Method for separating and purifying fidaxomicin
A non-damicin, separation and purification technology, applied in the field of separation and purification, can solve the problems of poor separation effect, low purification efficiency, complicated operation, etc., and achieve the effects of improved purity and recovery rate, simple operation and good separation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Sample solution preparation
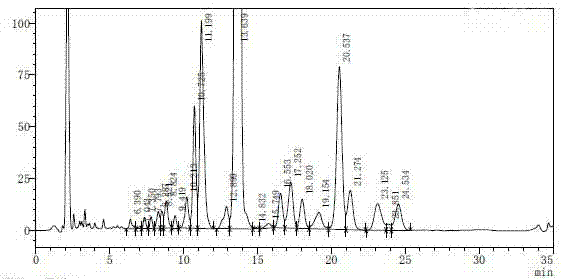
[0024] Get 65g non-damicin crude product (HPLC spectrogram is attached figure 1 shown), dissolved in 650ml glacial acetic acid, filtered with 0.45μm polytetrafluoroethylene ethylene filter membrane after dissolving, and the filtrate was further mixed with 650ml acetonitrile-1% glacial acetic acid aqueous solution (the volume ratio of acetonitrile to 1% glacial acetic acid aqueous solution is 45:55) was diluted to 50 mg / ml as a next step to prepare a sample solution of non-damicin.
[0025] (2) Separation and purification:
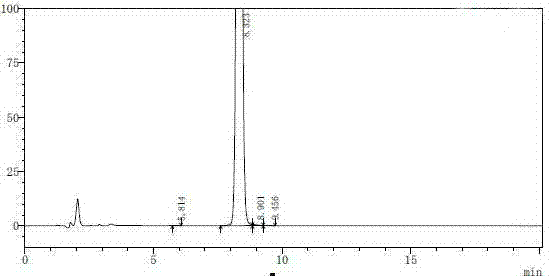
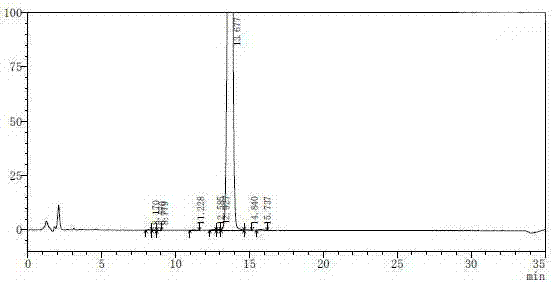
[0026] Inject 1300ml of the solution diluted in step (1) into a reversed-phase HPLC preparative chromatograph (using a glass column with an inner diameter of 200cm and a height of 25cm, and UniSil 10-120-C8 reversed-phase silica gel as a chromatographic filler, the dosage is 4kg, first Rinse 2 column volumes (CV) with 45% acetonitrile in water, then equilibrate with 20% acetonitrile for 2 CV), the mobile phase con...
Embodiment 2
[0030] According to the method of step (1) in Example 1, prepare the sample solution of non-damicin, and inject 13000ml of the diluted solution into the reversed-phase high-performance liquid phase preparative chromatograph (using a glass column with an inner diameter of 200cm and a height of 25cm, UniSil 10-120-C8 reversed-phase silica gel is used as a chromatography filler, the dosage is 4kg, first wash 2 column volumes CV with 45% acetonitrile aqueous solution, and then equilibrate 2 CVs with 20% acetonitrile), the mobile phase consists of acetonitrile-water ( Containing 1% glacial acetic acid), gradient elution is carried out on the stationary phase, that is, during 0~50min, the volume ratio of acetonitrile-1% glacial acetic acid aqueous solution is 40:60; during 51~150min, the volume ratio of acetonitrile-1% ice The volume ratio of the acetic acid aqueous solution is 50:50; the flow rate is 800ml / min, the ultraviolet detector is detected online, and the detection wavelengt...
Embodiment 3
[0033] (1) Preparation of sample solution:
[0034] Get 65g non-damicin crude product (HPLC spectrogram is attached figure 1shown), dissolved in 650ml of glacial acetic acid, filtered with a 0.45μm polytetrafluoroethylene ethylene filter membrane after dissolution, and the filtrate was further mixed with 650ml of acetonitrile-1% glacial acetic acid aqueous solution (the volume ratio of acetonitrile-1% glacial acetic acid aqueous solution is 45 :55) was diluted to 50 mg / ml as a next step to prepare a sample solution of non-damicin.
[0035] (2) Separation and purification of samples
[0036] Inject 1300ml of the solution diluted in step (1) into a reversed-phase HPLC preparative chromatograph (using a glass column with an inner diameter of 200cm and a height of 25cm, and SP-100-8-C8-HP reversed-phase silica gel as a chromatographic filler, with an amount of 4kg, wash 2 column volume CVs with 45% acetonitrile aqueous solution, and then equilibrate 2 CVs with 20% acetonitrile)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com