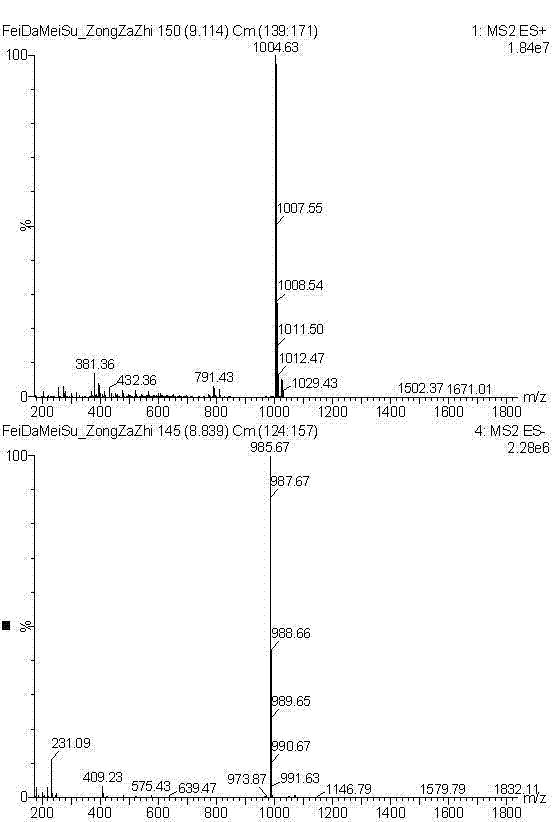Preparationmethod of macrolide compound
A technology of macrolides and compounds, applied in the field of biochemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
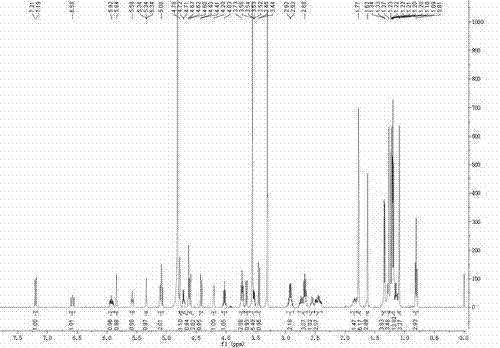
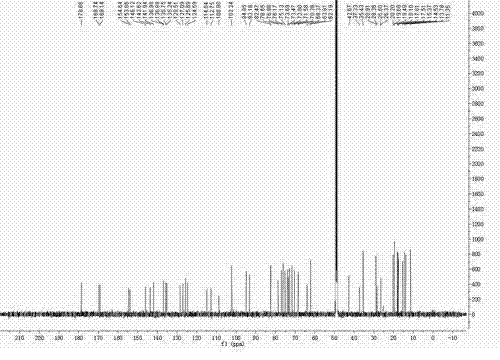
[0026] a. Disperse 1 g of fidaxomicin coarse powder in 10 ml of 0.1 mol / L NaOH aqueous solution, react at 20°C for 24 hours, filter, wash with distilled water, and filter under reduced pressure to obtain 0.9 g of OP-1118 coarse powder.
[0027] b. Add 3ml of acetonitrile to the OP-1118 coarse powder, sonicate for 2 minutes, and filter with a 0.45 μm filter membrane to obtain the sample solution.
[0028] c. Inject the sample solution into a medium-pressure chromatography column equipped with 90ml ODS, add acetonitrile:acid water mixed solution for elution, the ratio of trifluoroacetic acid and water in acid water is 5:10000 (v / v), HPLC On-line detection, collect the eluate with content ≥98.5%, concentrate to dryness under reduced pressure at 30°C to obtain 0.5g solid, add 5ml of crystallization solvent composed of acetonitrile and water with a volume ratio of 4:1, heat to dissolve, and then slowly cool down At 2°C, crystallize for 14 hours, and filter with suction to obtain OP...
Embodiment 2
[0033] a. Disperse 3 g of fidaxomicin coarse powder in 30 ml of 0.15 mol / L NaOH aqueous solution, react at 10°C for 48 h, filter, wash with distilled water, and filter under reduced pressure to obtain 2.6 g of OP-1118 coarse powder.
[0034] b. Add 15ml of methanol to the OP-1118 coarse powder, sonicate for 5 minutes, and filter with a 0.45 μm filter membrane to obtain the sample solution.
[0035] c. Inject the sample solution into a medium-pressure chromatography column equipped with 130ml ODS, add a mixed solution of methanol:acid water for elution, the ratio of acetic acid and water in acid water is 1:5000 (v / v), HPLC online detection , collect the eluent with content ≥98.5%, concentrate to dryness under reduced pressure at 40°C to obtain 1.4g solid, add 7ml of crystallization solvent composed of methanol and water with a volume ratio of 4:1, heat to dissolve, and slowly cool down to 5 ℃, crystallized for 20 hours, and filtered with suction to obtain OP-1118 crystalline po...
Embodiment 3
[0037] a. Disperse 5 g of fidaxomicin coarse powder in 50 ml of 0.2 mol / L NaOH aqueous solution, react at 30°C for 12 hours, filter, wash with distilled water, and filter under reduced pressure to obtain 4.6 g of OP-1118 coarse powder.
[0038] b. Add 20ml of ethanol to the OP-1118 coarse powder, sonicate for 5 minutes, and filter with a 0.45 μm filter membrane to obtain the sample solution.
[0039]c. Inject the sample solution into a medium-pressure chromatography column equipped with 320ml ODS, add ethanol: acid water mixed solution for elution, the ratio of acetic acid and water in acid water is 3:10000 (v / v), HPLC online detection , collect the eluent with content ≥98.5%, concentrate to dryness under reduced pressure at 50°C to obtain 2.5g of solid, add 37ml of crystallization solvent composed of ethyl acetate and ethanol with a volume ratio of 1:10, heat to dissolve, and then slowly cool down to 10°C, crystallize for 28 hours, and filter with suction to obtain OP-1118 cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com