Application of Fidaxomicin in preparation of medicines treating related diseases and/or symptoms caused by Zika virus infection
A technology for Zika virus and fidaxomicin, which is applied in the field of medicine, can solve the problems of lack of effective drugs, etc., and achieve the effect of inhibiting Zika virus with high activity, high safety and strong binding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Fidaxomicin inhibits ZIKV activity at the cellular level
[0023] Virus strain: Zika virus ZIKV (Z16019)
[0024] Cell line: A549
[0025] Detection method:
[0026] Fidaxomycin antiviral half effective dose (50% Effective Concentration, EC 50 ): DMSO, 6, 9, 12, 18, 24 μM Fidaxomycin saturated cells 1 hour in advance, 2 hours after virus infection, changed to virus-free medium containing corresponding concentration of drugs for 48 hours; collected cell supernatant, detected by plaque test Plaque formation inhibition rate of different doses of fidaxomicin group relative to solvent group (DMSO) after virus infection.
[0027] Inhibition rate (%) = (1-the number of virus plaques in the administration group / the number of plaques in the solvent control group) 100%, calculated using the Forcast formula of EXCEL2013, when the inhibition rate is equal to 50%, the corresponding fidaxomicin Concentration, as EC 50 . The average value was obtained from three repea...
Embodiment 2
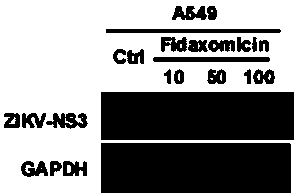
[0031] Example 2 The inhibitory activity of fidaxomicin to ZIKV-NS3 protein in A549 cell line
[0032] Virus strain: Zika virus ZIKV (Z16019)
[0033] Cell line: A549
[0034] Detection method:
[0035] DMSO, 10, 50, 100 μM Fidaxomycin saturated the cells 1 hour in advance, and 2 hours after the virus infection, the virus-free medium containing the corresponding concentration of drugs was maintained for 48 hours; the cell pellet was collected, and Western blot electrophoresis was used to detect ZIKV in the cells under different treatments -The relative expression level of NS3 protein, using GAPDH as an internal reference protein. like figure 1 As shown, fidaxomicin can effectively inhibit the expression of ZIKV NS3 protein at 10-50 μM, thereby preventing the reproduction of ZIKV.
Embodiment 3
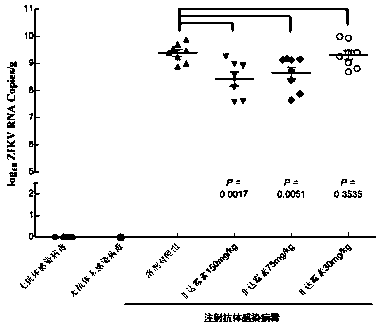
[0036] Example 3 Anti-ZIKV activity of Fidaxomycin in mice
[0037] Mouse strain: C57BL / 6J WT
[0038] Model: anti-IFNAR1 antibody blocking method
[0039] Experimental method: 5~6W mice were intraperitoneally injected with antibodies one day in advance, and intraperitoneally infected with ZIKV 1×10 5 PFU virus was administered intraperitoneally after 4 hours, and Mock group, solvent control group, and fidaxomicin dose groups of 30, 75, and 150 mg / kg were set up, and the administration was continued for 7 days. On the 8th day, the mice were killed and the spleen was dissected for qRT-PCR Methods The copy number of ZIKV virus RNA in the spleen was detected. The result is as figure 2 , in mice, the doses of 75mg / kg and 150mg / kg have obvious anti-ZIKV effect (p<0.01). Correspondingly, there was no death in the dose of 150 mg / kg for 7 consecutive days, and no obvious toxic and side effects in the dose of 75 mg / kg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com