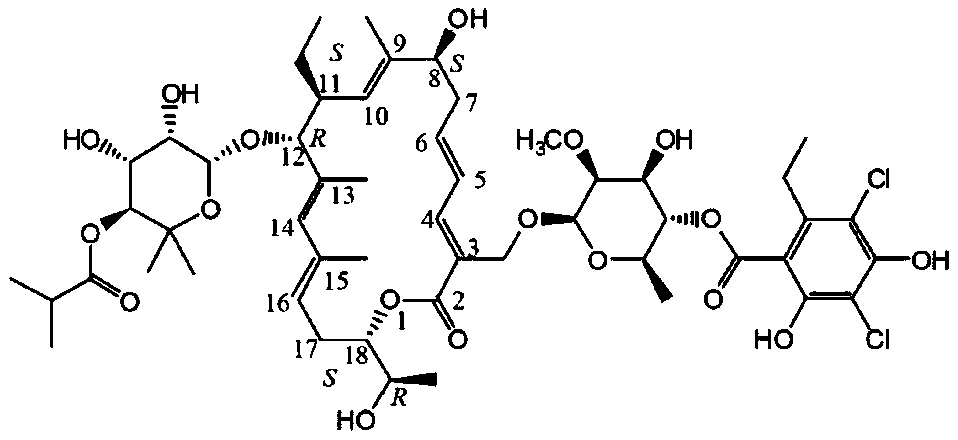
Stable fidaxomicin composition
A technology for fidaxomicin and composition, applied in the field of stable pharmaceutical composition and preparation thereof, can solve the problem of high F heterogeneity of drugs, achieve the requirements of lowering temperature, stable dissolution, and less increase of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
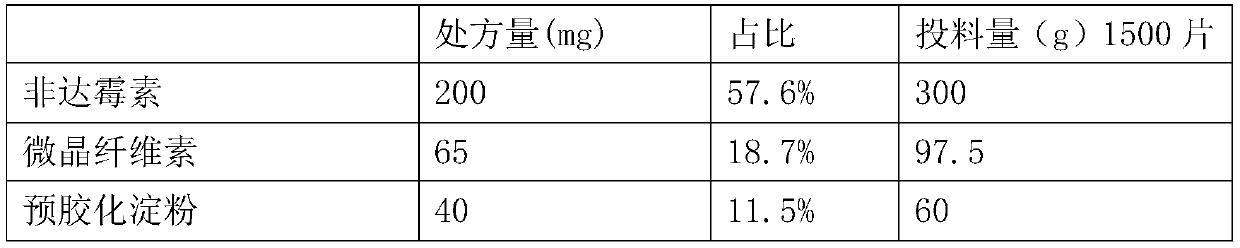
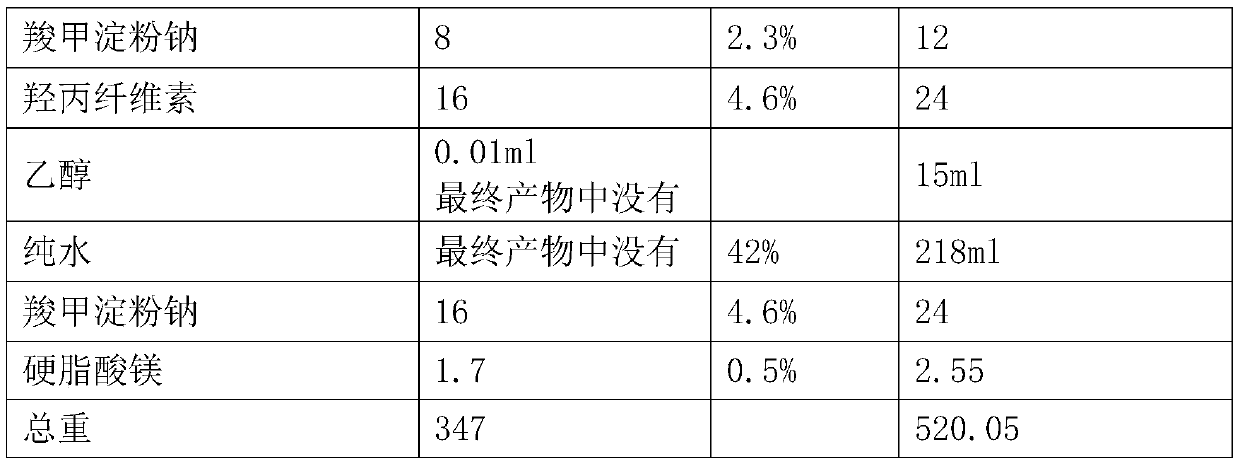
[0027] prescription:
[0028]
[0029]
[0030] Preparation:
[0031] 1. Weigh the raw materials, microcrystalline cellulose, pregelatinized starch, sodium carboxymethyl starch, and hydroxypropyl cellulose into the granulator BoschMycromix, pre-mix for 3 minutes, and stir at a speed of 60m / min;
[0032] 2. After premixing, add ethanol and continue stirring, slowly add pure water and continue stirring, the stirring speed is 115m / min, and the granulation time is 6 minutes. After the granulation is completed, the wet granules are sized, the screen is 4*4mm square holes, and the speed is 1000rpm.
[0033] 3. Fluidized bed Solidlab drying, air inlet temperature 60°C, moisture 1.0-3.0%.
[0034] 4. Whole grain QUADRO COMIL U5-0437R, sieve aperture Φ1.5mm, rotation speed 1000rpm.
[0035] 5. Add additional sodium carboxymethyl starch and magnesium stearate according to the prescription amount, mix well, and compress the tablet with a rotary tablet machine ZPS8.
Embodiment 2-7
[0037] Embodiments 2-7 are the same as Example 1 except that the amount of water added, the stirring speed of granulation, and the granulation time are as shown in the table below.
[0038] Example Add water % Amount of water added g Linear speed(m / min) Granulation time (min) 2 52% 270 61 6 3 42% 220 61 3 4 52% 270 115 3 5 42% 220 115 6 6 52% 270 61 3 7 47% 245 88 4.5
Embodiment 8
[0040] prescription:
[0041]
[0042]
[0043] Preparation:
[0044] 1. Weigh raw materials, microcrystalline cellulose, pregelatinized starch, sodium carboxymethyl starch, and hydroxypropyl cellulose and pour them into the granulator Pilotmix 50T granulation line wet granulator, pre-mix for 3 minutes, and stir at a speed of 88m / min;
[0045] 2. After premixing, add ethanol and continue stirring, slowly add pure water and continue stirring, the stirring speed is 88m / min, and the granulation time is 4.5min. After the granulation is completed, the wet granules are sized, the screen is 4*4mm square holes, and the speed is 1000rpm.
[0046] 3. Pilotlab L granulation line fluidized bed drying, air inlet temperature 60 ℃, MB35 fast moisture analyzer to measure moisture 1.0-3.0%.
[0047] 4. The aperture of the screen is Φ1.5mm, and the rotation speed is 1000rpm.
[0048] 5. Add additional sodium carboxymethyl starch and magnesium stearate according to the prescription am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com