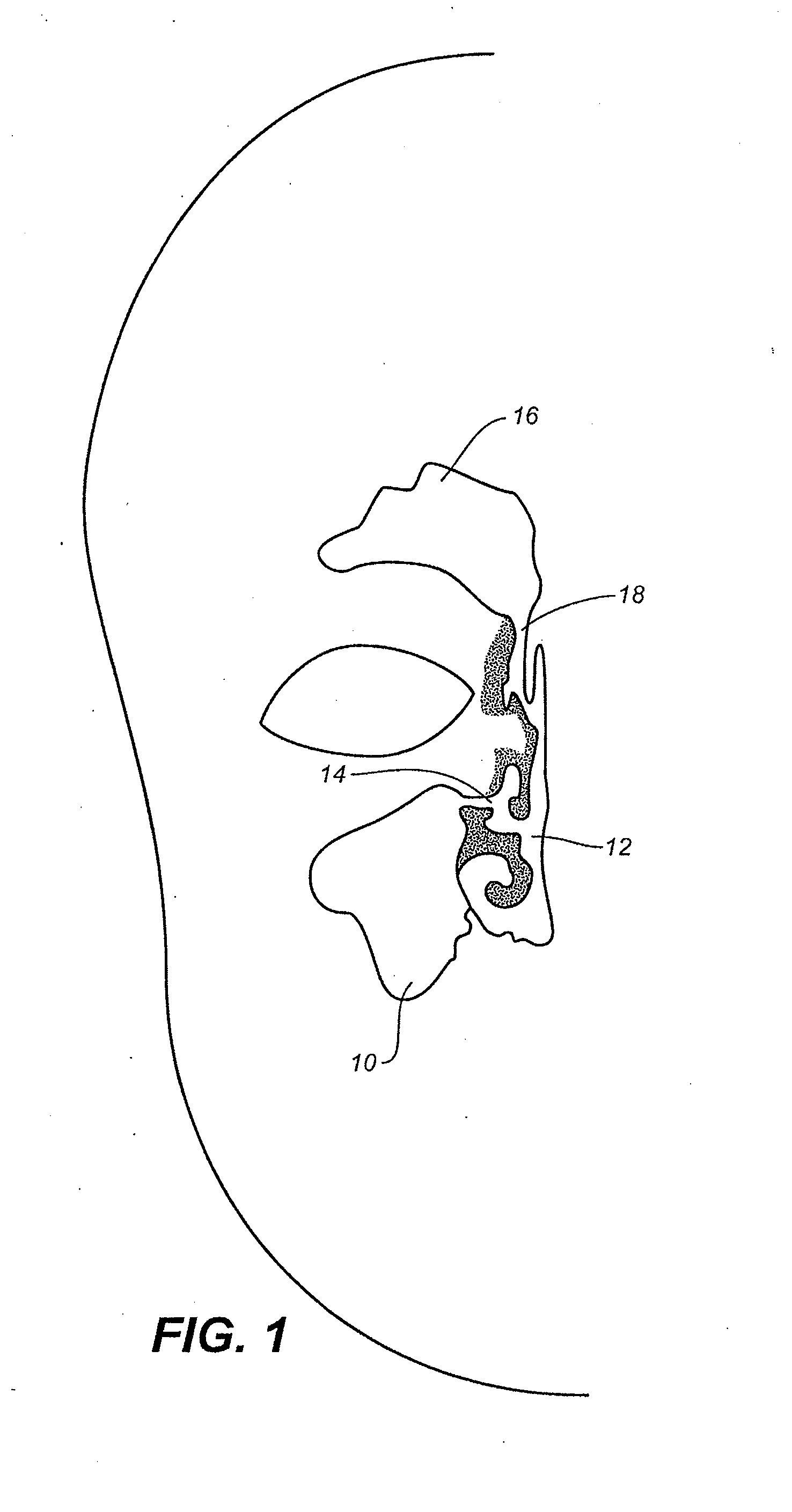
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Devices, Systems and Methods For Diagnosing and Treating Sinusitis and Other Disorders of the Ears, Nose and/or Throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Devices, systems and methods for diagnosing and treating sinusitis and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Sphenoid sinus stent
A stent specifically designed for use in treating chronic sphenoid sinusitis comprises a soft compressible plastic tube of a predetermined diameter having a proximal end, a distal end and a lumen extending therebetween. The stent has a generally hemispherical hollow dome integrally molded to its distal end. The diameter of the dome is greater than the predetermined diameter of the plastic tube. The stent further includes an integrally molded annular flange located a short predetermined distance proximal to the hemispherical dome. The device is designed to be fitted through a surgically enlarged ostium of the sphenoid sinus such that the dome resides within the sinus cavity and the flange abuts the bony wall surrounding the ostium. The stent maintains the ostium patent and permits irrigation / suctioning via the stent's lumen.
Owner:MICROMEDICS
Device and methods for treating paranasal sinus conditions
ActiveUS8025635B2Reduce needRecovery functionAntibacterial agentsOrganic active ingredientsActive agentNose
Described here are paranasal sinus devices for treating paranasal sinus conditions. The devices include a cavity member, ostial member, and nasal portion. One or more of the cavity member, ostial member, and nasal portion may deliver an active agent for sustained release to treat the paranasal sinus condition. Exemplary paranasal sinus conditions are sinus inflammation due to functional endoscopic sinus surgery (FESS) and rhinosinusitis.
Owner:INTERSECT ENT INC
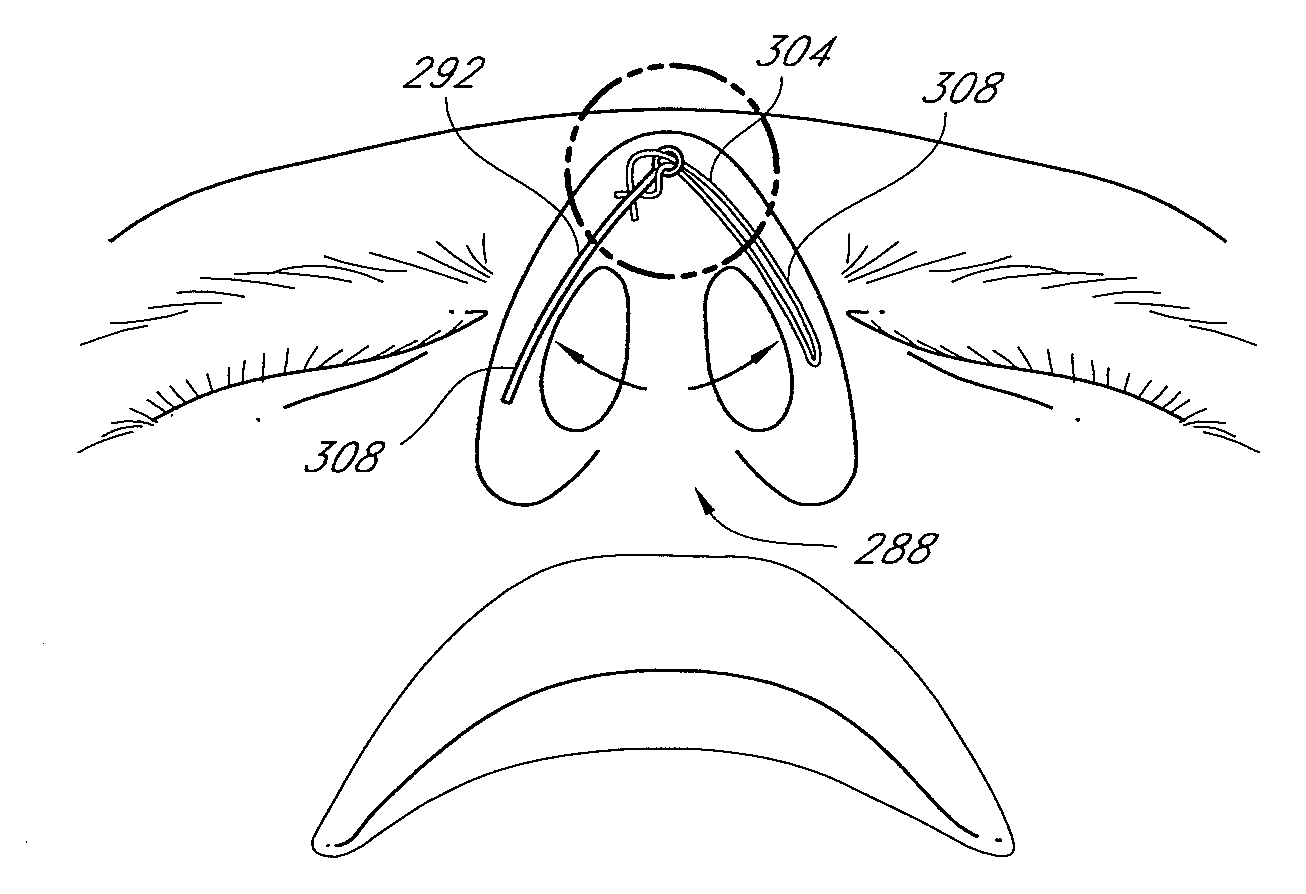
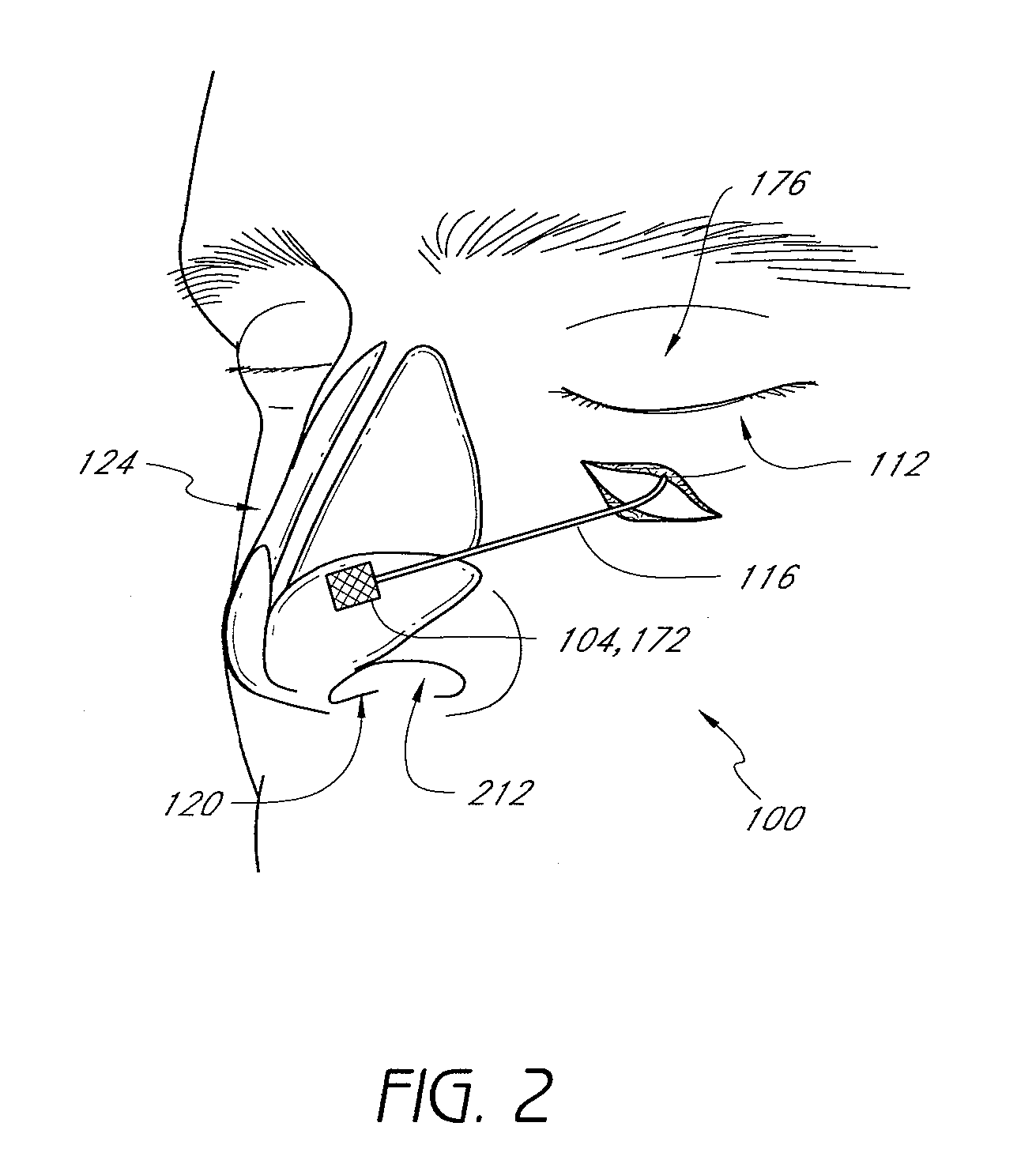
Methods and devices for rhinoplasty and treating internal valve stenosis
ActiveUS20080027480A1Increase the cross-sectional areaIncrease exposureSuture equipmentsNose implantsNasal cavityMitral valve stenosis
Methods and devices for rhinoplasty and treating nasal valve stenosis are disclosed herein. The nasal valve acts as a flow-limiter and can contribute to airway obstruction if resistance within the nasal valve is excessive. In one embodiment, a system for treating nasal valve stenosis includes a first elongate implant and a second elongate implant configured to support the nasal valves when implanted. The implants can be coupled together by connecting elements, such as eyelets, tethers, complementary socket joints, and button-rivet supports.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
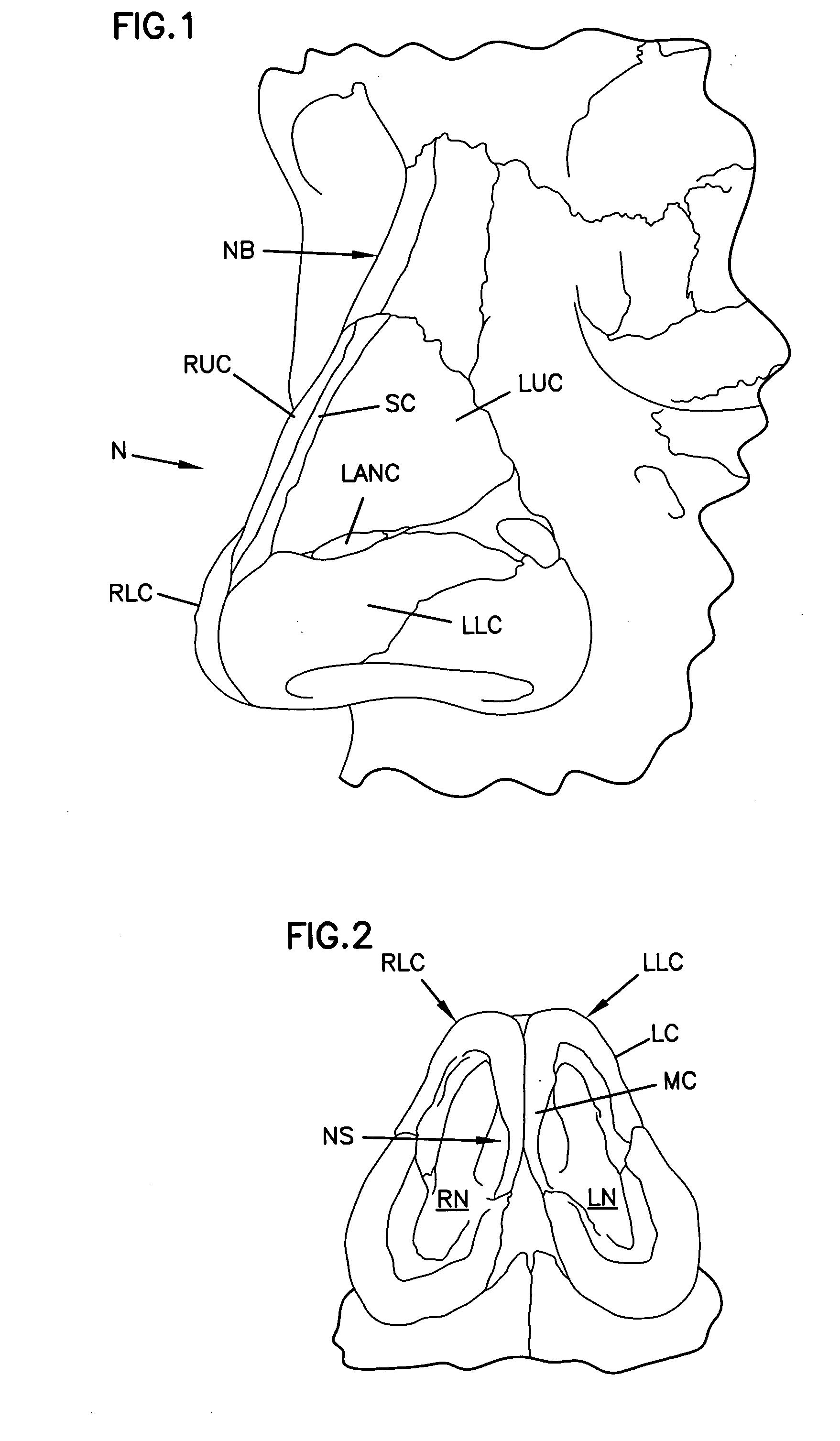
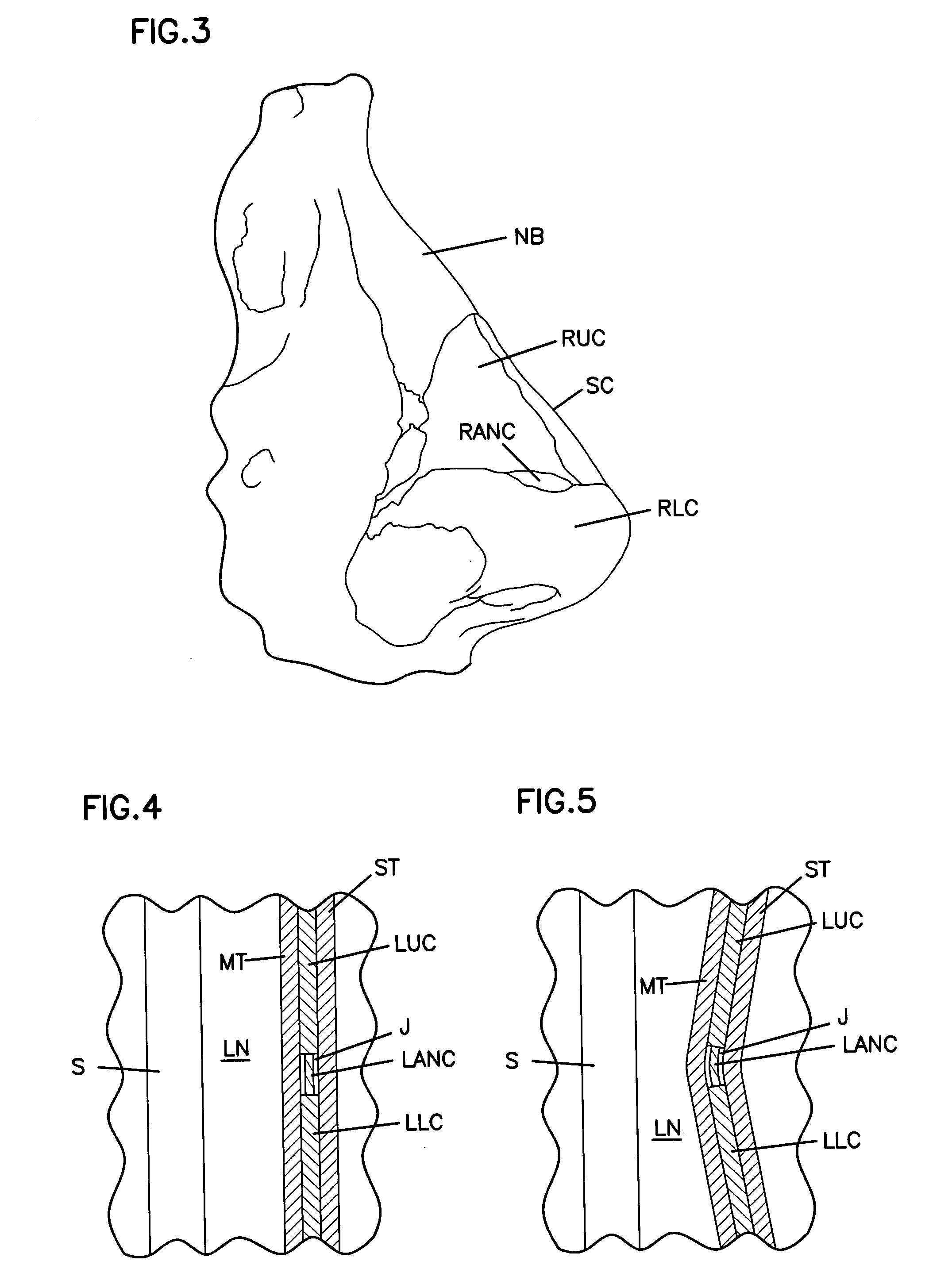
Apparatus and Methods for Dilating and Modifying Ostia of Paranasal Sinuses and Other Intranasal or Paranasal Structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
Apparatus and Methods for Dilating and Modifying Ostia of Paranasal Sinuses and Other Intranasal or Paranasal Structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
Nasal valve treatment method & apparatus
ActiveUS20060276817A1Maintaining material strengthThin thicknessIncision instrumentsNose implantsSurgical operationMedicine
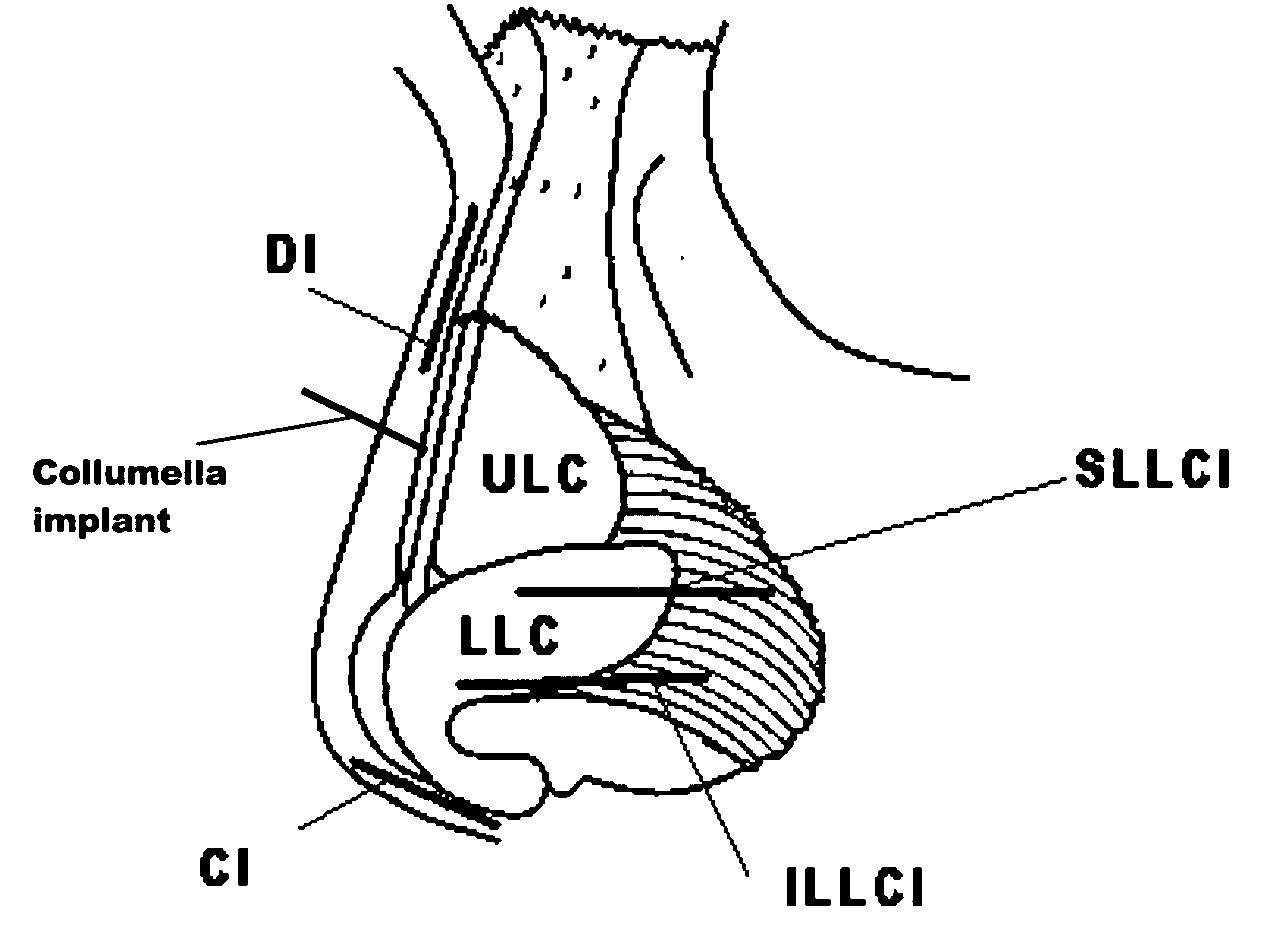
A method and apparatus for treating a nasal valve condition include surgically forming an access path to create a pocket on a side of the patient's nose. The pocket is positioned between a soft tissue layer and opposing surfaces of upper and lower cartilages of the nose. The pocket spans a junction between the upper and lower cartilages. An implant is selected having a length, width and thickness sized to reside within the pocket. The length is sized to span the junction. The width is sized to be less than the width of the upper and lower cartilages. The thickness of the implant is as thin as possible while maintaining material strength to resist bending in response to inhalation pressures at the nasal valve. The implant is placed through the access path into the pocket with the length oriented spanning the junction. A delivery system for placement of the implant includes a surgical tool for forming the access path and for delivering the implant into the access path.
Owner:MEDTRONIC XOMED INC
Device and methods for treating paranasal sinus conditions
ActiveUS20090227945A1Reduce needRecovery functionAntibacterial agentsOrganic active ingredientsDiseaseActive agent
Described here are paranasal sinus devices for treating paranasal sinus conditions. The devices include a cavity member, ostial member, and nasal portion. One or more of the cavity member, ostial member, and nasal portion may deliver an active agent for sustained release to treat the paranasal sinus condition. Exemplary paranasal sinus conditions are sinus inflammation due to functional endoscopic sinus surgery (FESS) and rhinosinusitis.
Owner:INTERSECT ENT INC
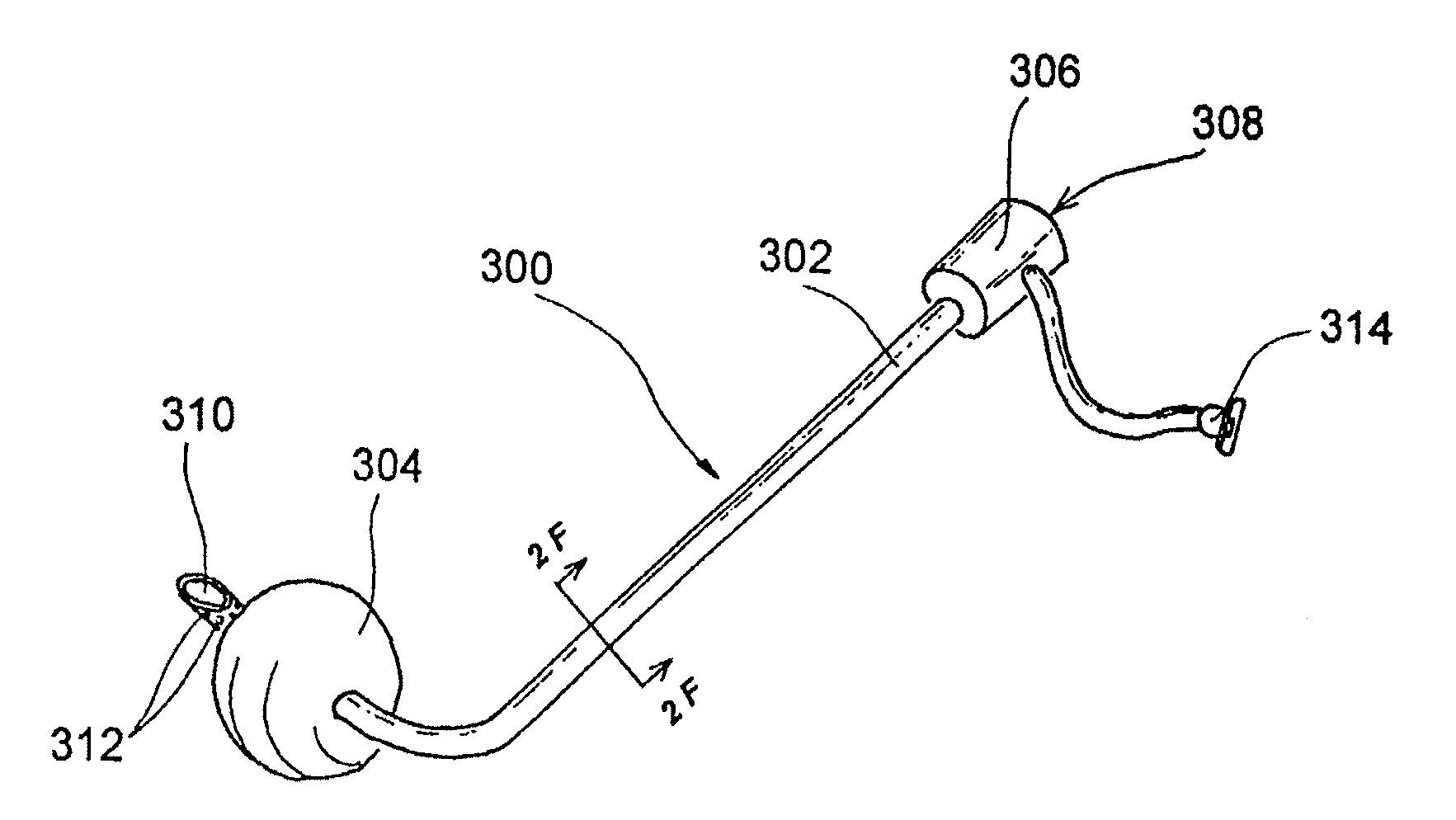
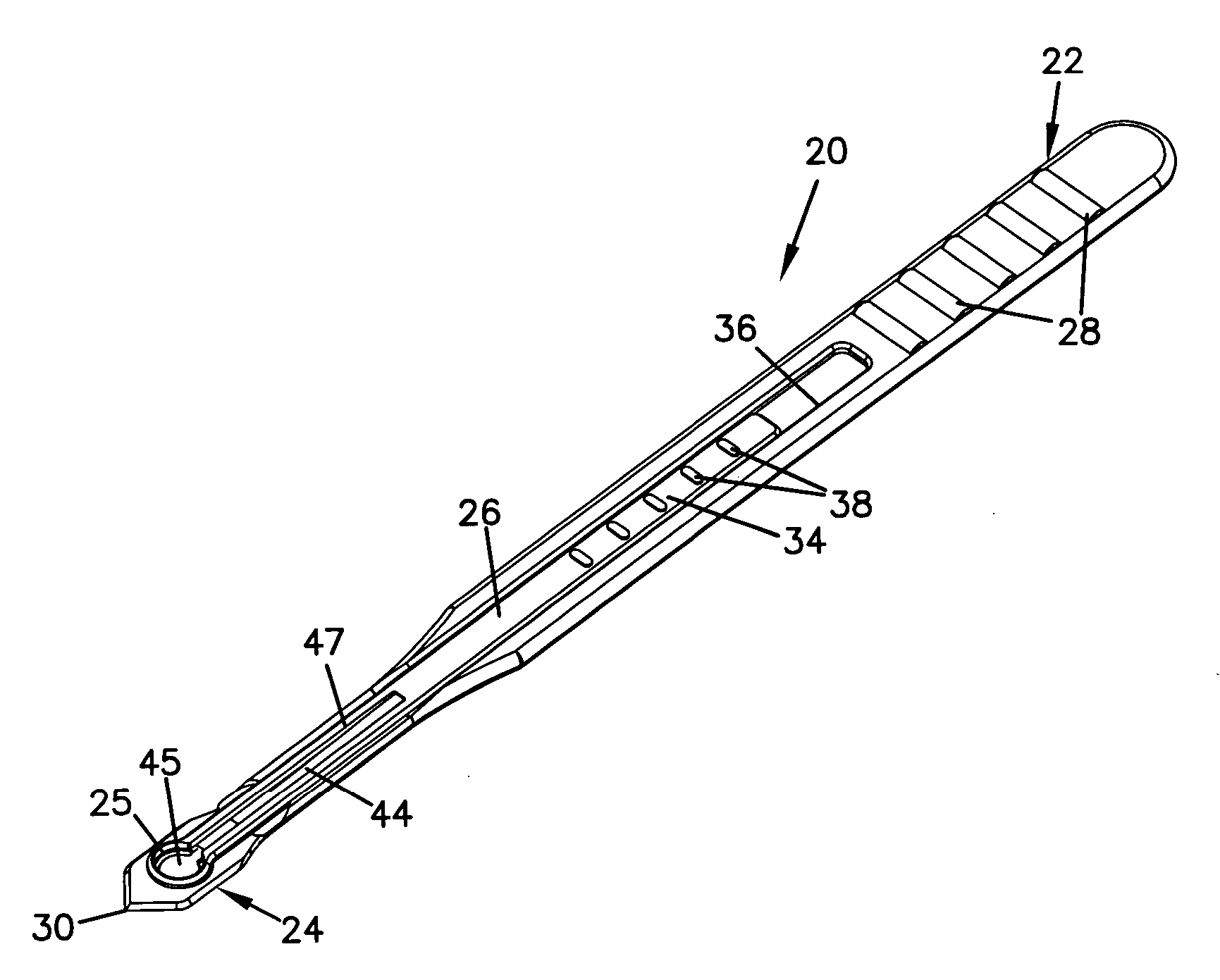
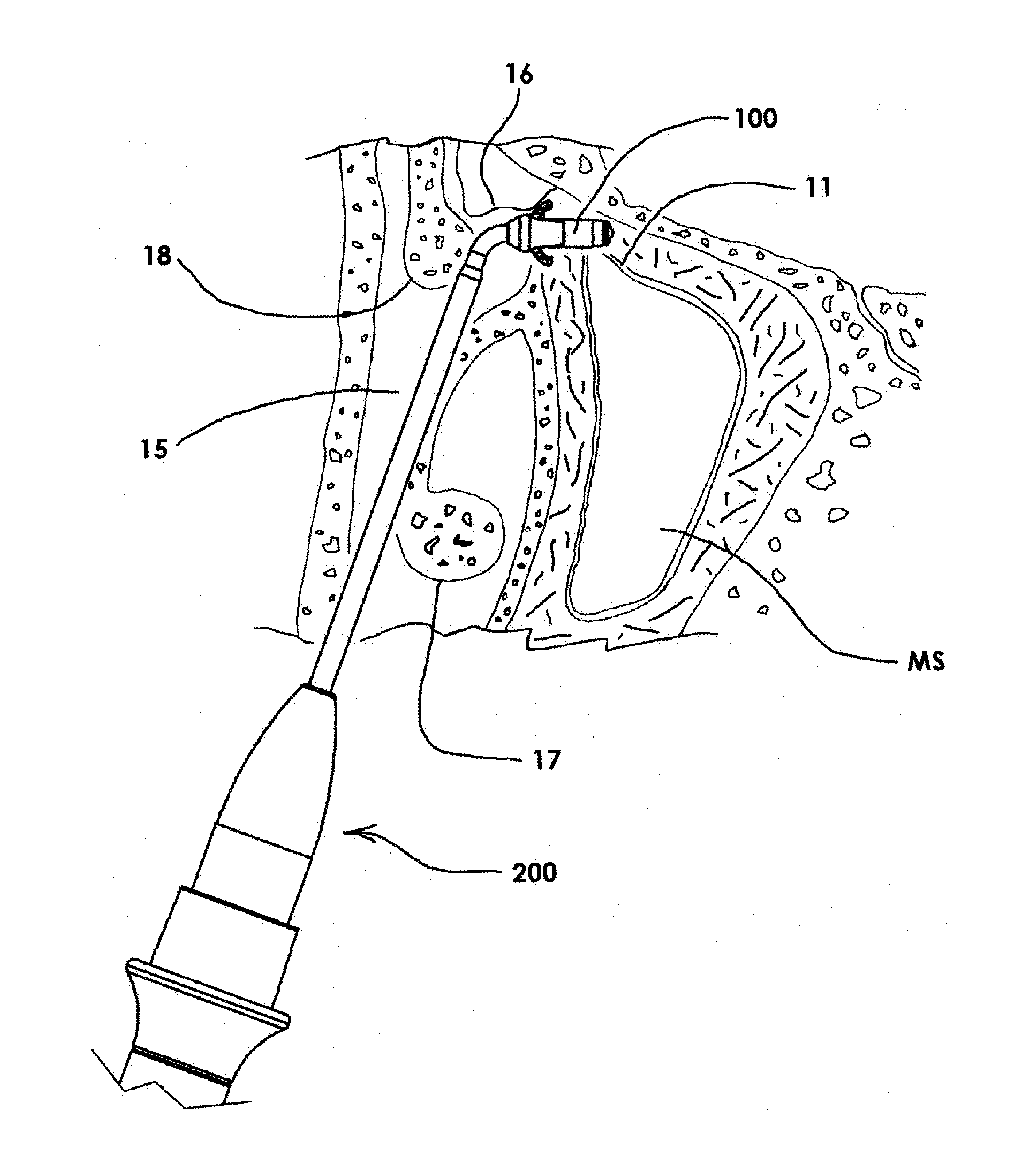
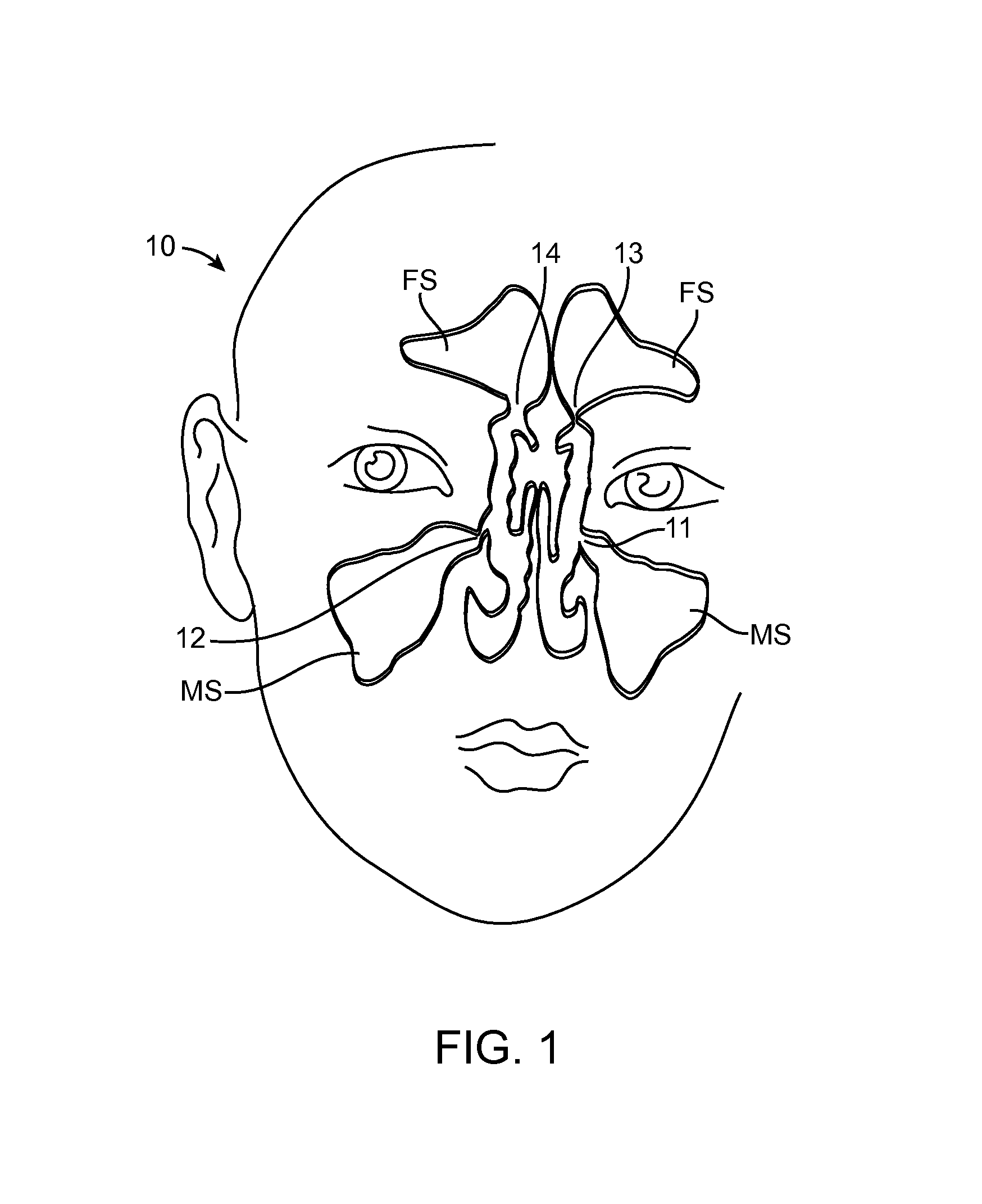
Guide catheter and method of use
Owner:ENTELLUS MEDICAL
Guide catheter and method of use
A system for manipulating a guide catheter within a patient's nasal passages or sinus cavities includes a guide catheter formed from an elongate flexible member having a lumen passing there through. A wire guide is slidably disposed within the lumen of the guide catheter. The system further includes a steering member fixedly secured to a proximal end of the wire guide and a proximal hub secured to a proximal end of the guide catheter. The system further includes a recessed handle having a first recess for fixedly receiving the proximal hub of the guide catheter and a second recess for receiving the steering member, the second recess being dimensioned to permit axial and rotational movement of the steering member while disposed in the second recess.
Owner:ENTELLUS MEDICAL
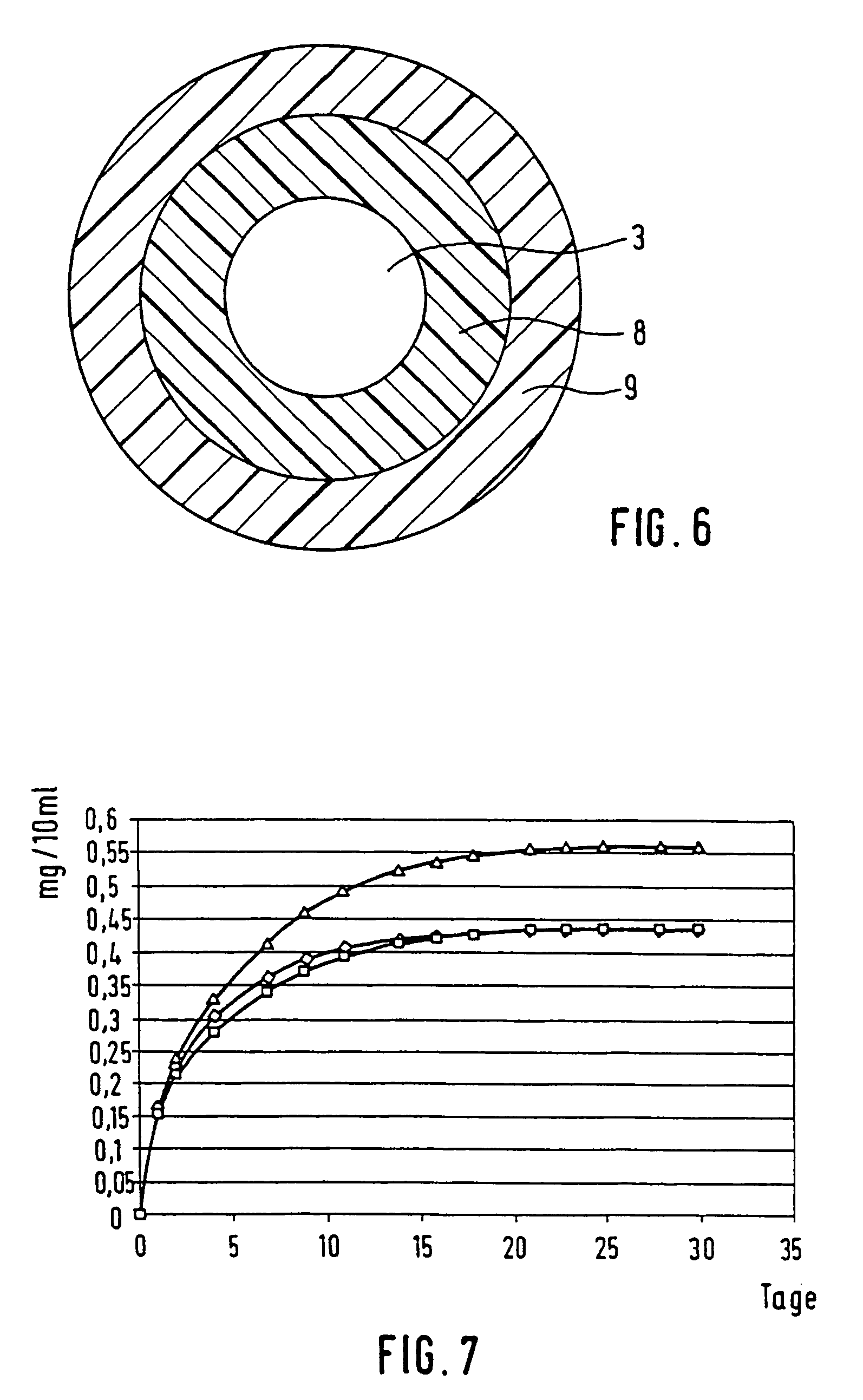
Spacing device for releasing active substances in the paranasal sinus
A spacing device for use in fenestrations of the paranasal sinus, the device including a sheath which forms a hollow body defining at least two apertures. The sheath includes at least one layer loaded with an active substance. The ratio q of the external diameter ra of the hollow body to the internal diameter ri of the hollow body is about 1.2 to 3.0.
Owner:ACCLARENT INC
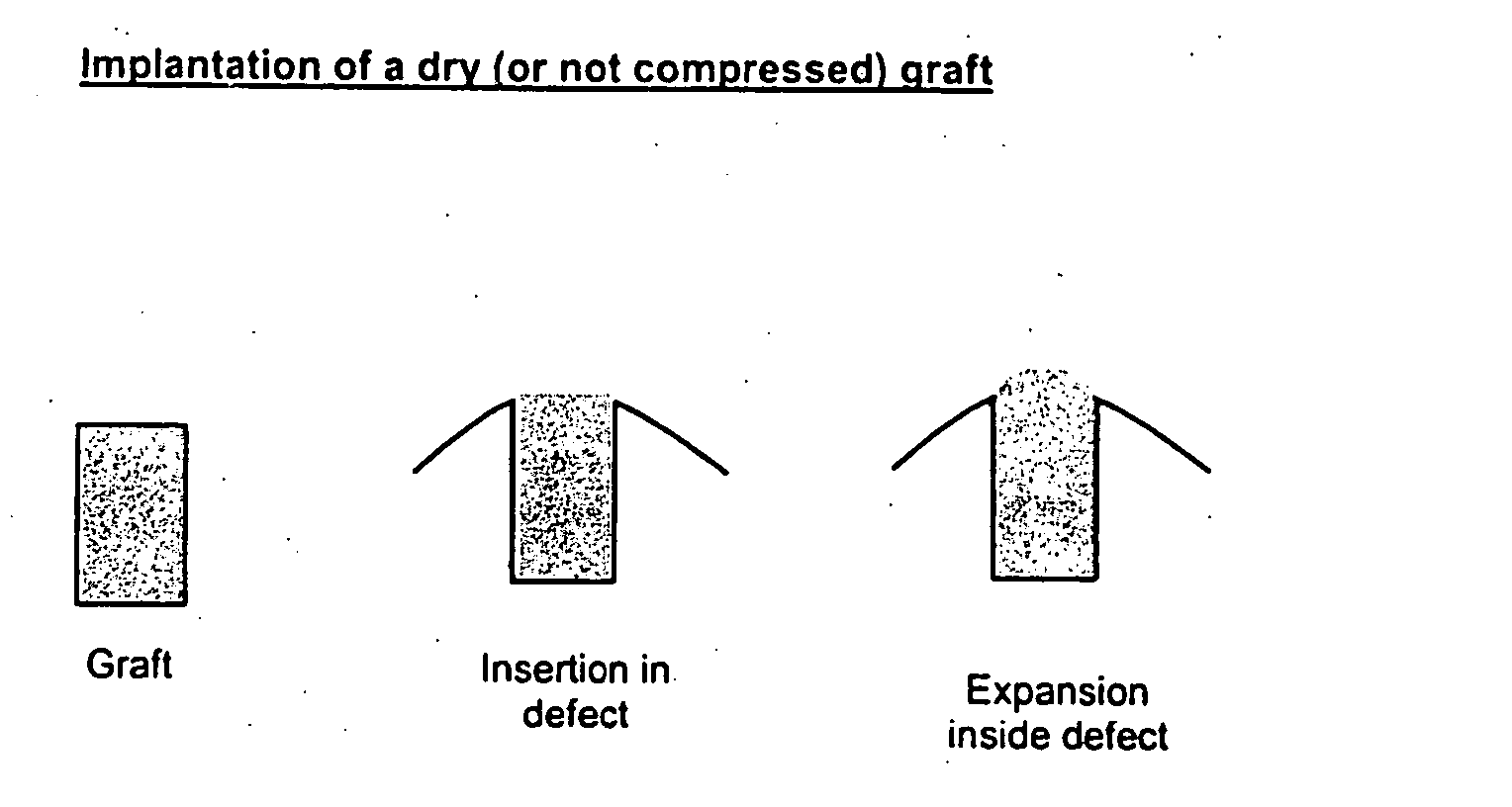
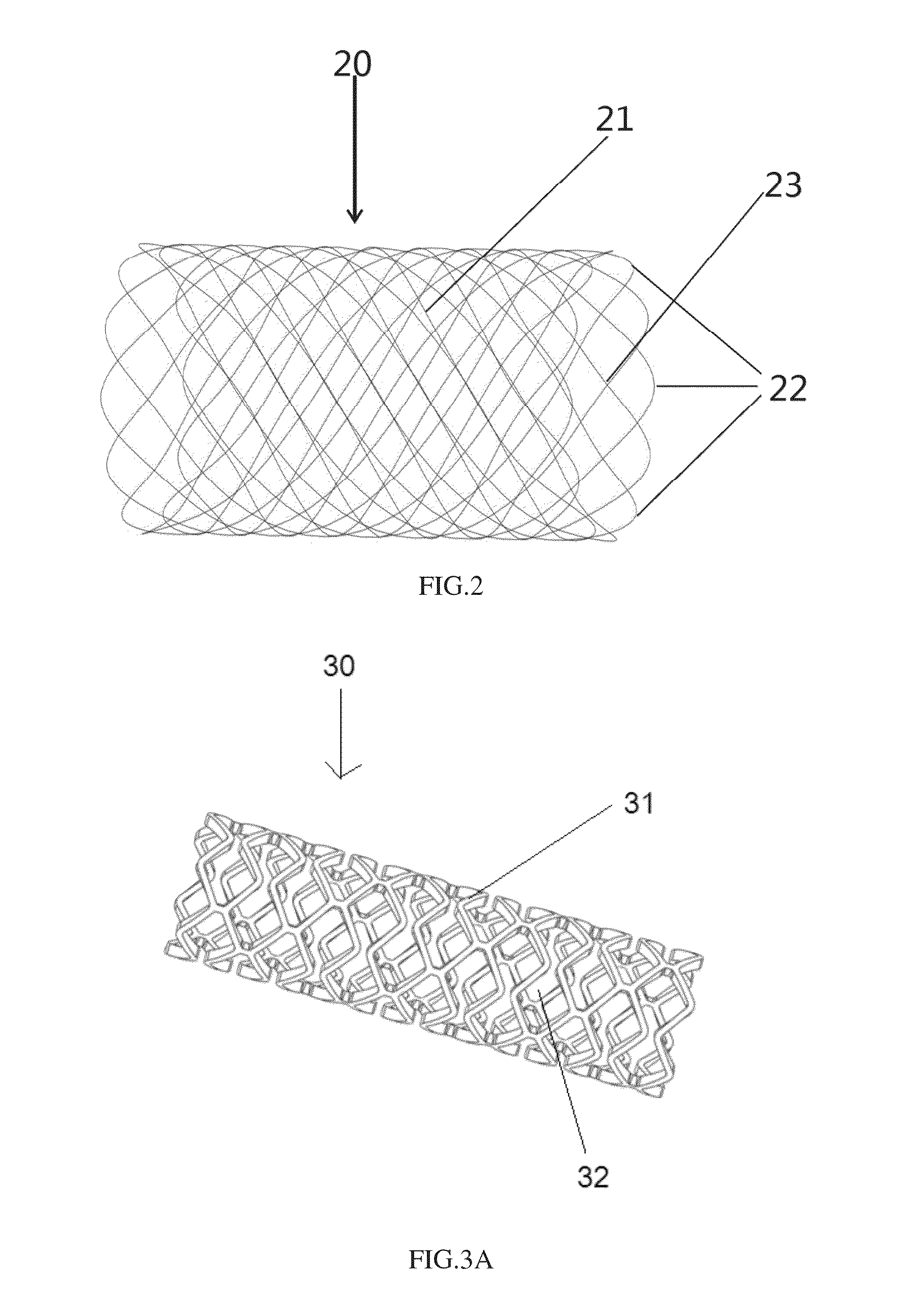
Device for splinting a cavity, organ duct and/or vessel
ActiveUS20130090720A1Easy to disassembleEasy to insertMechanical cleaningPressure cleaningSurgeryAnimal body
The invention relates to a device for splinting and / or maintaining a cavity, an organ duct and or a vessel in a human or animal body, said device including at least one compressible and self-expanding stent that is composed of at least three phases.
Owner:DURING KLAUS
Nasal implant introduced through a non-surgical injection technique
ActiveUS20090292358A1Increase in internal nasal valve angleImprove structural strengthNose implantsDiagnosticsNoseEngineering
Owner:SPIROX INC
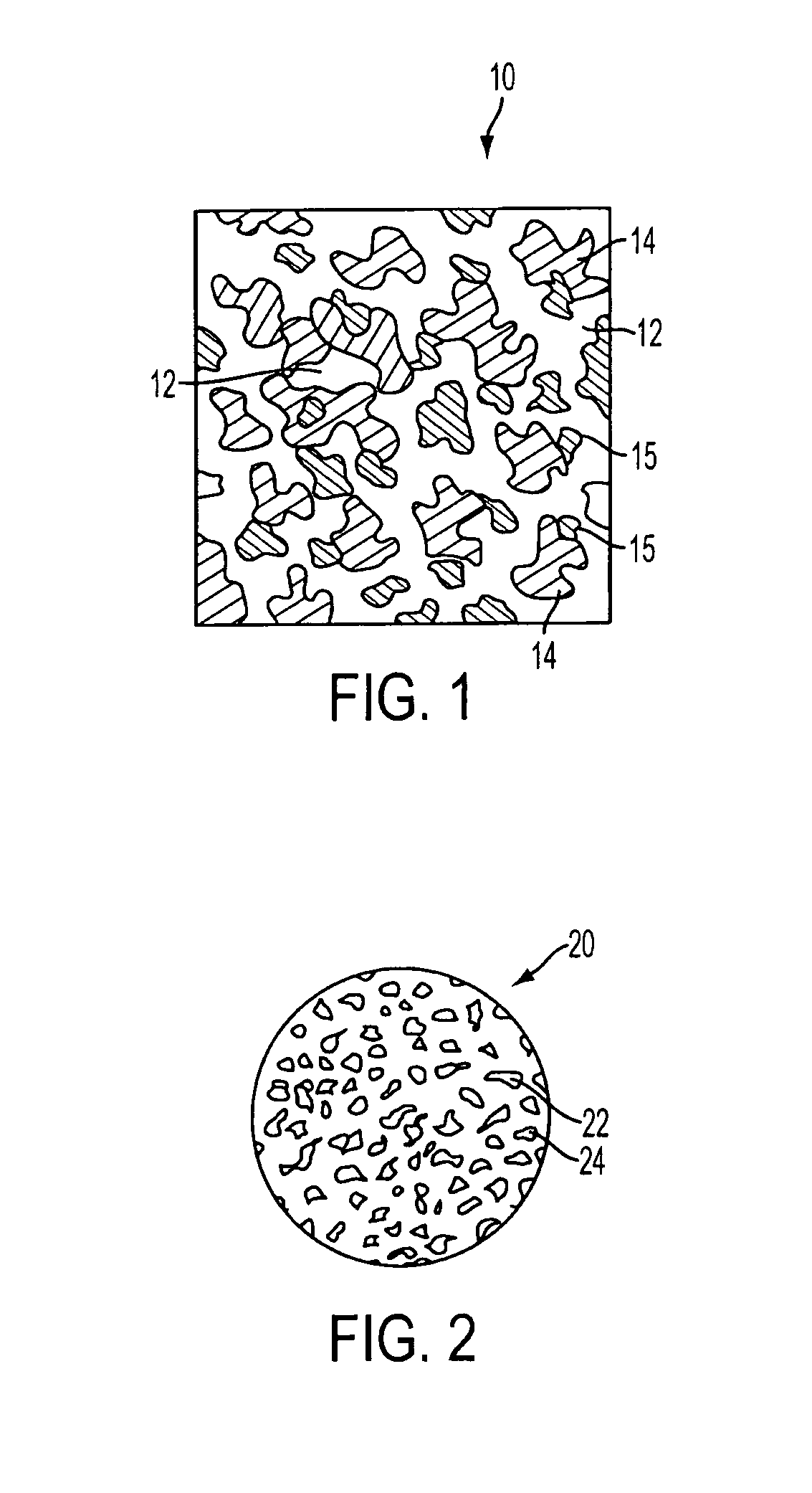
Composite surgical implant made from macroporous synthetic resin and bioglass particles
A cranio-maxillofacial implant material that is made of a macroporous (greater than 100 microns in diameter) interconnecting porous polyethylene structure with bioactive glass particles dispersed throughout the porous polyethylene structure, is disclosed. The Implant provides augmentation or replacement of cranio-maxillofacial tissues when implanted subperiosteally or within cranio-maxillofacial soft tissue. The addition of the bioactive glass particles to the porous polyethylene implant structure provides for faster fibrovascular ingrowth into the implant material.
Owner:ORTHOVITA INC
Device and methods for treating paranasal sinus conditions
ActiveUS20110004194A1Reduce needRecovery functionAntibacterial agentsOrganic active ingredientsActive agentNose
Described here are paranasal sinus devices for treating paranasal sinus conditions. The devices include a cavity member, ostial member, and nasal portion. One or more of the cavity member, ostial member, and nasal portion may deliver an active agent for sustained release to treat the paranasal sinus condition. Exemplary paranasal sinus conditions are sinus inflammation due to functional endoscopic sinus surgery (FESS) and rhinosinusitis.
Owner:INTERSECT ENT INC
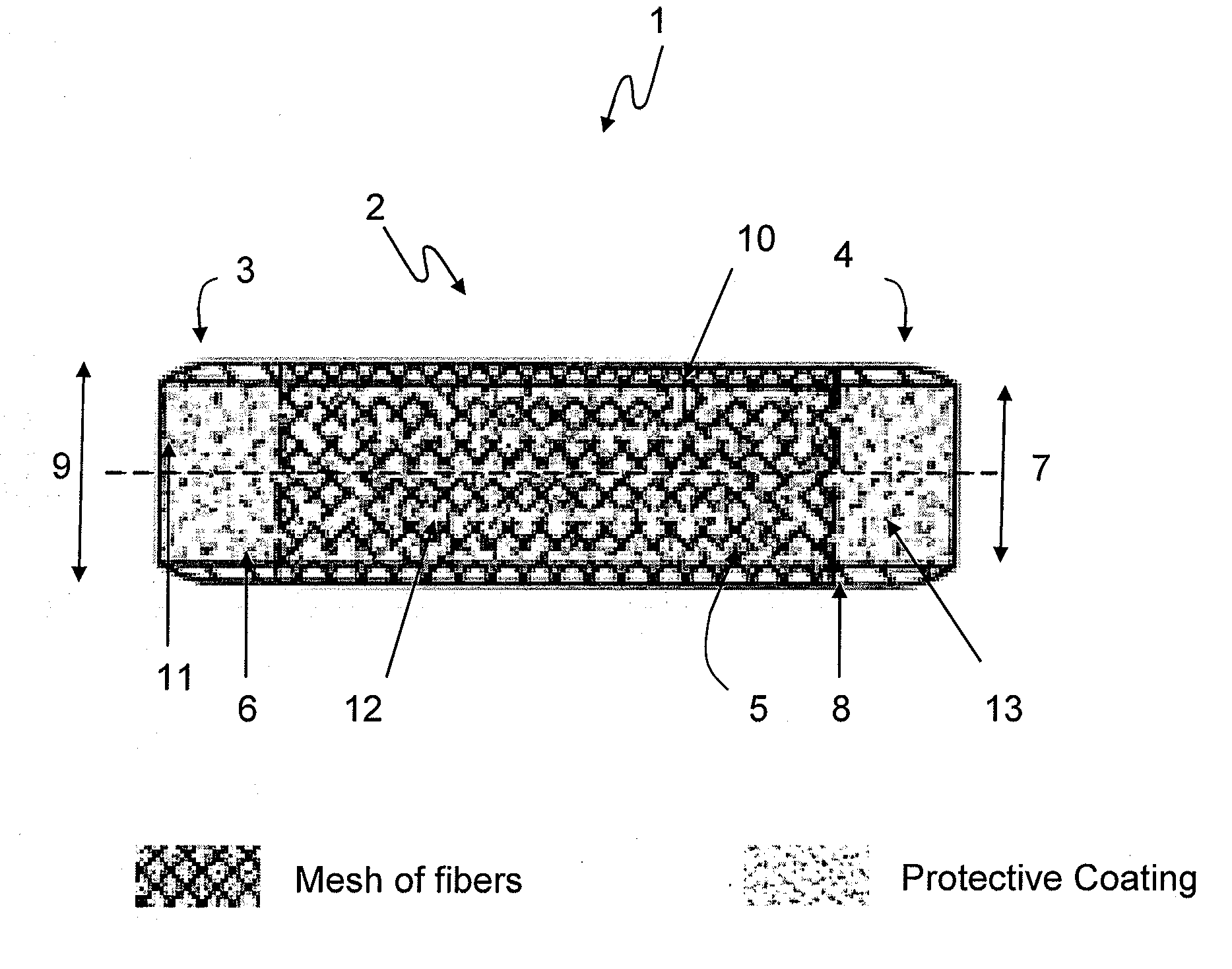
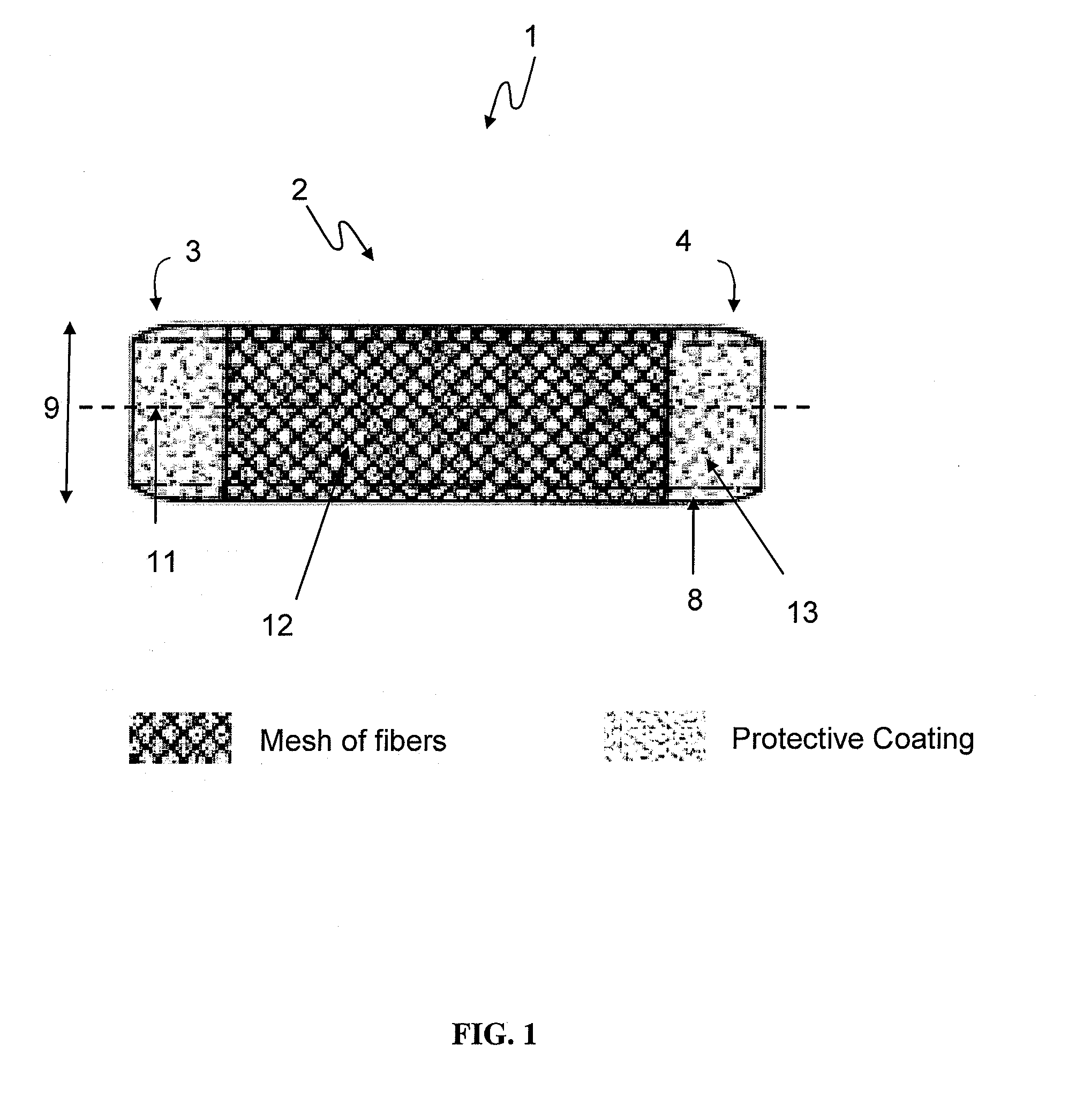
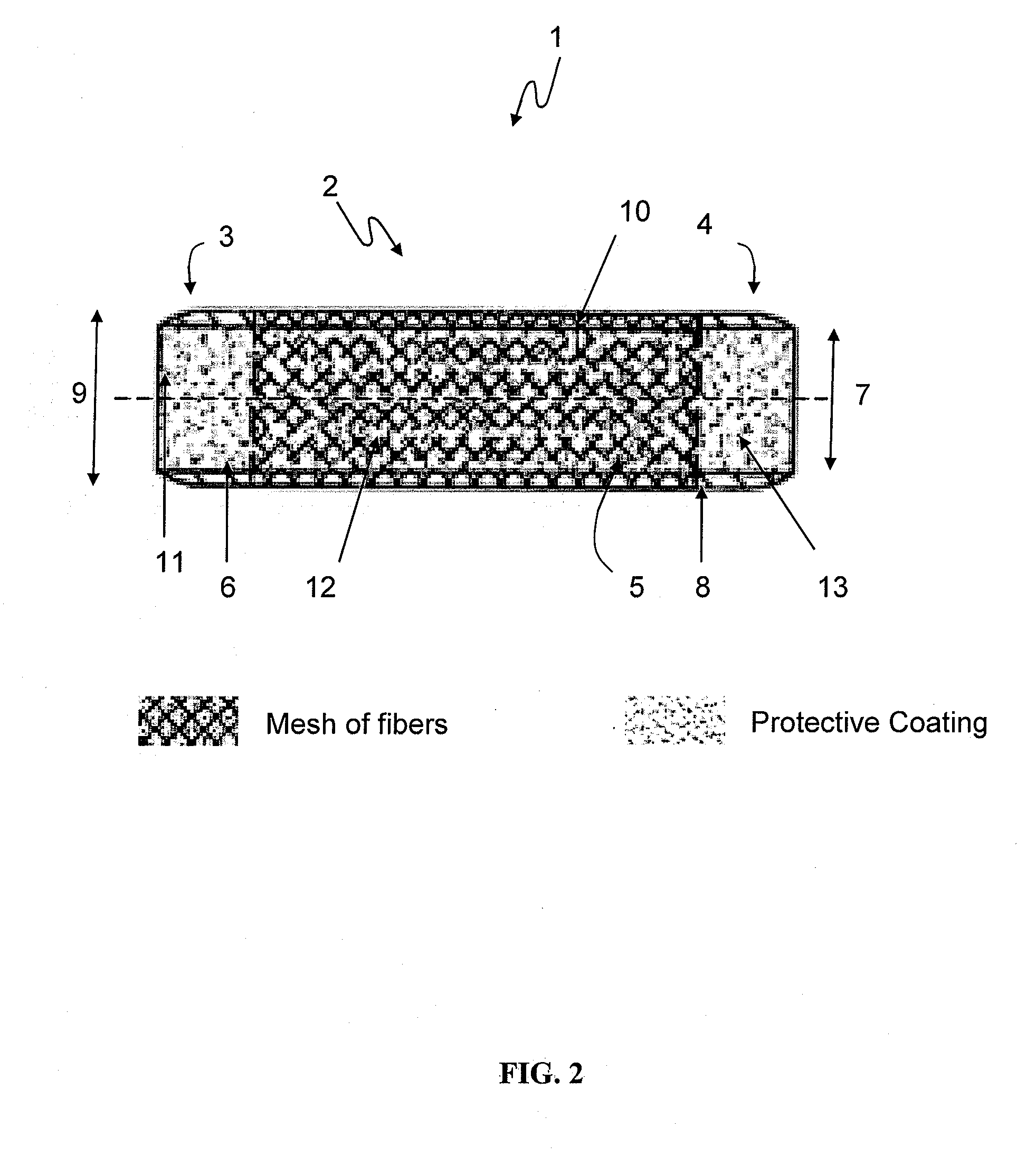
Coated devices comprising a fiber mesh imbedded in the device walls
Devices used in the management of bodily airways including bronchial and tracheal stents, bronchial Y-tubes, bronchial TY-tubes, nasal septal buttons, and nasal stents. The present invention also relates to devices used to manage the esophagus, such as salivary bypass tubes. These devices may comprise a fiber mesh imbedded in the walls of the device in order to provide integrity to the device wall and permit anchoring and suturing of the device.
Owner:E BENSON HOOD LAB
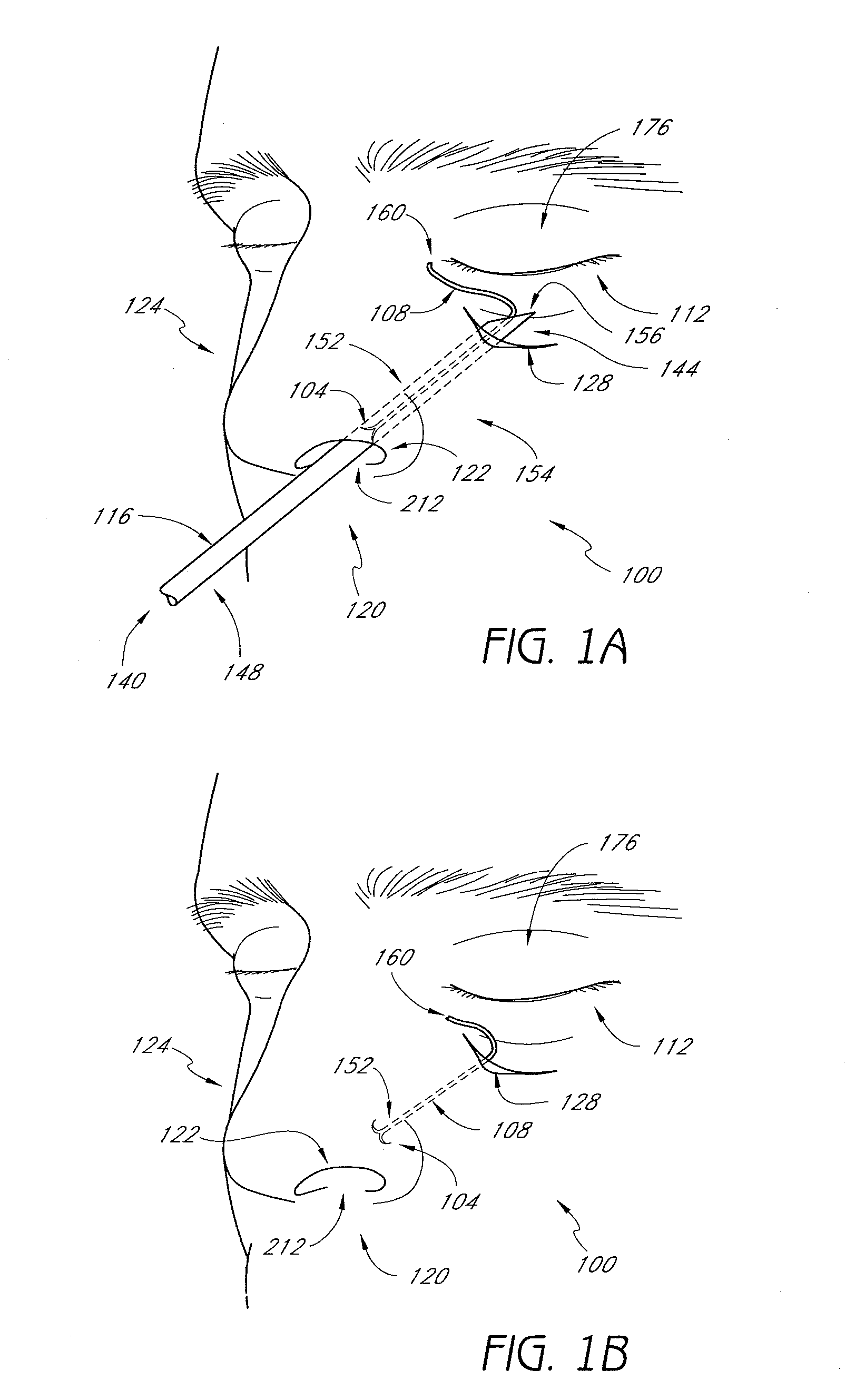
Nasal implants and systems and method of use
ActiveUS20160058556A1Promote recoveryIncrease heightNose implantsSurgical needlesHand heldBiomedical engineering
Described are implants for placing in a body, tools for delivering the implants, and systems and methods for using implants and tools for placing in a body and more particularly to nasal implants, tools for delivering nasal implants, and systems and methods for using such implants and tools. A tool may include a hand-held implant delivery device that holds, moves, orients, inserts, or shapes an implant. An implant may be a biodegradable, longitudinal implant that may be oriented for implantation by an implant delivery device.
Owner:SPIROX INC
Nasal implants and systems and methods of use
ActiveUS20140243975A1Increase implant flexibilityPrecise positioningNose implantsSurgical needlesMedicineHand held
Described are implants for placing in a body, tools for delivering the implants, and systems and methods for using implants and tools for placing in a body and more particularly to nasal implants, tools for delivering nasal implants, and systems and methods for using such implants and tools. A tool may include a hand-held implant delivery device that cuts, holds, moves, orients, inserts, or shapes an implant. An implant may be a biodegradable, longitudinal implant that may be oriented for implantation by an implant delivery device.
Owner:SPIROX INC
Medical Implants
InactiveUS20090283701A1Relieve symptomsIncrease surface areaDental implantsNanotechBiomedical engineeringMedical treatment
Owner:RGT UNIV OF CALIFORNIA
Nasal implant introduced through a non-surgical injection technique
ActiveUS20080077240A1Increase in internal nasal valve angleImprove structural strengthNose implantsSurgical needlesNoseNasal valve
A method for non-surgically treating an internal nasal valve of a patient comprising, injecting a working device into the internal nasal valve of the patient, wherein the injected working device in the nasal tissue causes an alteration of an internal or external nasal valve. A device introduced by injection into the nose, allowing for structural support or filling of defects in the nose, and causing a change in external shape of the nose. The device and inserts and implants described also have use in cosmetic applications relating to the facial tissue.
Owner:SPIROX INC
Apparatus and methods for correcting nasal valve collapse
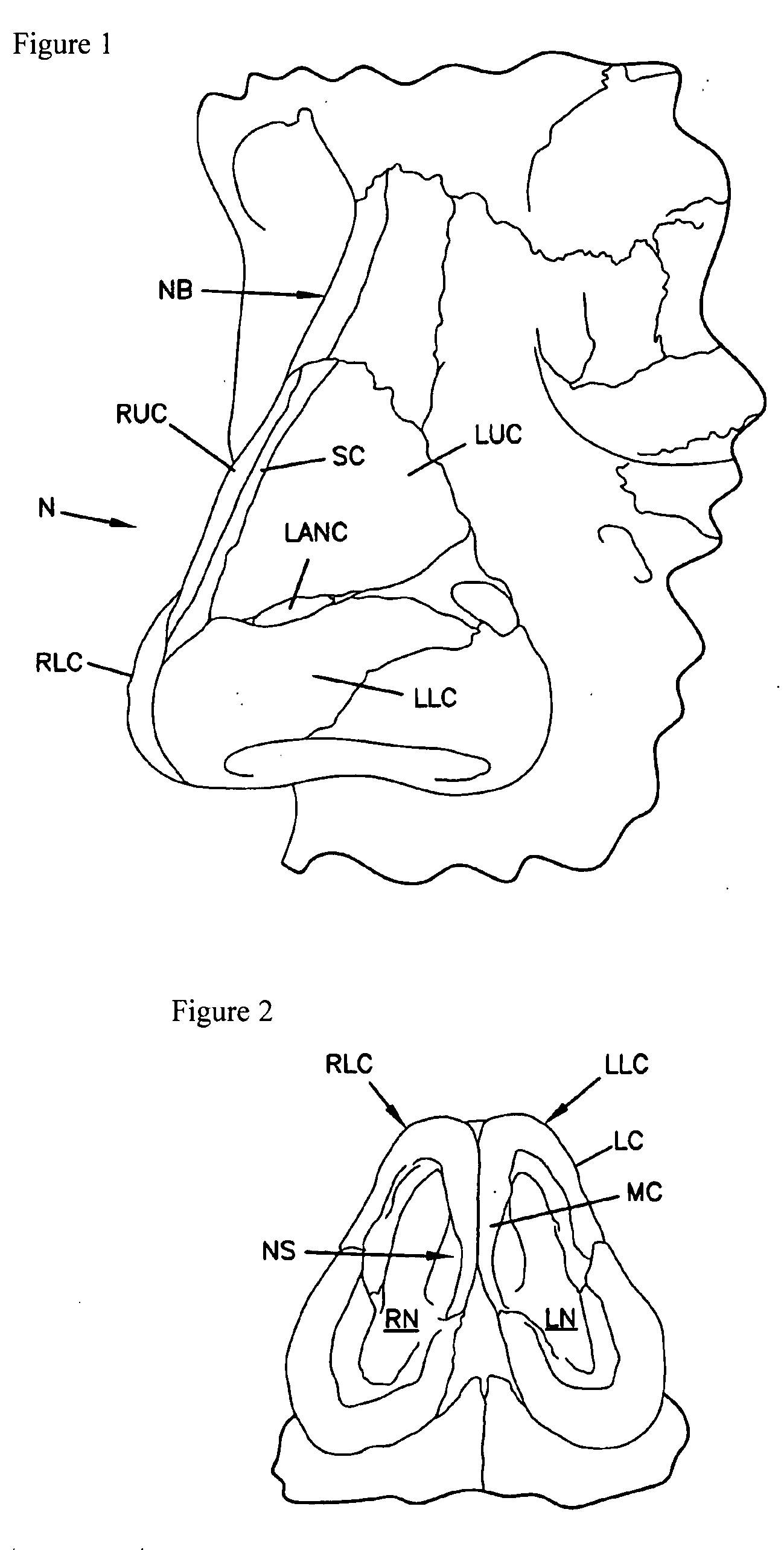
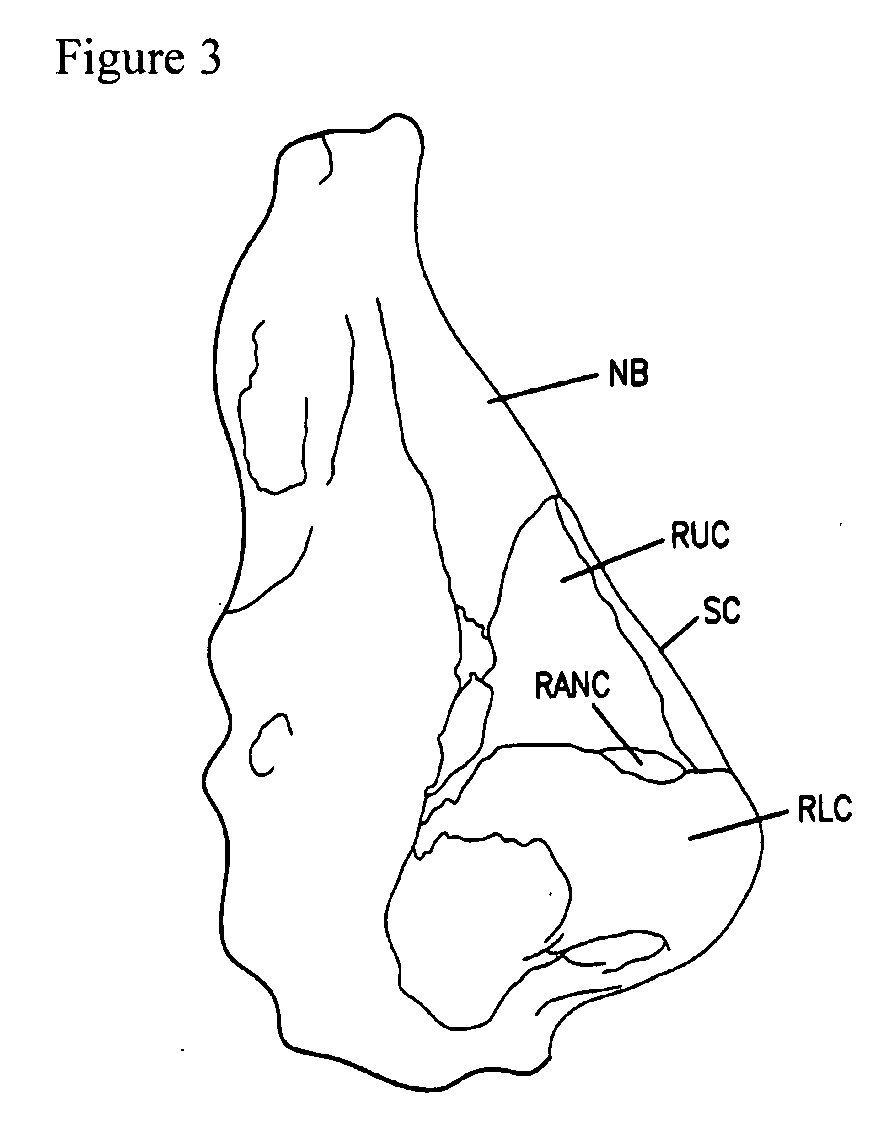
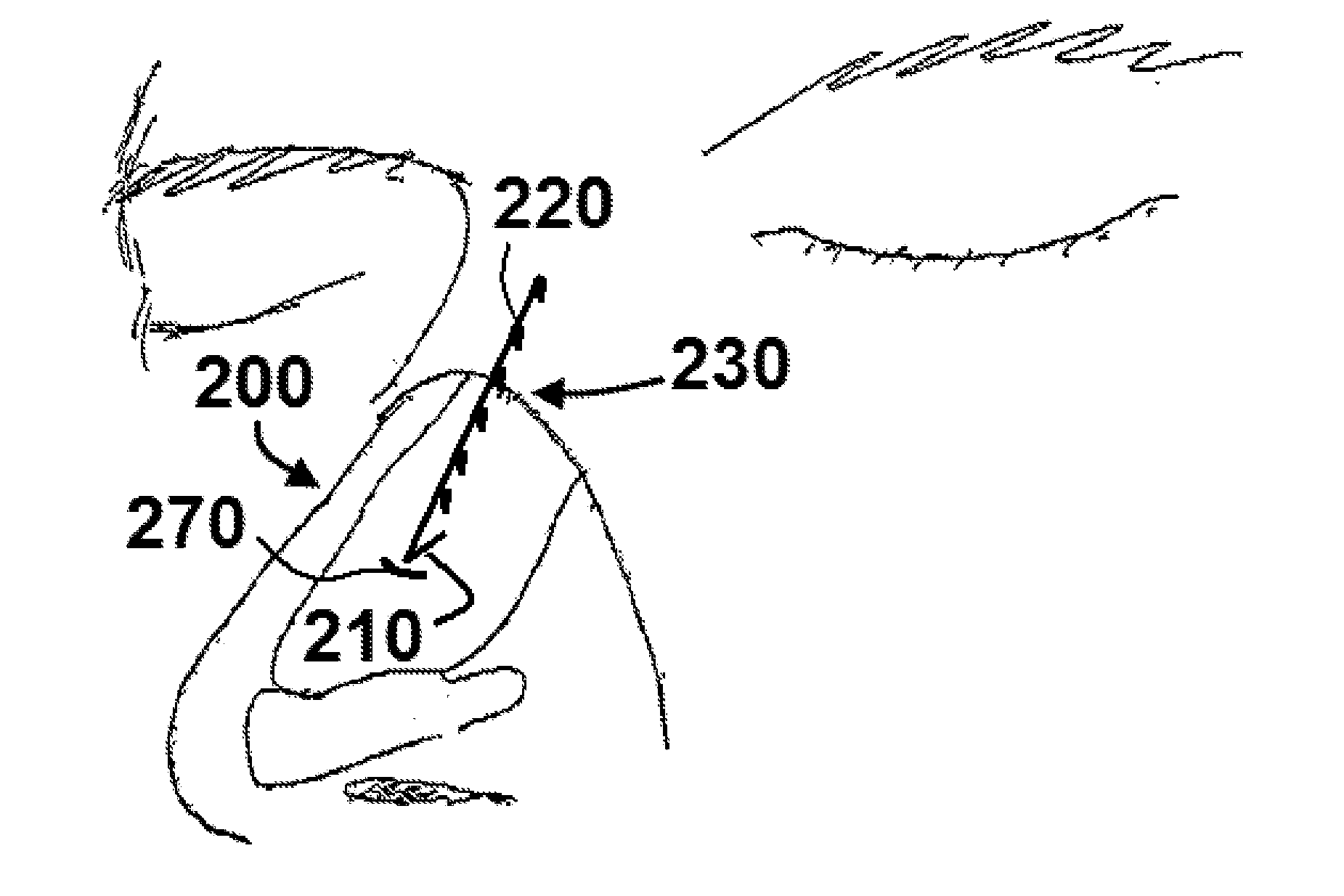
The present invention relates to apparatus ad methods for correcting nasal valve collapse comprising a device configured for suspending a nasal valve, the device comprising a body portion comprising a proximal end, a distal end, and a plurality of barbs, wherein the body portion is configured to be inserted through or underneath the upper lateral cartilage of a patient and wherein the plurality of barbs are configured to engage a soft tissue overlying a bony tissue proximal to the upper lateral cartilage, and a head portion coupled to the proximal end of the body portion wherein the head portion is configured to engage the upper lateral cartilage of the patient when the plurality of barbs are engaged with the soft tissue overlying the bony tissue proximal to the upper lateral cartilage
Owner:SPIROX INC
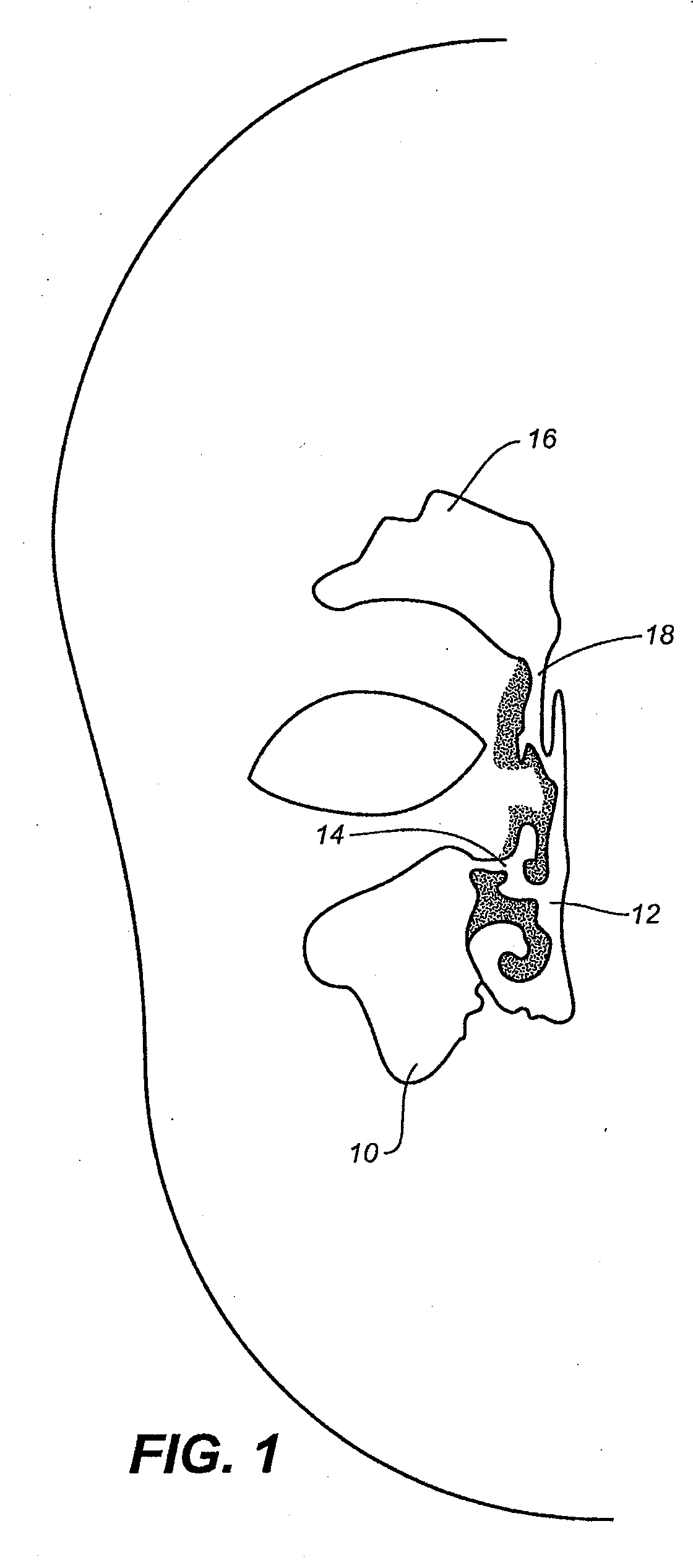
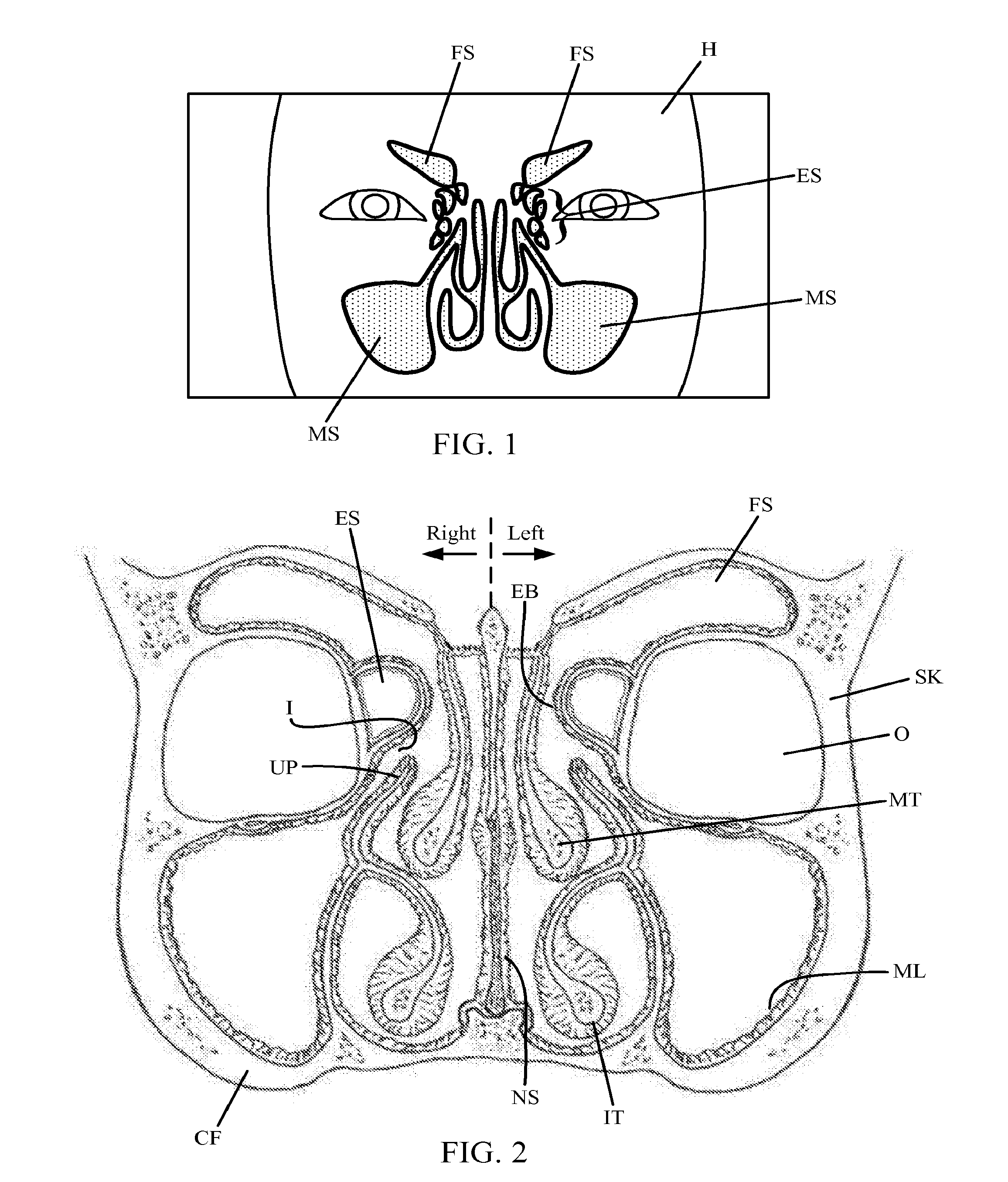
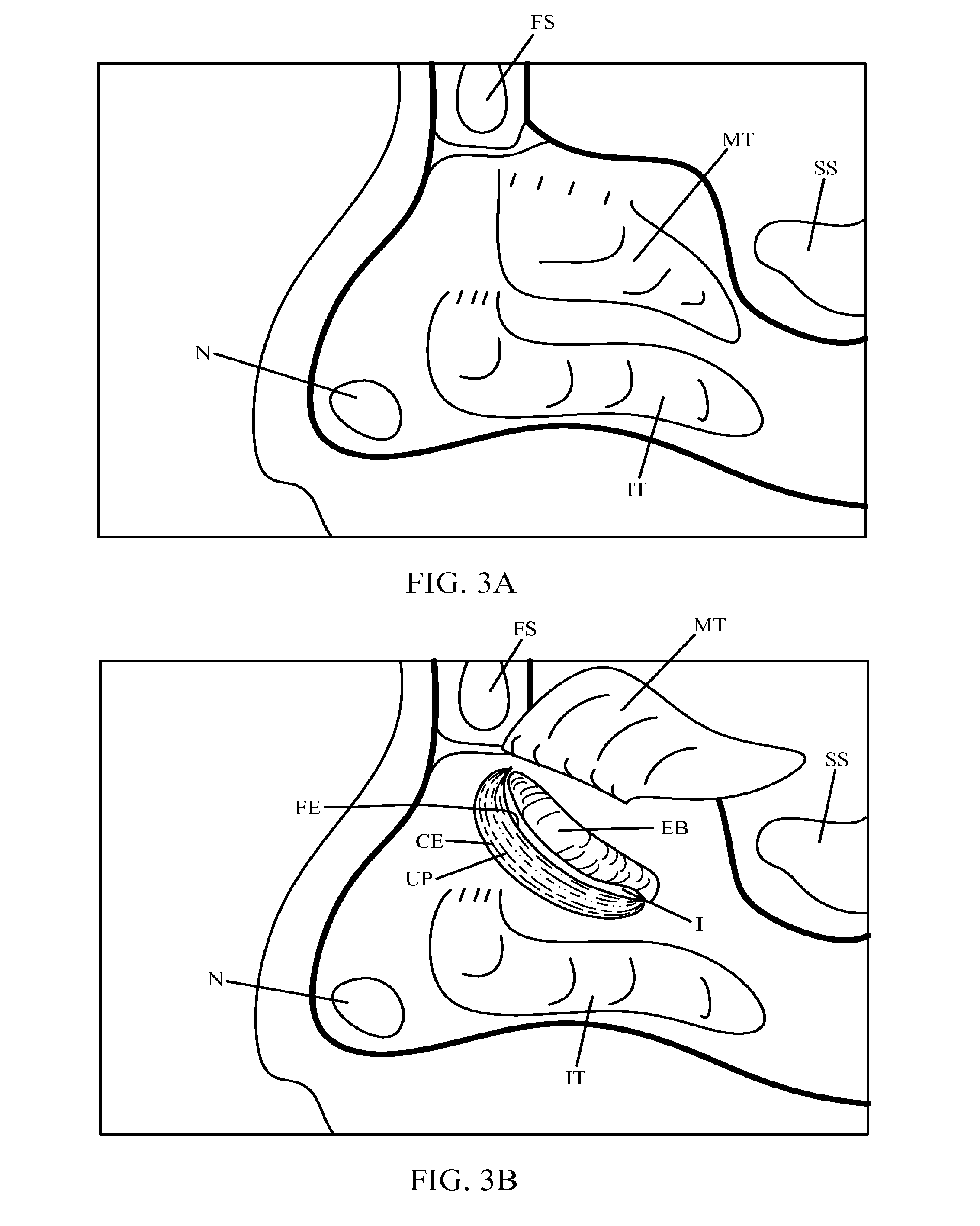
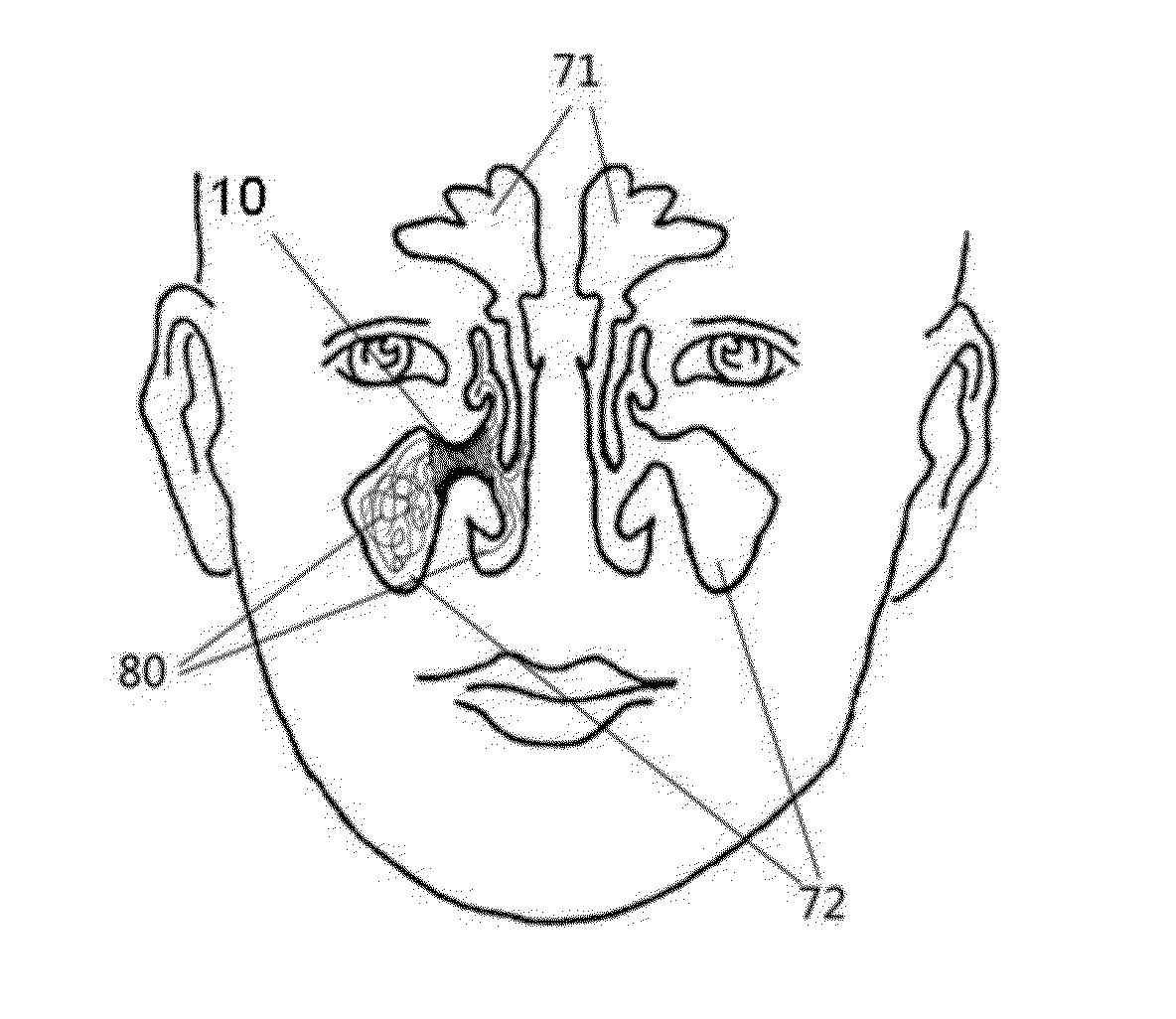
Devices and Methods for Dilating a Paranasal Sinus Opening and for Treating Sinusitis
Medical devices which are adapted to be inserted into a patient for a limited period of time using minimally invasive insertion procedures for dilating a stenotic opening, such as a stenotic sinus opening, are provided. The devices and methods can be used for treating sinusitis and other nasal and / or sinus disorders.
Owner:SINUSYS CORP
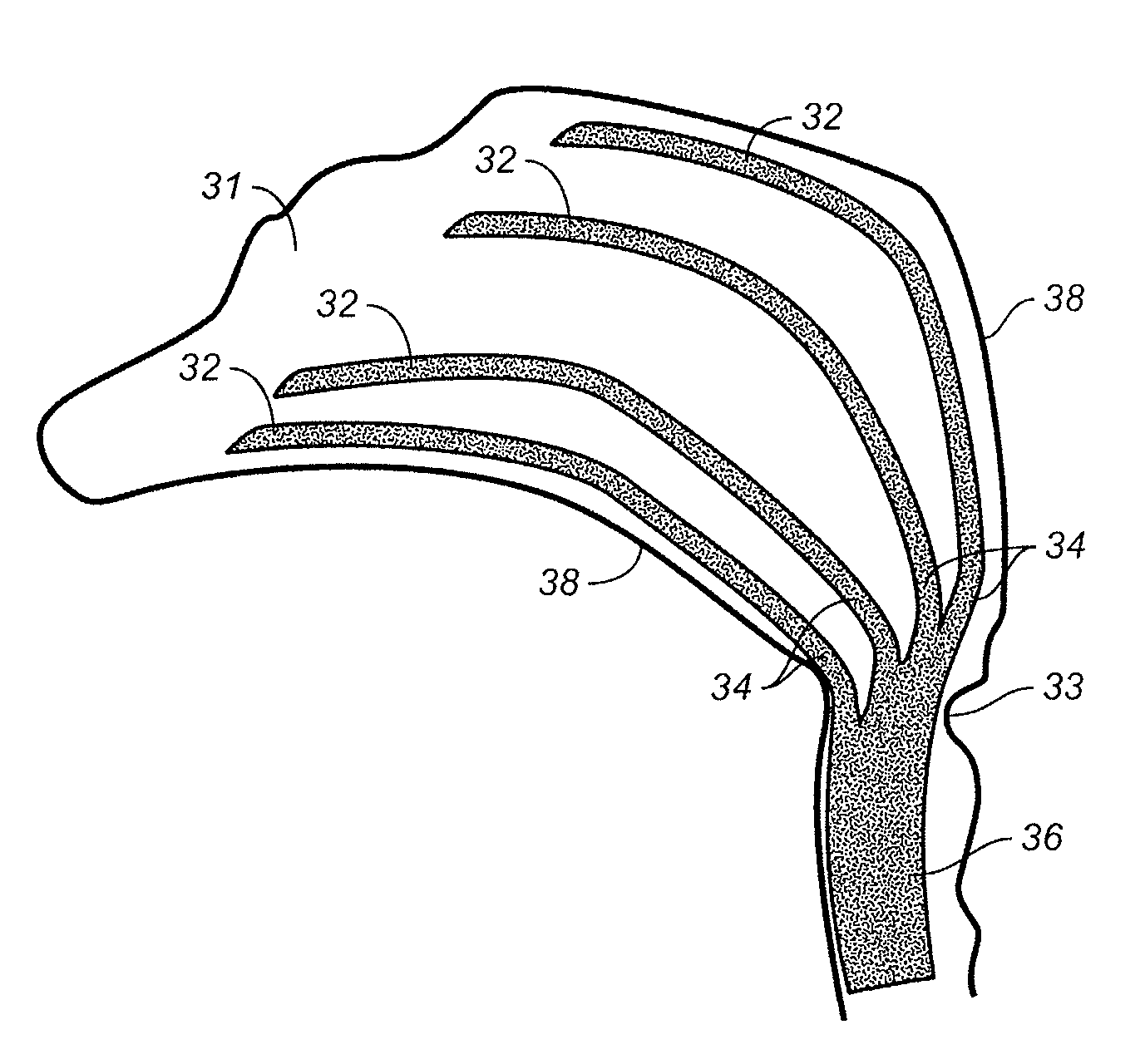
Prosthetic system for treating nasosinusitis or allergic rhinitis
The invention provides a prosthetic system for treating the nasosinusitis or allergic rhinitis. The prosthetic system is provided with a wall surface which is formed by a degradable polymer and extends along the circumferential direction; the wall surface is provided with a plurality of gaps; and degradable fiber tows containing medicines are penetrated and inserted into the gaps. The prosthetic system formed by the degradable polymer can provide enough normal force vertical to the outer surface when being compressed, and after the planted part (the diseased part or the paranasal sinus opening) is released, an opened channel can be maintained with less closing possibility; and the prosthetic system is provided with a hole with the axially-hollow interior, so that after the prosthetic system is planted, the nasal cavity maintains a natural ventilating state. The prosthetic system can be naturally degraded in the nasal cavity, the degradable fiber tows containing the medicines, which are hung on the prosthetic system, can contact the diseased part which can not be directly contacted by a bracket, so that the treating effect is optimized.
Owner:PUYI (SHANGHAI) BIOTECHNOLOGY CO LTD
Devices and Methods for Dilating a Paranasal Sinus Opening and for Treating Sinusitis
InactiveUS20130231693A1Maintain moisture contentPrevented from expandingNose implantsMedical devicesDiseaseSinusitis
Medical devices which are adapted to be inserted into a patient for a limited period of time using minimally invasive insertion procedures for dilating a stenotic opening, such as a stenotic sinus opening, are provided. The devices and methods can be used for treating sinusitis and other nasal and / or sinus disorders.
Owner:SINUSYS CORP
Implanted system for treating sinusitis or allergic rhinitis
An implanted system for treating sinusitis or allergic rhinitis is provided, wherein the implanted system has a circumferentially extending wall formed of a biodegradable polymer, and the wall includes a plurality of interspaces, with biodegradable fiber bundles containing drugs interspersed in the interspaces. The implanted system formed of the biodegradable polymer can provide a sufficient normal force perpendicular to the external surface during a compression, and prevent the supported channel from being closed after the release in a location for the implantation (a location of pathological changes or ostium of the sinus). The implanted system formed of the biodegradable polymer has an axial internal bore to guarantee the aeration for the nasal cavity after the implantation. The implanted system formed of the biodegradable polymer can be naturally degraded in the nasal cavity. Compared with prior stents which cannot directly contact with certain locations of pathological changes, the biodegradable fiber bundles containing drugs carried on the implanted system can reach those locations of pathological changes to optimize the therapeutic effect.
Owner:PUYI (SHANGHAI) BIOTECHNOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com