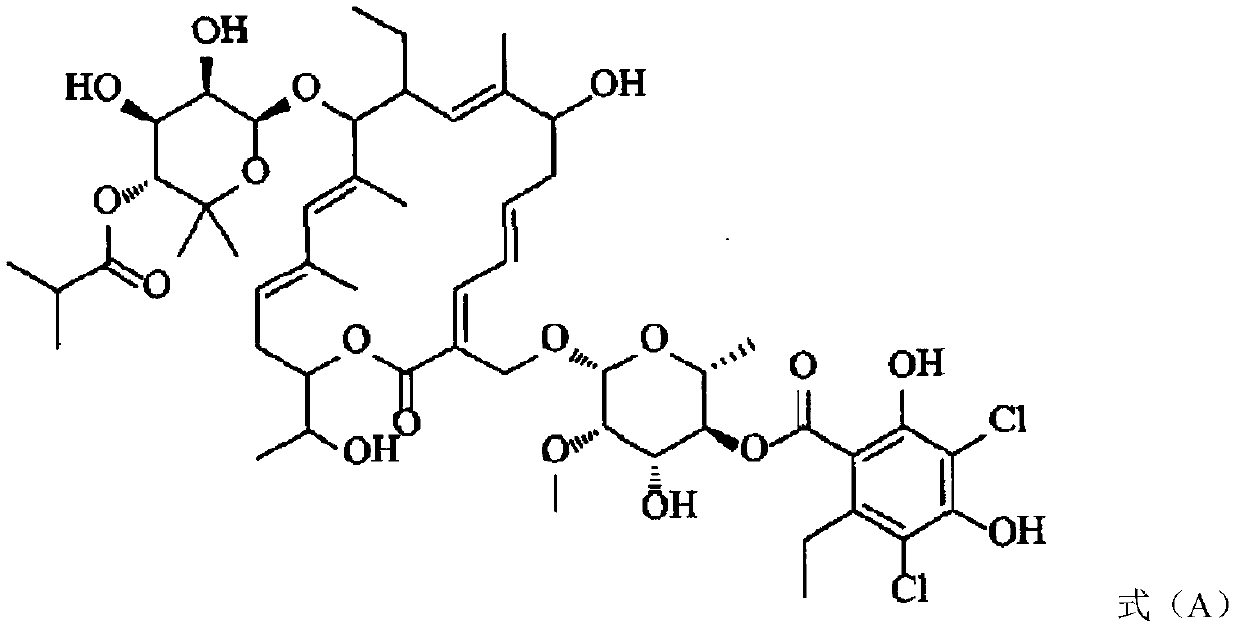
Fidaxomicin impurities and preparation method thereof
A technology for fidaxomicin and impurities, which is applied in the field of pharmaceutical analysis, can solve the problems of few studies on degrading impurities, and achieve the effect of improving quality controllability and quality improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The detection of embodiment 1 impurity F
[0036] Instrument: Agilent 1260 HPLC
[0037] Chromatographic conditions: Chromatographic column: Agilent Poroshell 120EC-C18 (100*4.6mm, 2.7μm); mobile phase: 0.05M potassium dihydrogen phosphate solution-acetonitrile (50:50), isocratic elution; column temperature: 15°C; Flow rate: 0.5ml / min; detection wavelength: 230nm.
[0038] Dissolve the fidaxomicin sample in methanol solution to prepare a sample solution with a concentration of 0.5 mg / ml; inject the sample solution into a liquid chromatograph. Detected by HPLC, the impurity peak at relative retention time (RRT) 1.33 is impurity F, see attached figure 1 .
Embodiment 2
[0039] The preparation of embodiment 2 impurity F
[0040] Instrument: Gilson GX-281 preparative chromatography system, rotary evaporator: Shensheng rotary evaporator LABOROTA4001
[0041] Chromatographic conditions:
[0042] Chromatographic column: Agilent ZORBAX RX-C18, with octadecylsilane bonded silica gel as filler, the column length of this chromatographic column is 250 mm, and the inner diameter is 21.2 mm.
[0043] Detection wavelength: 230nm; flow rate: 15ml / min, injection volume: 8ml
[0044] Mobile phase: acetonitrile-water-acetic acid (52:48:1)
[0045] Mix 40ml of acetonitrile, 60ml of water and 1ml of acetic acid to prepare a mixed solution of acetonitrile-water-acetic acid, dissolve the pretreated fidaxomicin sample at 60°C in the mixed solution of acetonitrile-water-acetic acid to make a concentration of 20mg / ml solution. According to the above-mentioned chromatographic conditions, the fractions for 28-32 minutes were collected, and the impurity target frac...
Embodiment 3
[0047] The preparation of embodiment 3 impurity F
[0048] Apparatus and reagents are the same as in Example 2.
[0049] Detection wavelength: 230nm; flow rate: 10ml / min, injection volume: 2ml
[0050] Mobile phase: acetonitrile-water-acetic acid (52:48:1)
[0051] Mix 20ml of acetonitrile, 40ml of water and 0.5ml of acetic acid to prepare a mixed solution of acetonitrile-water-acetic acid, and dissolve the pretreated fidaxomicin sample at 60°C in the mixed solution of acetonitrile-water-acetic acid to make a concentration of 5mg / ml The solution. The fractions collected for 43-47 minutes were collected according to the above-mentioned chromatographic conditions, and the impurity target fraction was confirmed by the fidaxomicin liquid chromatography analysis method. Samples were injected continuously, the target components were collected and combined, concentrated and dried by rotary evaporation to obtain a white solid.
[0052] The obtained white solid was detected by HPLC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com