Patents
Literature
49 results about "Polio virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Poliovirus is a virus that causes the medical condition polio. The virus is a single-stranded RNA virus from the family Picornaviridae and genus enterovirus.
Acellular pertussis vaccines and methods of preparation thereof
InactiveUS6696065B1Increase contentEnhance immune responseBiocideSsRNA viruses positive-sensePoliomyelitisTetanus toxoids
A multi-component vaccine composition is described comprising acellular pertussis vaccine components, diphtheria toxoid, tetanus toxoid and inactivated poliovirus. The composition also may contain a conjugate of a capsular polysaccharide on Haemophilus influenzae type b and tetanus toxoid or diphtheria toxoid, which may be reconstituted from a lyophilized state by the other component. The administration of the multiple component vaccine resulted in no diminution of the immunogenicity of any component as a result of interference by other components of the vaccine.
Owner:SANOFI PASTEUR LTD
Polytetrafluoroethylene multi-layer composite film and its protective material preparation method
InactiveCN101104314ASimple processLow costLaminationLamination apparatusMicroorganismPolymer science
The invention relates to a multi-layer polytetrafluoroethylene composite film and protective material making method thereof. The method is characterized in that a polytetrafluoroethylene base band and a thermoplastic permeable film are drawn longitudinally together under the temperature of 100 to 200 DGE C and then are drawn widthwise together on an expander under the temperature of 50 to 300 DGE C and at last are sintered to stereotype under the temperature of 200 to 400 DGE C, in this way the multi-layer composite film is made. With the method of laminating, the composite film is made into a protective material with machine woven fabric, knitted fabric or non-woven fabric through bonds. The protective material has a steam permitting capability of 3000 to 12000g per square meters per 24 hours, a hydraulic pressure bearing capability of 50 to 220 thousand pa, the filtration efficiency for the microorganism in the air equal to or more than 99 percent, and the filtration efficiency of the polioviruses in liquid equal to or more than 99 percent. The capability of the invention does not impair after washing for a plurality of times. The protective material can be widely used as biochemical protection material, waterproof and permeable material, and windproof and warm keeping material.
Owner:THE QUARTERMASTER EQUIPMENT RESEARCH INSTITUTE OF THE GENERAL LOGISITIC DEPARTME
Recombinant poliovirus for the treatment of cancer
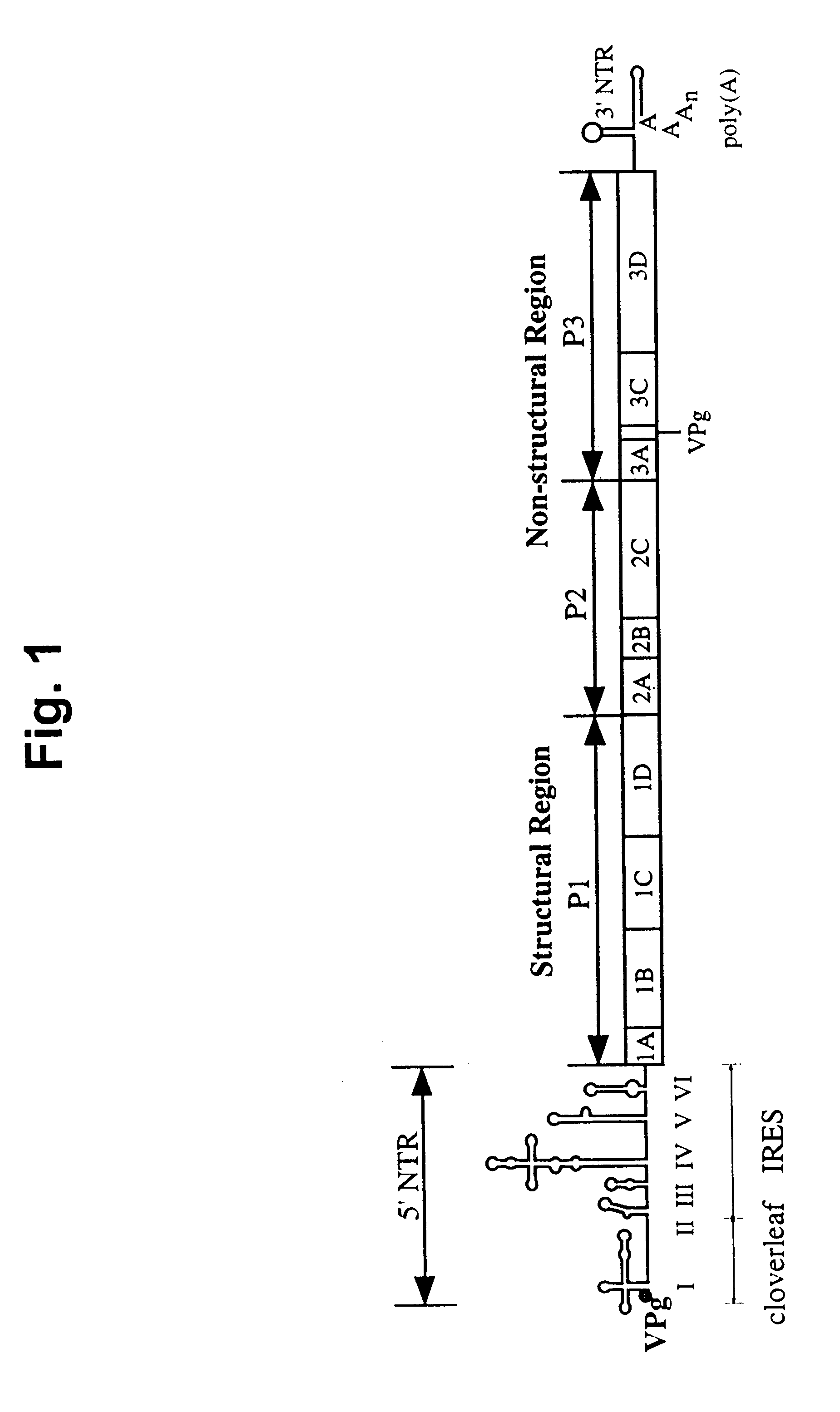
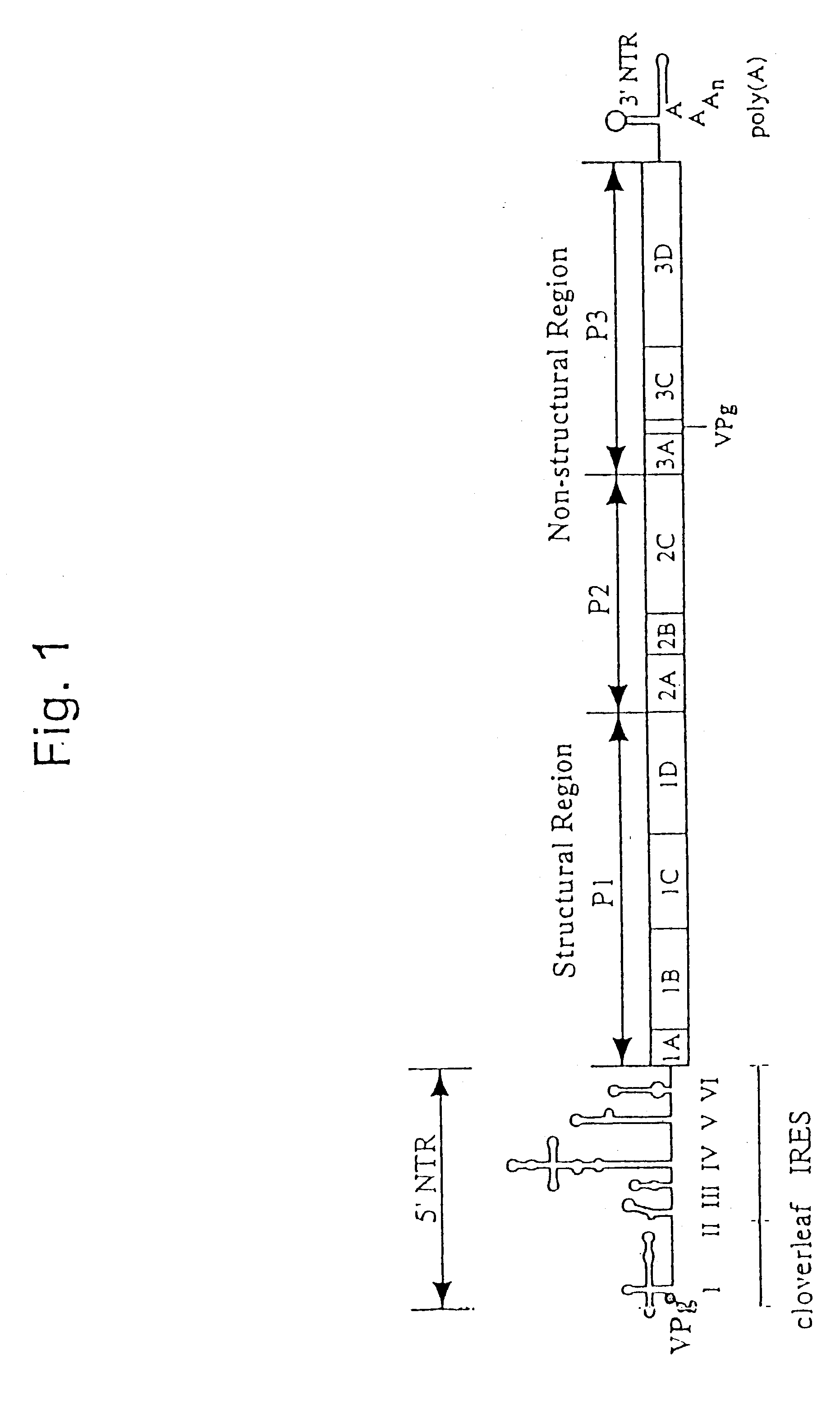
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:NEW YORK UNIV OF RES FOUND OF THE
Fatty acid composition
ActiveCN101233852AImprove stabilityImprove the bactericidal effectBiocideSurface-active detergent compositionsAlkaneForm solution
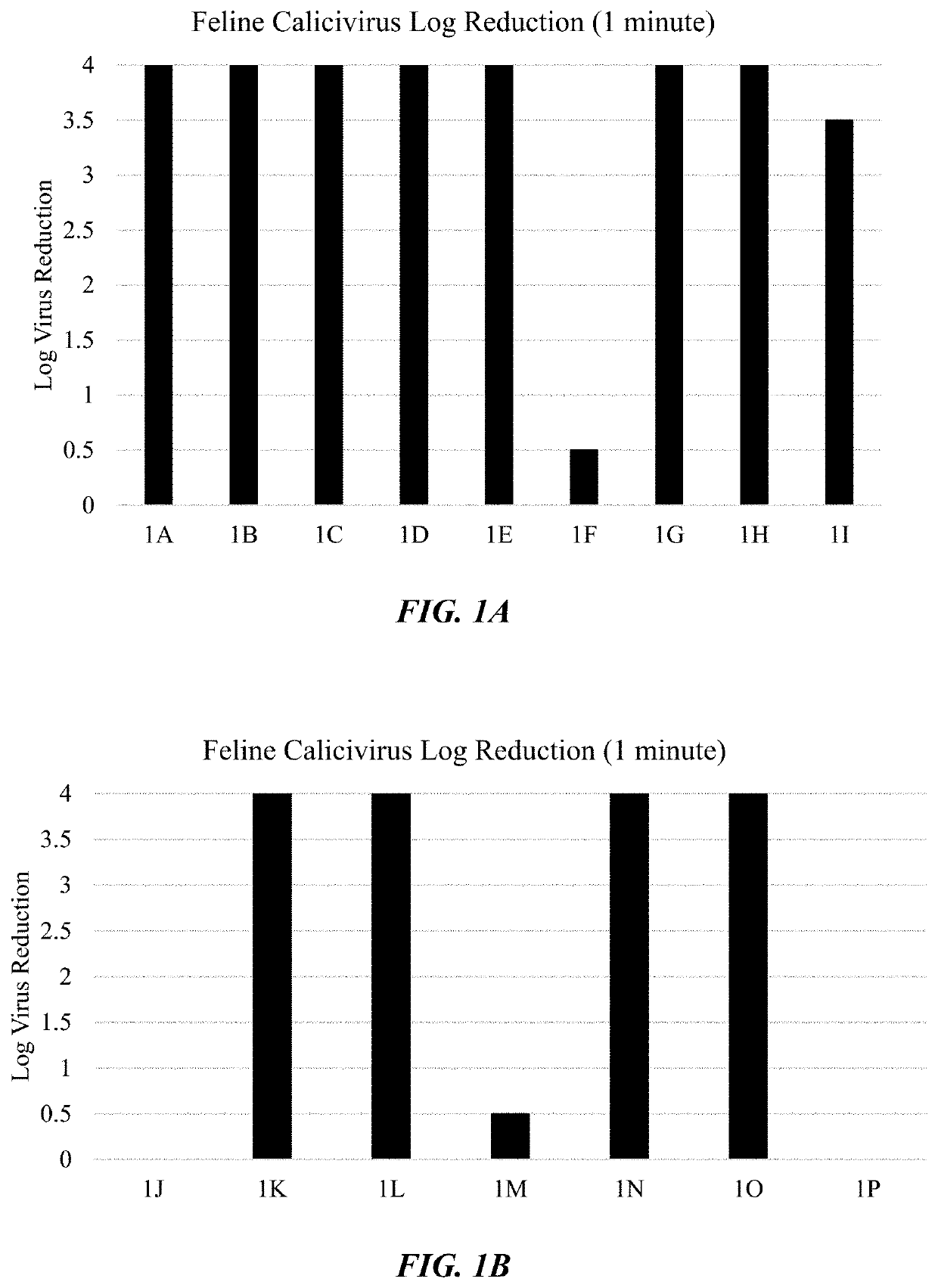
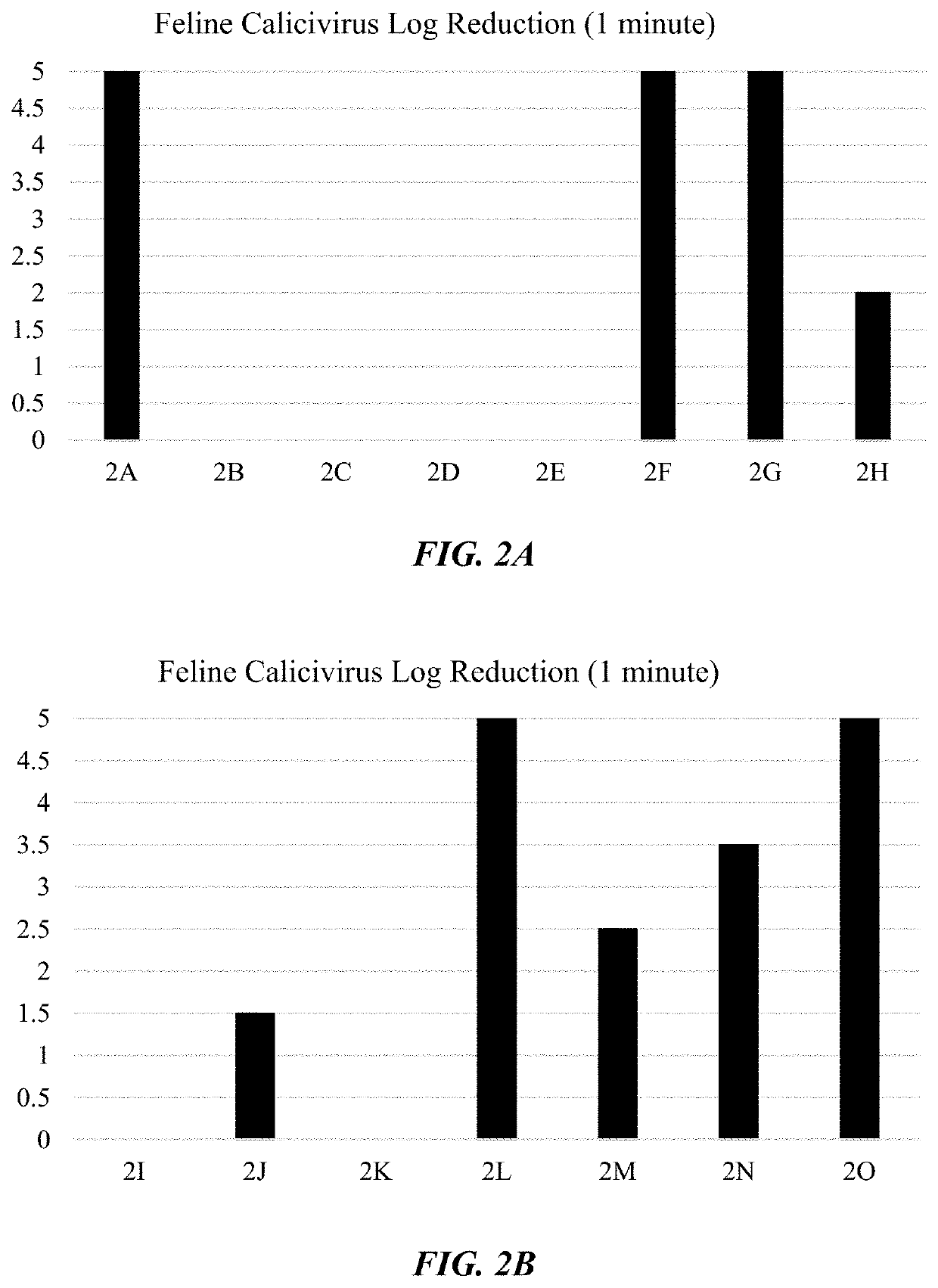
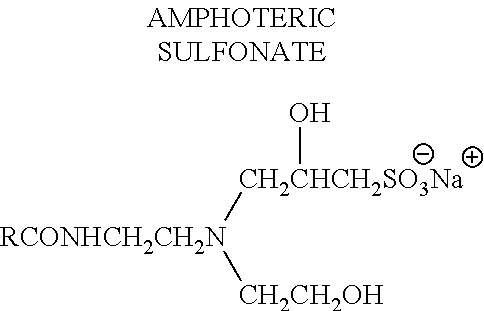
The invention relates to a fatty acid composition, which is diluted at normal temperature and in cold water to form solution used for cleaning of sterilization, fungicide and antivirus. The fatty acid composition comprises at least the following components: component A: food fatty acid comprising at least octyl decanoic acid and nonanoic acid; component B: surfactant comprising alkane sulfonate; component C: acid. The fatty acid composition has the advantages that the fatty acid composition or the diluted solution thereof has good stability, bactericidal and antiviral activity under the water temperature of 4-20 DEG C, no phase separation or crystallization, thus having safer use performance, and active components are low in cost, having more broad-spectrum bactericidal activity which can kill various bacteria, epiphytes and viruses. The fatty acid composition or the diluted solution thereof can be used for sterilization, fungicide and antivirus, which has high-efficient and quick killing effect; the sterilizing rate can reach more than 99.999 percent with contact time less than 1 minute; The fatty acid composition or the diluted solution thereof also has strong cleaning ability, and can keep effective even in hard water; the killing ratio for poliovirus reaches 99.9999 percent and the poliovirus can be killed immediately.
Owner:SICHUAN LOMON BIO TECH CO LTD +1
Recombinant poliovirus for the treatment of cancer
The present invention is directed to non-pathogenic, oncolytic, recombinant polioviruses for the treatment of various forms of malignant tumors. The recombinant polioviruses of the invention are those in which the internal ribosomal entry site (IRES) of the wild type poliovirus was exchanged with the IRES of other picornaviruses, and optionally P1, P3 or the 3'NTR thereof was exchanged with that of poliovirus Sabin type. More particularly, the present invention is directed to the administration of the non-pathogenic, oncolytic, recombinant poliovirus to the tumor directly, intrathecally or intravenously to cause tumor necrosis. The method of the present invention is particularly useful for the treatment of malignant tumors in various organs, such as: breast, colon, bronchial passage, epithelial lining of the gastrointestinal, upper respiratory and genito-urinary tracts, liver, prostate and the brain. Astounding remissions in experimental animals have been demonstrated for the treatment of malignant glioblastoma multiforme, an almost universally fatal neoplasm of the central nervous system.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Cell lines for virus production and methods of use
InactiveUS20150374812A1SsRNA viruses positive-senseViral antigen ingredientsExpression geneEnterovirus 71
Provided herein are engineered cell lines. In some embodiments, cells of an engineered cell line have altered expression of a gene and / or altered expression of an miRNA, wherein the altered expression results in increased or decreased production of a virus. The virus is a picomavirus, such as a poliovirus or Enterovirus 71. Also provided herein are methods for using the engineered cells to produce virus, and methods for treating a subject having or at risk of having a viral infection.
Owner:UNITED STATES OF AMERICA +2
Multivalent immunogenic composition
ActiveCN103394082AImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
Multi-component vaccine comprising at least three antigens to protect against disease cased by Haemophilus influenzae
InactiveUS6342232B1No suppression of anti-rHMW responseStimulate immune responseAntibacterial agentsSenses disorderProteolysisDiphtheria vaccination
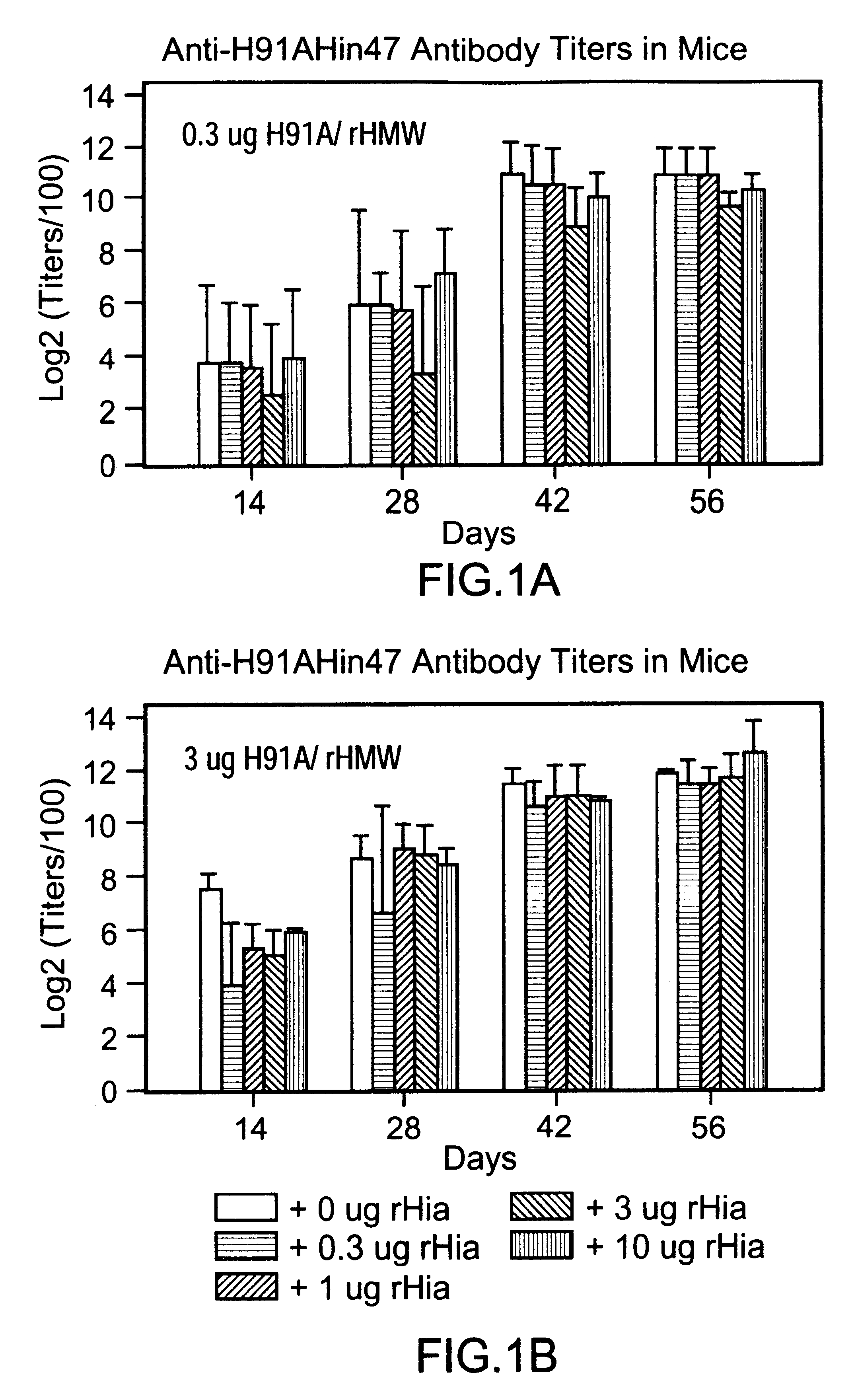
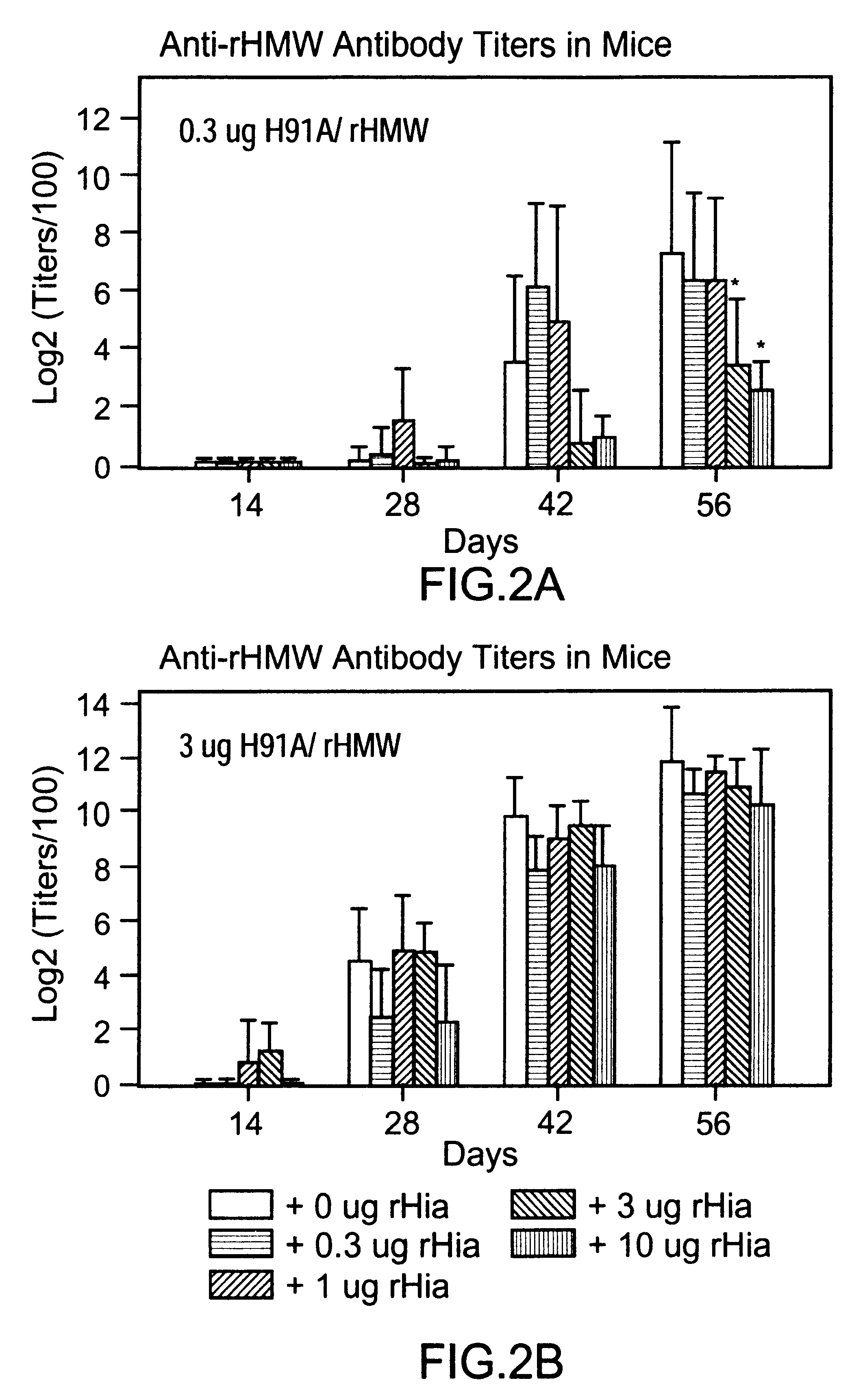
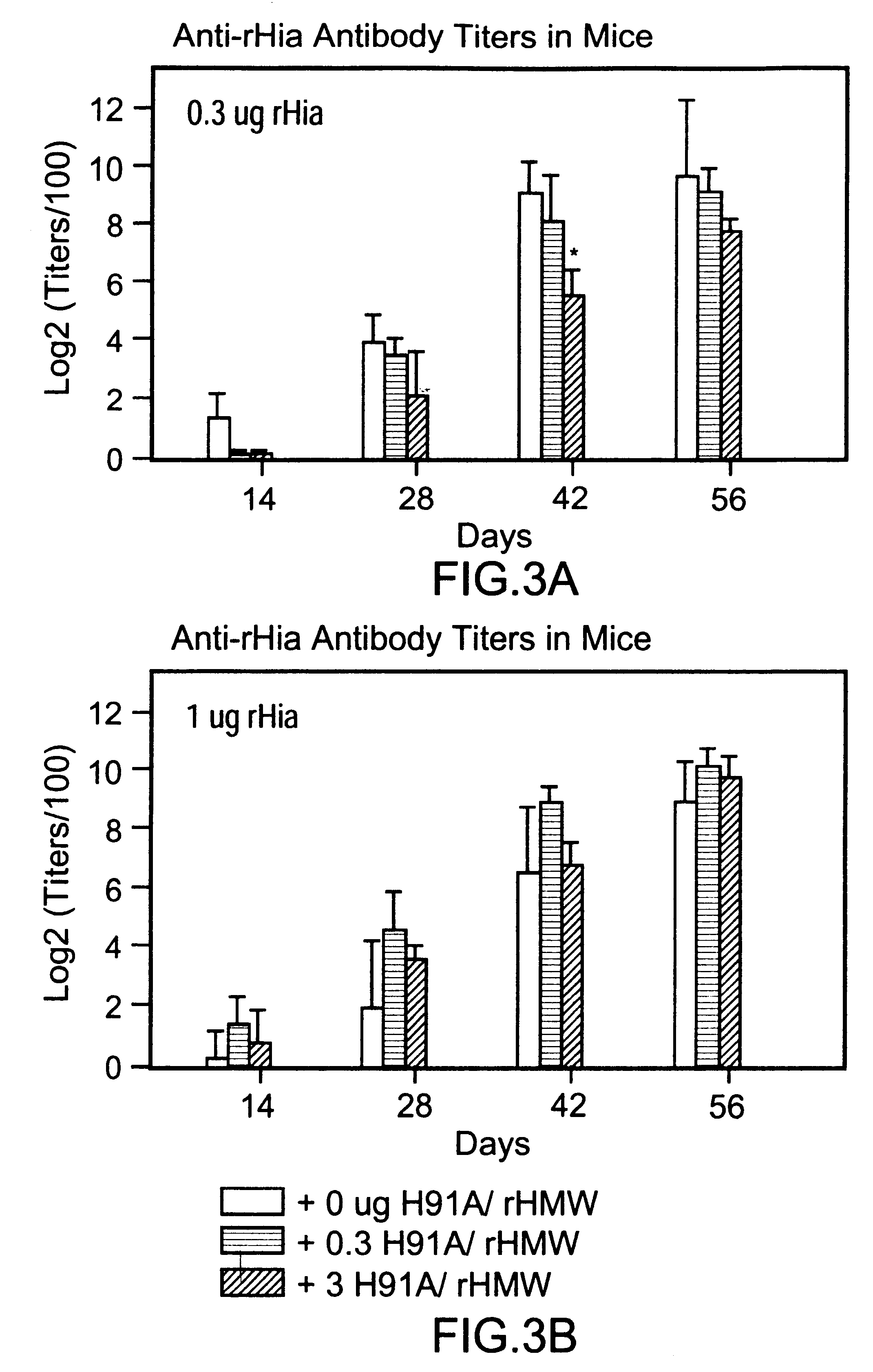
A multi-component immunogenic composition confers protection on an immunized host against infection caused by Haemophilus influenzae. Such composition comprises at least three different antigens of Haemophilus influenzae, two of which are adhesins. High molecular weight (HMW) proteins and Haemophilus influenzae adhesin (Hia) proteins of non-typeable Haemophilus influenzae comprise the adhesin components while the other antigen is a non-proteolytic analog of Hin47 protein. Each component does not impair the immunogenicity of the others. The Haemophilus vaccine may be combined with DTP component vaccines, which may contain inactivated poliovirus, including type 1, type 2 and / or type 3, and / or a conjugate of a capsular polysaccharide of Haemophilus influenzae and tetanus or diphtheria toxoid, including PRP-T, to provide a multi-valent component vaccine without impairment of the immunogenic properties of the other antigens.
Owner:AVENTIS PASTUER LTD
Gene chip and reagent box for detecting food-borne virus
InactiveCN101328505AGuaranteed reproducibilityGuaranteed accuracyMicrobiological testing/measurementAgainst vector-borne diseasesFood borneRotavirus RNA
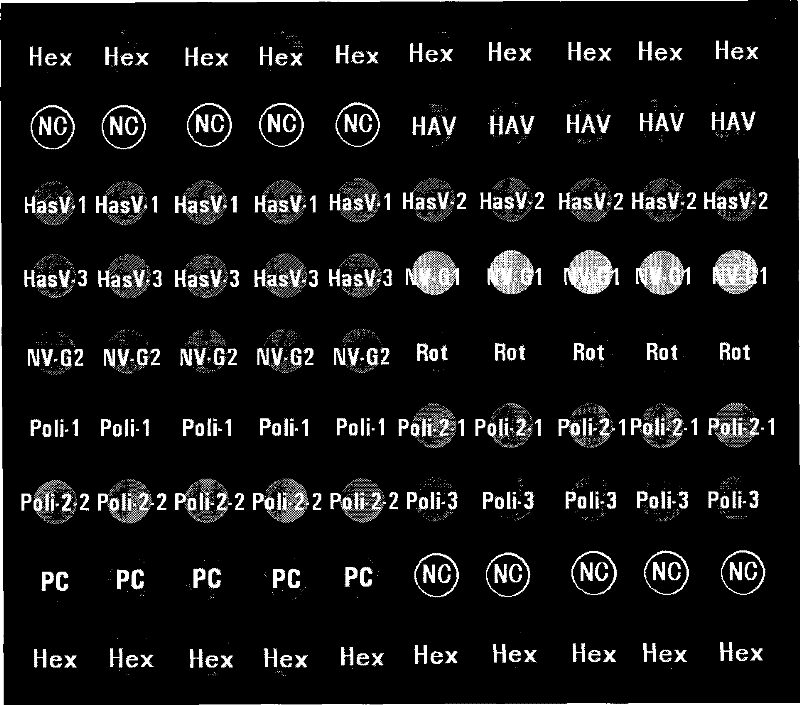
The invention relates to a gene chip for detecting food-home virus and a kit, belonging to the inspection field. The surface of a solid phase carrier is fixed with a plurality of detection probes and quality control contrast probes, wherein, the detection probes consist of the probes for detecting hepatitis A virus, human astrovirus, norwalk virus G1, norwalk virus G2, rotavirus, polio virus 1, polio virus 2 and polio virus 3; and the quality control contrast probes consist of a spotting positive quality control probe, a chip hybridization positive quality control probe and a chip negative quality control probe. The gene chip and the kit have the advantages of: (1) high throughout: five common viruses are integrated and detected simultaneously, and the practicability is strong; (2) rapidness: the detection time is only 4 hours; (3) specificity: the false positive caused by the cross reaction is avoided; (4) flexibility: the detection flexibility of the chip is 10<8> virus particles per gram of the tissue sample, which is higher than that of RT-PCR; and (5) good repetitiveness and stable results. The gene chip and the kit can be widely applied to the food safety inspection of the inspections system.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Broad Spectrum Inhibitors of the Post Proline Cleaving Enzymes for Treatment of Hepatitis C Virus Infections
InactiveUS20150202218A1Increase secretionBiocidePeptide/protein ingredientsEcho virusesCOXSACKIE A VIRUS
Disclosed are methods of treating, inhibiting, or preventing a viral infection in a mammal in need thereof by administering a therapeutically or prophylactically effective amount of an inhibitor of FAP, an inhibitor of DPPIV, an inhibitor of DPP8, or an inhibitor of DPP9. The inhibitor may act as both an inhibitor of DPPIV and an inhibitor of DPP8 / 9. The viral infection includes, but is not limited to, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, Polio virus, Coxsackie A virus, Coxsackie B virus, Rhino virus, respiratory syncytial virus, dengue virus, equine infectious anemia virus, Echo virus, small pox virus, Ebola virus, and West Nile virus.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
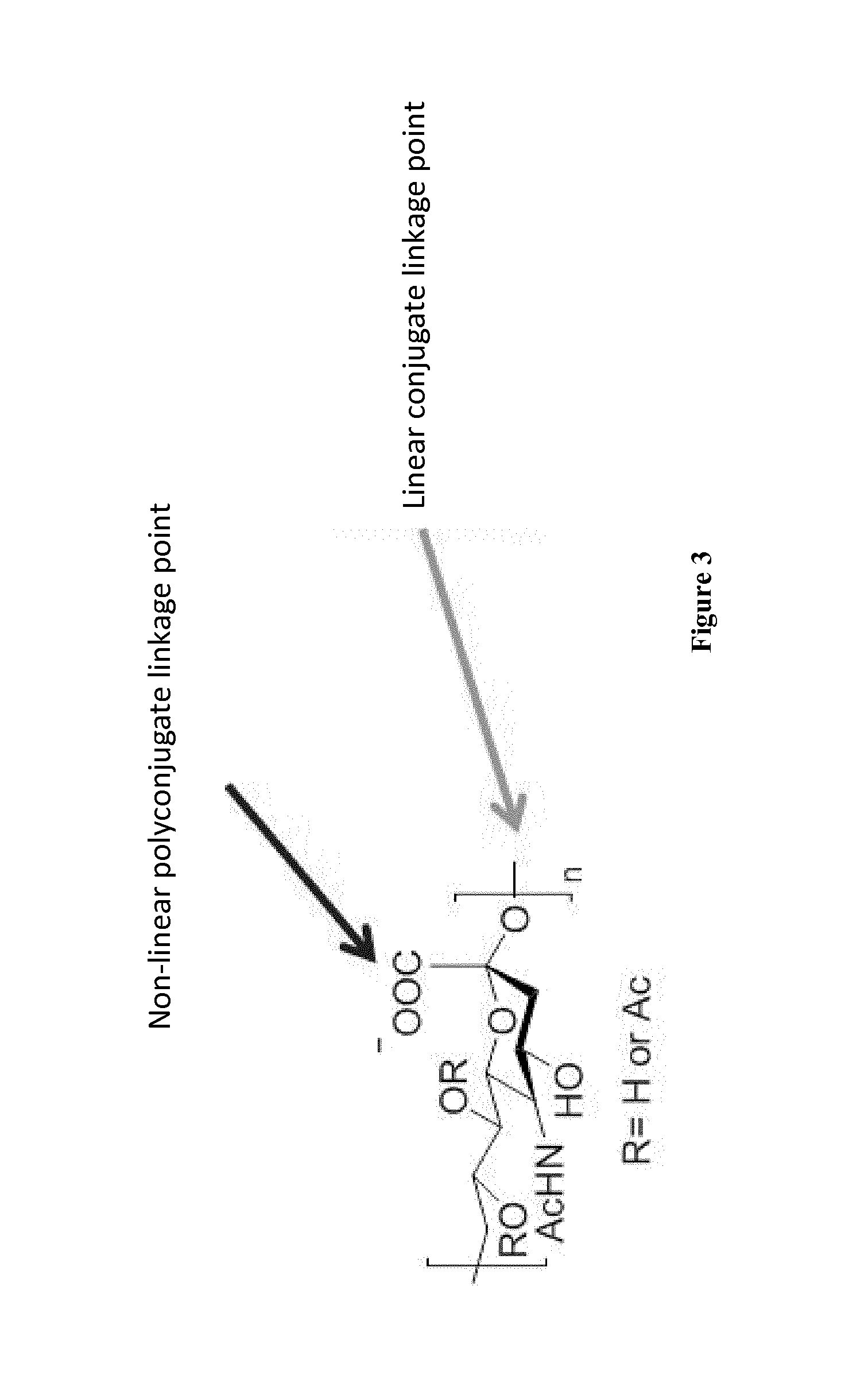
Nonlinear saccharide conjugates
InactiveUS20160101187A1Enhance immune responseIncrease in activated macrophagesAntibacterial agentsAntimycoticsB-Cell EpitopesPolysaccharide
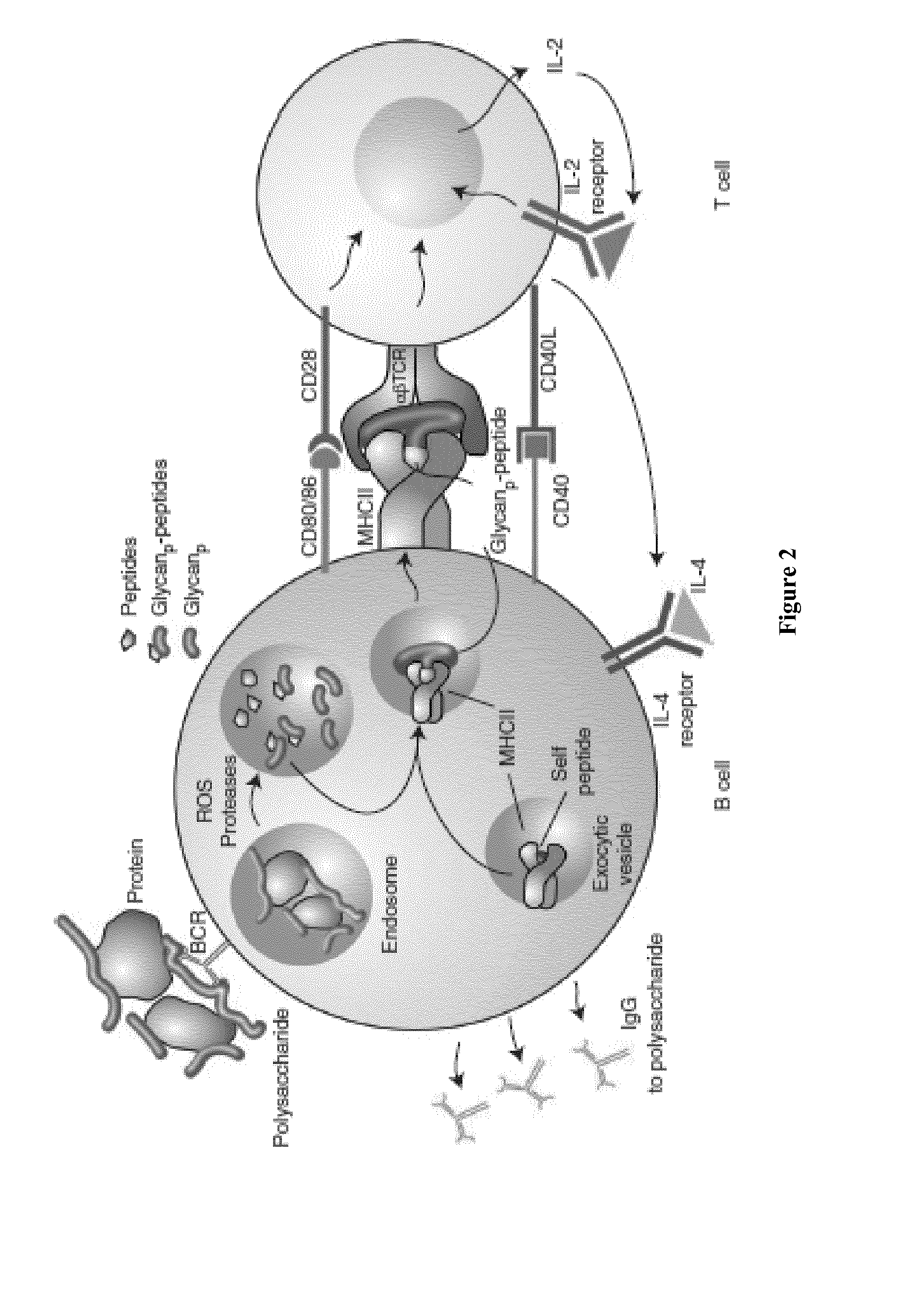
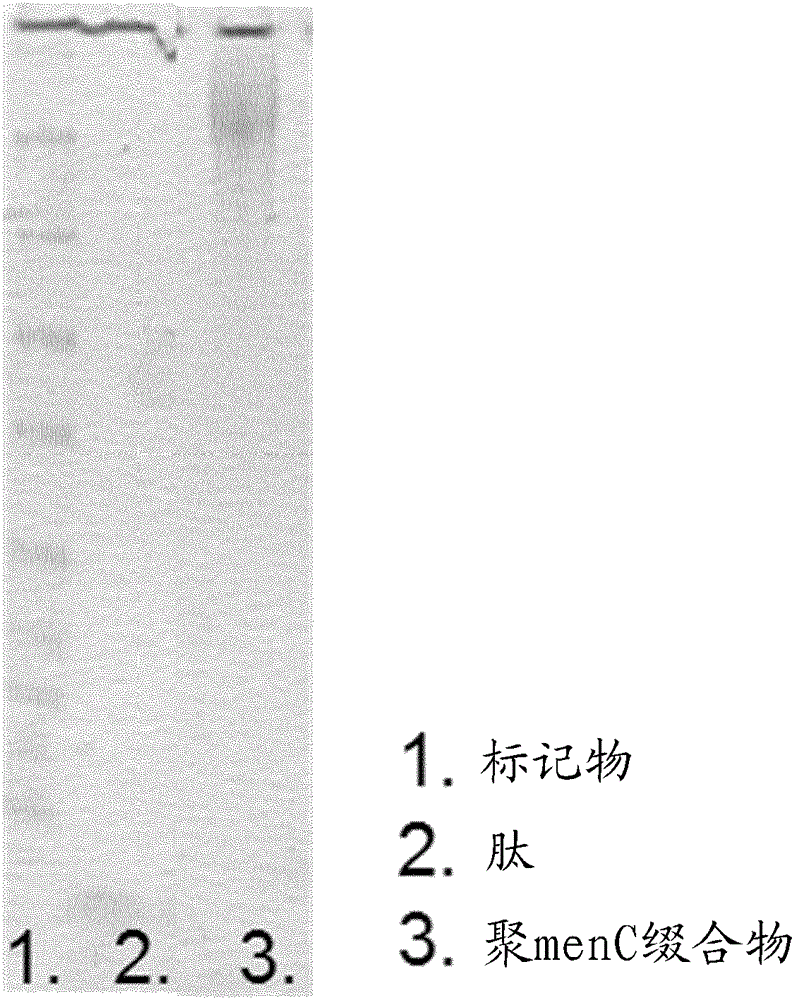
This specification is directed to nonlinear saccharide conjugates that comprise polysaccharides that are linked to at least two peptides that comprise T-cell epitopes and have no conformational B-cell epitopes where one of the peptides is linked to an internal saccharide so that the conjugates have a branched (i.e., nonlinear) structure. The specification also provides methods of manufacturing these conjugates, methods of formulating these conjugates in compositions for use as vaccines and methods of using the compositions to induce an immune response to the capsular saccharide. The specification also provides a new polyepitope carrier peptide comprising the PV1 epitope from polio virus. The new polyepitope carrier peptide can be used in both linear saccharide conjugates as well as the nonlinear saccharide conjugates.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Inactivated Poliomyelitis Vaccine Derived From Sabin Strain Of Polio Virus
InactiveUS20080193478A1Effective immunizationEffective and stableSsRNA viruses positive-senseViral antigen ingredientsAntigenHuman immunodeficiency
An inactivated Polio Vaccine derived from Sabin strain for safe and effective immunization against Poliomyelitis is provided. A process of preparation for such vaccine and formulations thereof are also provided. Administration of the vaccine of the present invention along with other antigens provides immunization not only against polio infection but also against other pathogens causing Hepatitis C. Hepatitis D. Hepatitis E. Meningitis A. Meningitis B. Meningitis C. Meningitis W. Meningitis Y. Pnemococcal (23 valent or more). Smallpox, Typhoid, Bacille Calmette Guerin, Tuberculosis. Human Immunodeficiency Virus. Anthrax or the like, to which children or adults not immunized earlier are susceptible, particularly to which children are susceptible.
Owner:PANACEA BIOTEC
Vaccine
InactiveCN101534854AIncrease doseIncreased level of protectionViral antigen ingredientsAgainst vector-borne diseasesDiseaseTetanus
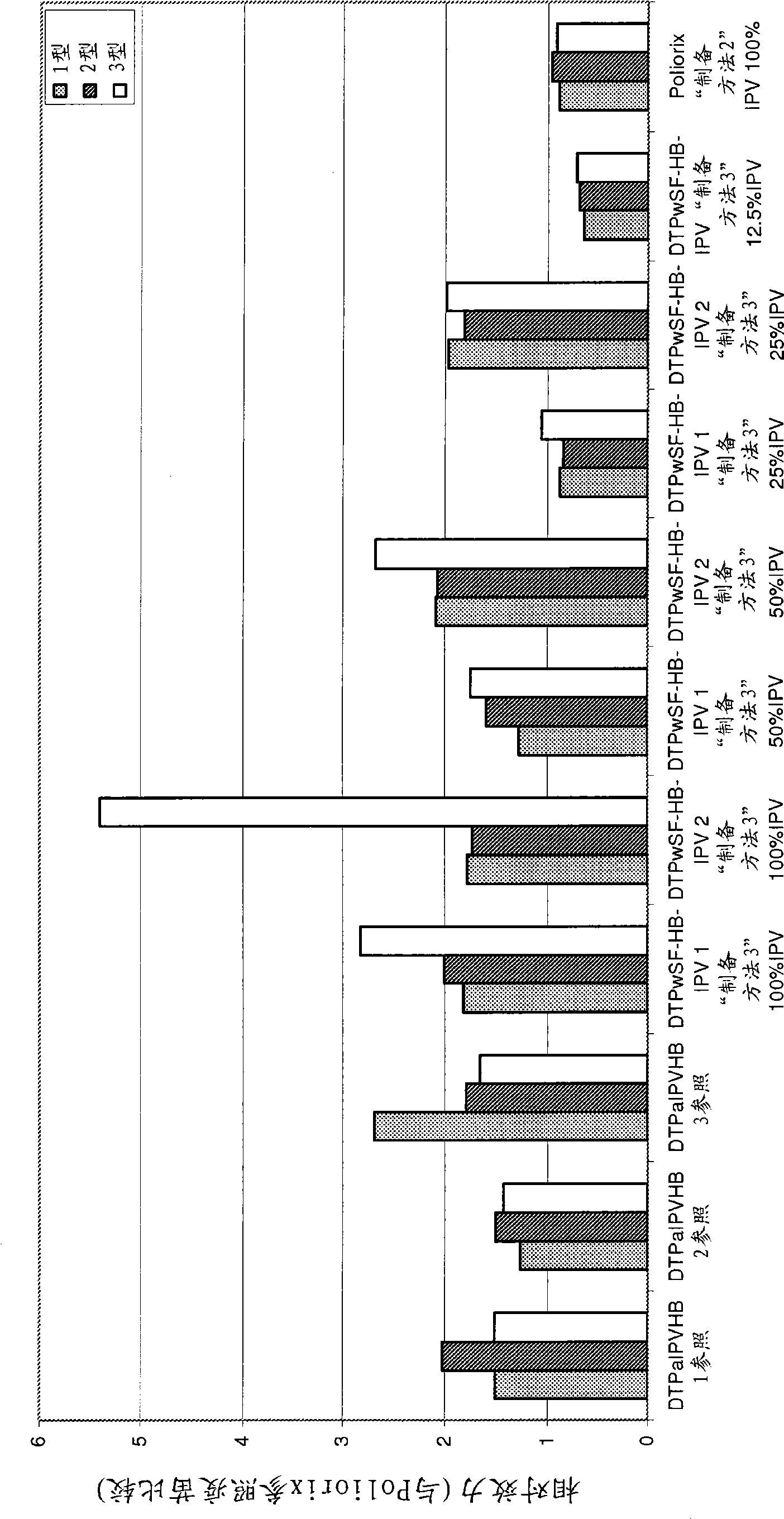
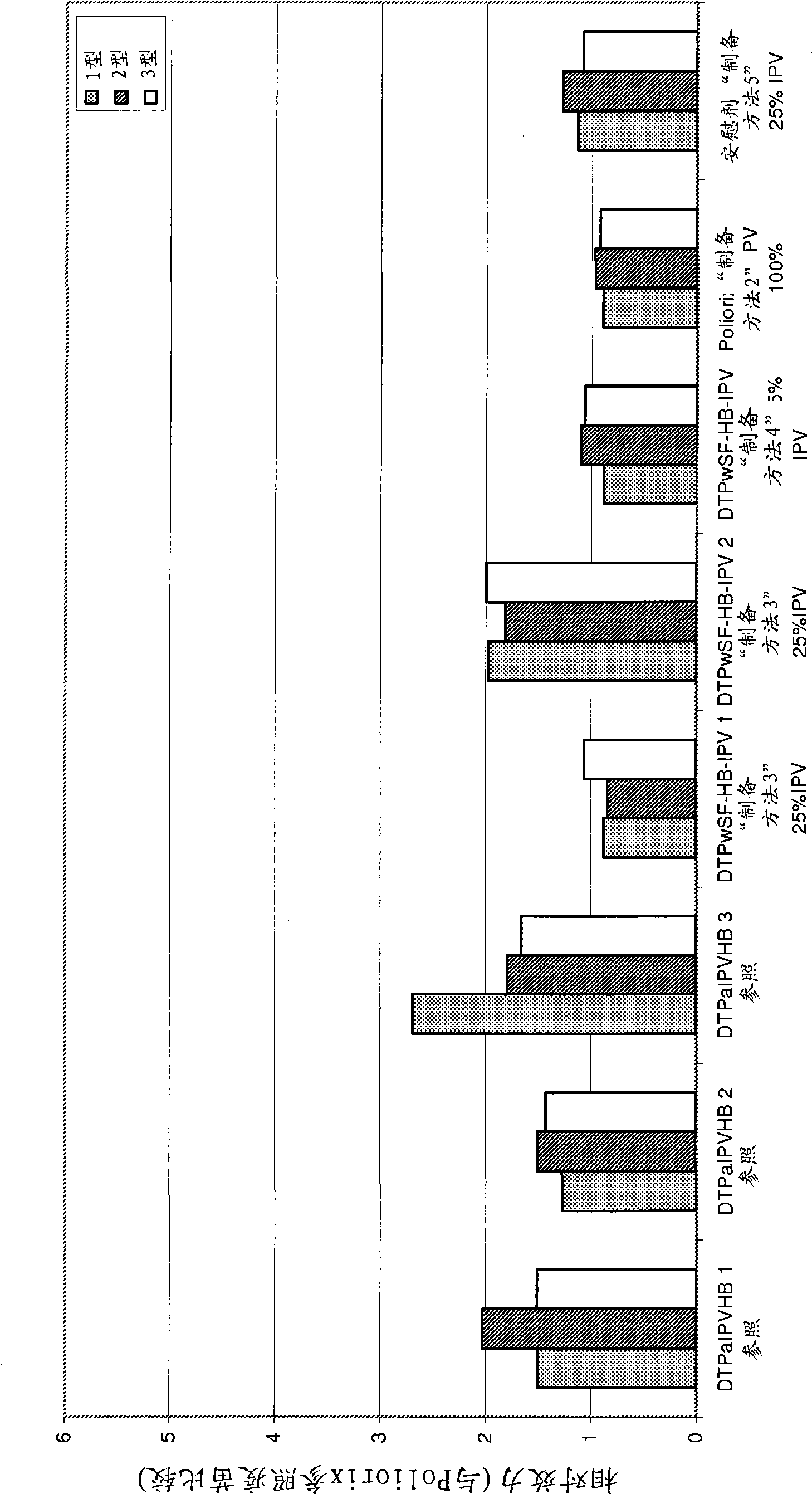
The present invention relates to the field of vaccines for protecting against polio, and in particular to combination vaccines for protecting against polio, diphtheria, tetanus, and pertussis diseases. Specifically, vaccines comprising reduced dose inactivated poliovirus (IPV) is provided, which can maintain an adequate or improved level of protection against polio.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Alcohol disinfectant
Owner:DR SCHUMACHER
Attenuated polio viruses
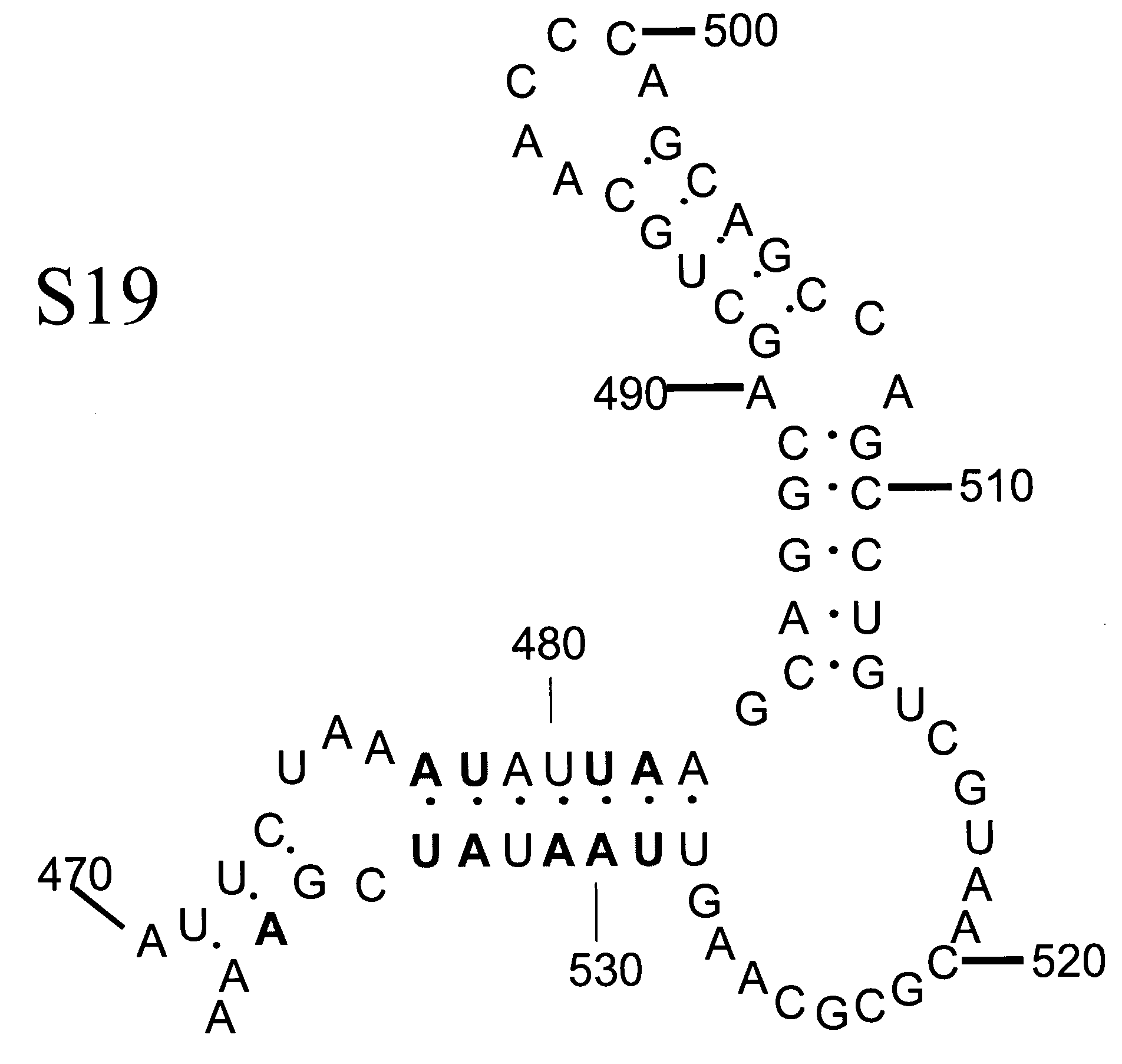
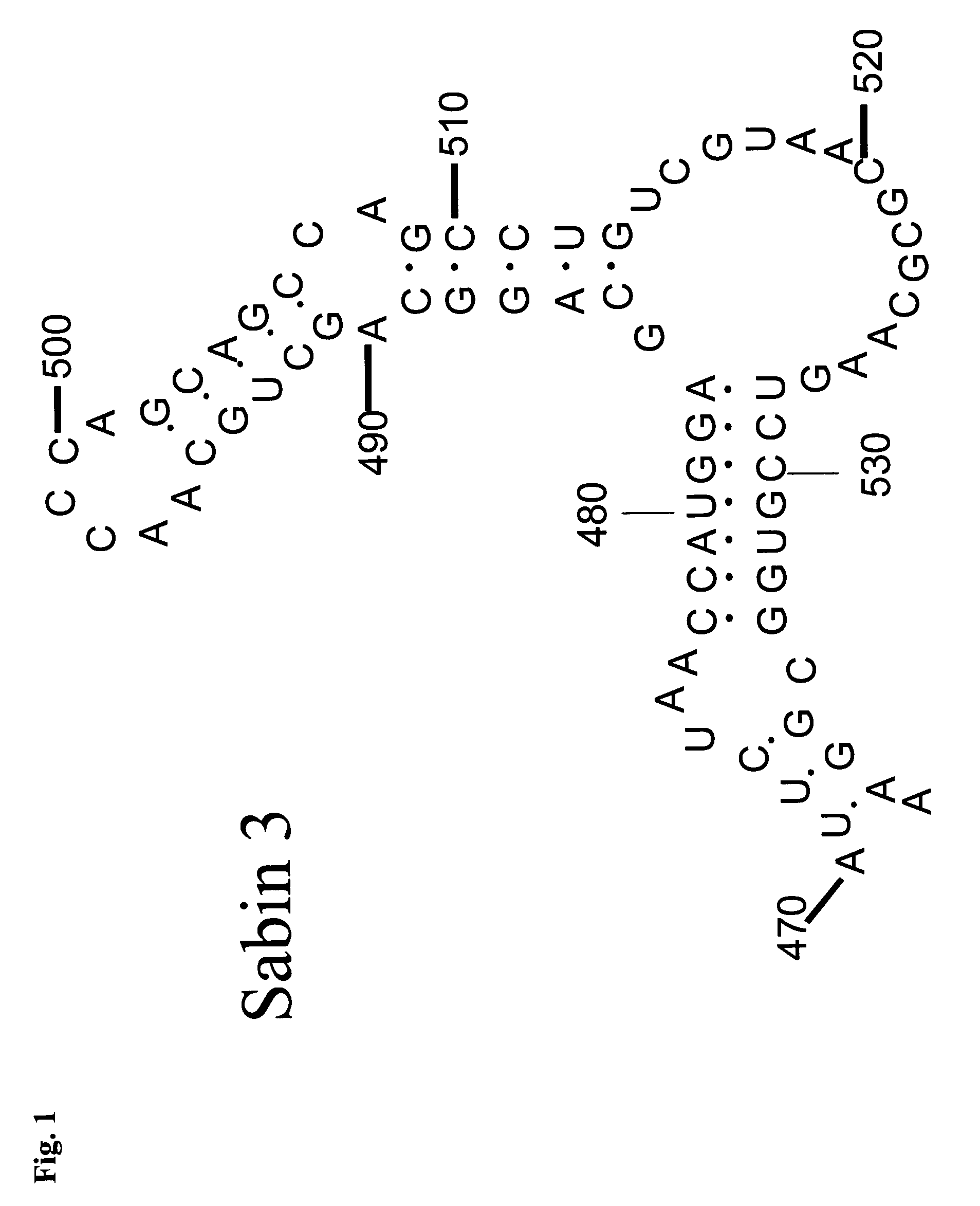
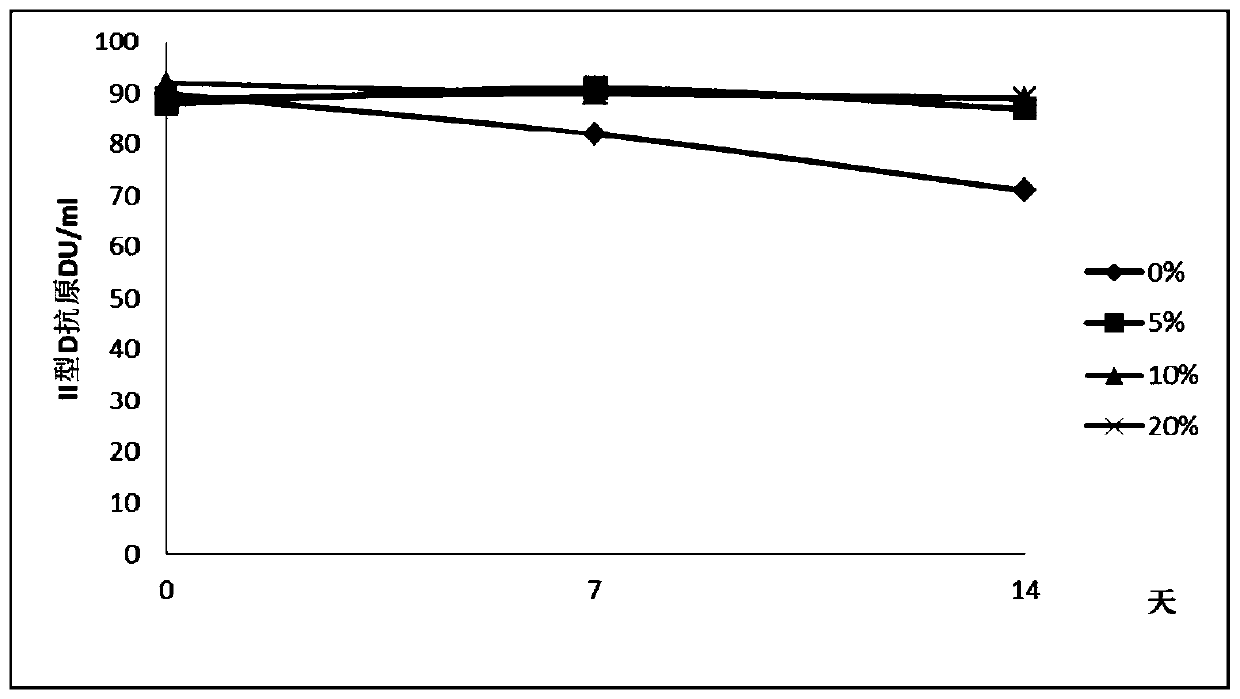
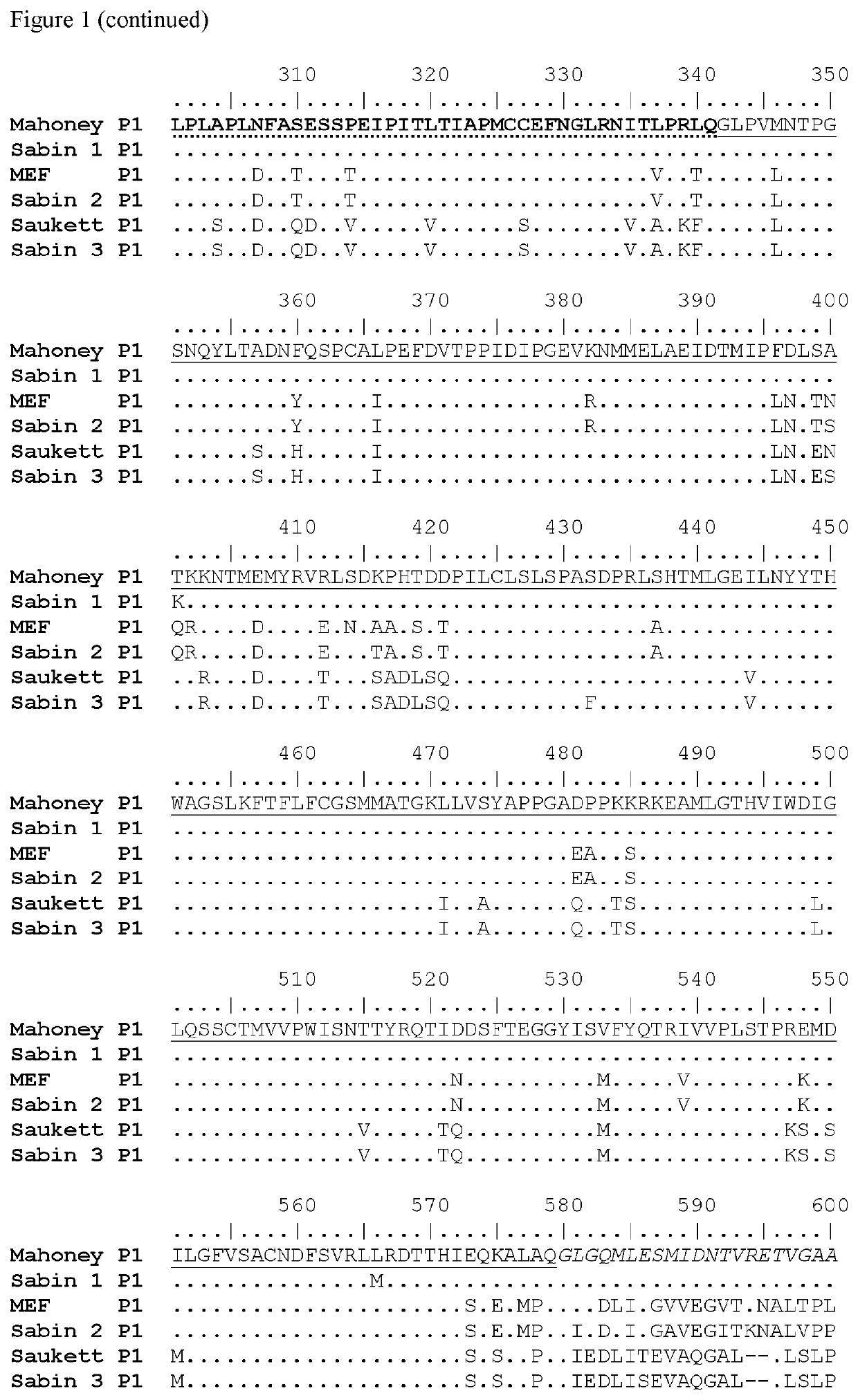
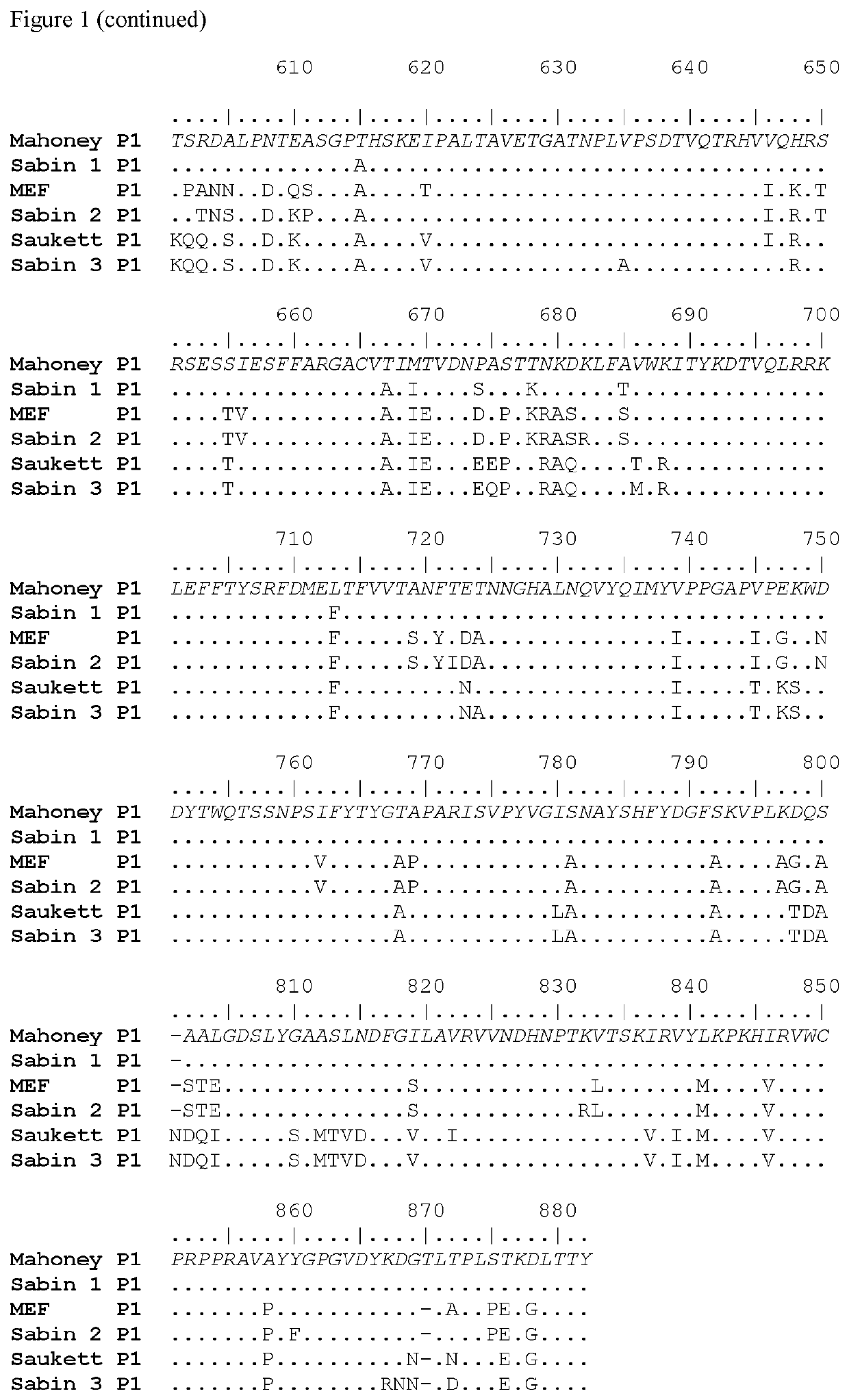
ActiveUS20100158945A1Reduce infectivitySsRNA viruses positive-senseViral antigen ingredientsBase Pair MismatchCoding region
The invention provides an attenuated poliovirus which does not have a base pair mismatch in stem (a) or (b) of domain V of the 5′ non-coding region of its genome, wherein at least seven of the base pairs in stems (a) and (b) are U-A or A-U base pairs.
Owner:SEC OF STATE FOR HEALTH & SOCIAL CARE
Antibodies specific to human poliovirus receptor (PVR)
ActiveUS20200040092A1Good effectReduce tumor cell viabilitySenses disorderAntipyreticTIGITSpecific antibody
The present invention provides monoclonal antibodies that recognize polio virus receptor (PVR) and inhibit its binding to T cell immunoreceptor with Ig and ITIM domains (TIGIT). The present invention further provides pharmaceutical compositions comprising the antibodies and methods for their use in cancer immunotherapy, treating infections and in diagnosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Construction method and application of recombinant enterovirus phenotype hybrid system containing cytokine adjuvant
InactiveCN106754758APractical and wide application valueSsRNA viruses positive-senseViral antigen ingredientsEnterovirusCytopathic effect
The invention provides a construction method of a recombinant enterovirus phenotype hybrid system containing a cytokine adjuvant. The construction method comprises the following steps: firstly respectively constructing a VP1 recombinant cell line and a recombinant attenuated enterovirus strain loaded with cytokine genes; then performing infection amplification on the recombinant attenuated enterovirus strain in the VP1 recombinant cell line; the VP1 recombinant cell line is one of or two of VP1 cell lines for expressing an enterovirus type 71 and a coxsackie virus A group type 16; the recombinant attenuated enterovirus strain loaded with the cytokine genes selects from a coxsackie virus group B type 3, the enterovirus type 71 or a poliovirus type I; a cytokine selects from cholera toxin or interferon gamma; recombinant attenuated enteric viruses loaded with the cytokine genes infect the VP1 recombinant cell line, and after a cytopathic effect, progeny viruses are obtained and can activate host immunity to generate immune protection aiming at a variety of intestinal viruses; therefore, the construction method has application values.
Owner:SHANTOU UNIV MEDICAL COLLEGE
An immunogenic composition having improved stability, enhanced immunogenicity and reduced reactogenicity and process for preparation thereof
ActiveUS20200206331A1Suitable preventionSuitable treatmentBacterial antigen ingredientsSsRNA viruses positive-senseHepatitis B immunizationAdjuvant
An immunogenic composition comprising of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen, Hepatitis B surface antigen (HBsAg), inactivated whole-cell B. pertussis (wP) antigen, Haemophilus influenzae type B (Hib) capsular saccharide conjugated to a carrier protein, Inactivated Polio Virus (IPV) antigen and additionally one or more antigens and the method of preparing the same. A fully liquid combination vaccine, showing improved immunogenicity, reduced reactogenicity and improved stability. Improved methods of formaldehyde inactivation, improved adsorption profile of Diphtheria toxoid antigen (D), tetanus toxoid (T) antigen and Hepatitis B (HepB) surface antigen adsorbed individually onto aluminium phosphate adjuvant, minimum total aluminum content (Al3+) and optimized concentration of 2-phenoxyethanol (2-PE) as preservative.
Owner:SERUM INST OF INDIA PTE LTD
Quaternary ammonium salt composition for killing microorganisms as well as preparation method and application of quaternary ammonium salt composition
The invention relates to the technical field of disinfection, and particularly discloses a quaternary ammonium salt composition for killing microorganisms as well as a preparation method and application of the quaternary ammonium salt composition. The quaternary ammonium salt composition contains or does not contain sodium free ions; when the quaternary ammonium salt composition contains sodium free ions, the mole number of the sodium free ions in the quaternary ammonium salt composition is less than 0.1 mol / 100g; when the quaternary ammonium salt composition contains sodium free ions and the mole number of the sodium free ions is greater than or equal to 0.001 mol / 100g, the quaternary ammonium salt composition also contains potassium free ions and ions / ligands with chelation. According to the invention, a quaternary ammonium salt synergistic system is adopted, ion species are added in a targeted manner, and a non-quaternary ammonium salt surfactant can be compounded, so that the quaternary ammonium salt composition has a very good killing effect on various microorganisms, and especially has very effective inactivation on hydrophilic viruses represented by poliovirus; and moreover, the method is safe to a processed object and wider in application range.
Owner:GUANGZHOU BLUE MOON IND
Nonlinear saccharide conjugates
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Liquid vacuum composition and application thereof
ActiveCN110917344AEfficient inoculationVaccination is done efficientlyAntibacterial agentsBacterial antigen ingredientsHemagglutininTetanus toxoids
The invention relates to a liquid vacuum composition which comprises diphtheria toxoid, tetanus toxoid, pertussis toxins, filamentous hemagglutinin, pertactin, inactivated poliovirus, type-b haemophilus influenzae capsular polysaccharide-protein conjugate and meningococcus capsular polysaccharide-protein conjugate, wherein the inactivated poliovirus comprises type-I, type-II and type-III poliovirus Sabin strains. By adopting the liquid vacuum composition provided by the invention, security risks caused by freeze-drying processes and limits of production capacities can be avoided, and meanwhile, a tedious redissolution process needed before immunization of a freeze-dried vacuum is avoided. The liquid vacuum composition provided by the invention is capable of preventing six infectious diseases simultaneously, so that the workload of inoculation and the burden of children can be greatly reduced.
Owner:SINOVAC RES & DEV
Inactivated poliovaccine
ActiveUS20130344108A1Improving immunogenicityImprove scalabilitySsRNA viruses positive-senseViral antigen ingredientsPoliomyelitisBase Pair Mismatch
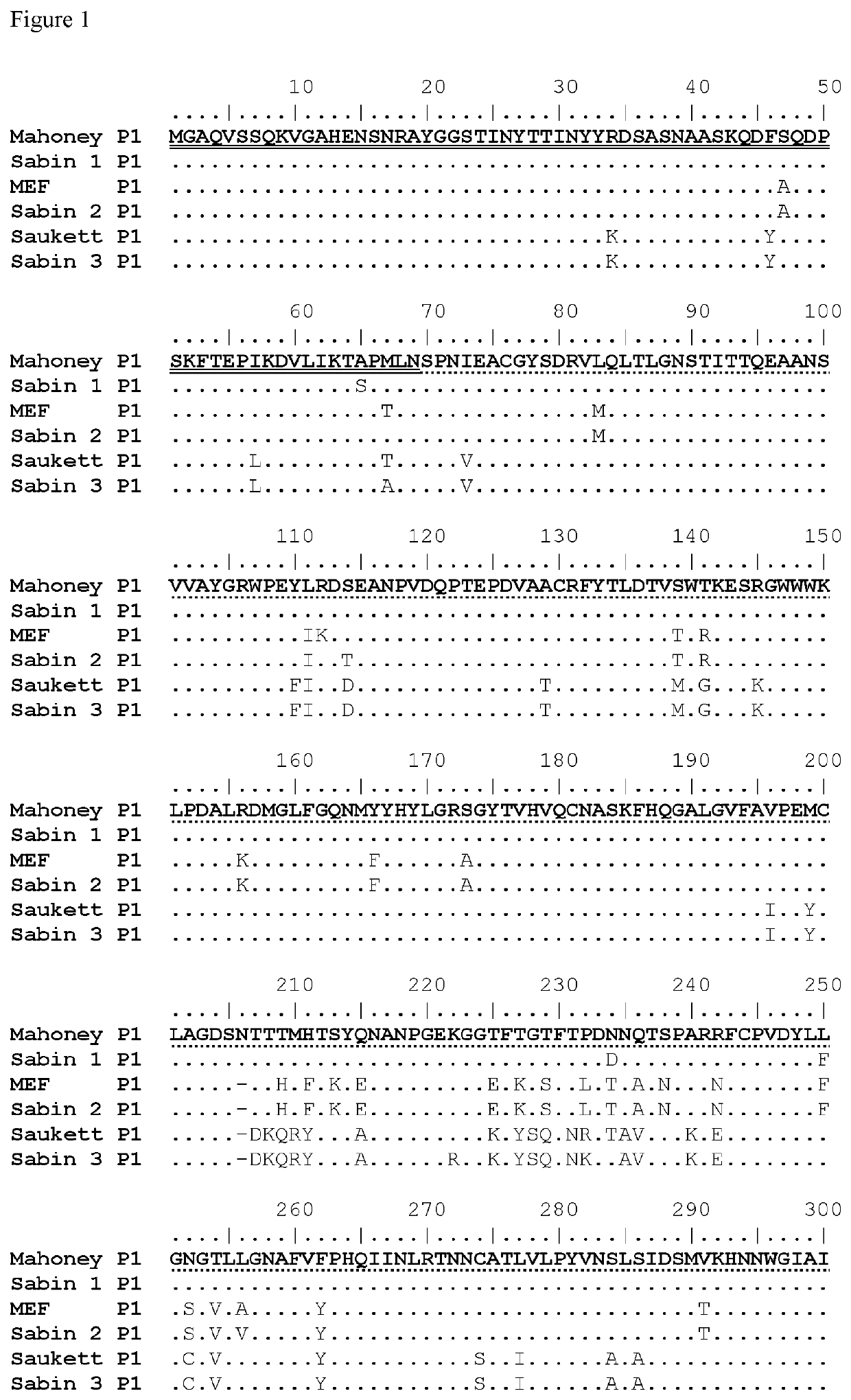
The invention provides an attenuated polio virus having a 5′ non-coding region consisting of the 5′ non-coding region of Sabin 3, modified so that it does not have a base pair mismatch in stem (a) or (b) of domain V, wherein seven or eight of the base pairs in stems (a) and (b) are U-A or A-U base pairs; and a capsid protein from the Sabin 1, Mahoney, MEF or Saukett strain.
Owner:SEC OF STATE FOR HEALTH & SOCIAL CARE
Process for the purification of poliovirus from cell cultures
ActiveUS10294460B2Increase cell densityHigh yieldSsRNA viruses positive-senseAgainst vector-borne diseasesPoliomyelitisVirus
Owner:JANSSEN VACCINES & PREVENTION BV
Hard surface cleaning solution with rapid viricidal activity
PendingUS20200323199A1Enhance killing activityStrong kill rateBiocideSurface-active detergent compositionsBiotechnologyPoliomyelitis
The invention relates to fast-acting viricidal compositions and methods of using the same, wherein the compositions are capable of removing soil and effectively inactivating a wide variety of viral pathogens, particularly small, non-enveloped viruses such as poliovirus and norovirus. The compositions are especially useful on hard surfaces, and can easily be applied to a service to provide rapid viricidal efficacy.
Owner:ECOLAB USA INC
Poliovaccine
PendingUS20190358315A1High protection levelModulate capsid stabilitySsRNA viruses positive-senseViral antigen ingredientsAntigenPoliomyelitis
Owner:SEC OF STATE FOR HEALTH & SOCIAL CARE MINISTERIAL CORRESPONDENCE & PUBLIC ENQUIRIES DEPT OF HEALTH & SOCIAL CARE
Inactivated poliovaccine
ActiveUS9402892B2Improving immunogenicityImprove scalabilitySsRNA viruses positive-senseViral antigen ingredientsPoliomyelitisBase Pair Mismatch
The invention provides an attenuated polio virus having a 5′ non-coding region consisting of the 5′ non-coding region of Sabin 3, modified so that it does not have a base pair mismatch in stem (a) or (b) of domain V, wherein seven or eight of the base pairs in stems (a) and (b) are U-A or A-U base pairs; and a capsid protein from the Sabin 1, Mahoney, MEF or Saukett strain.
Owner:SEC OF STATE FOR HEALTH & SOCIAL CARE
Composition and method for stabilising vaccines in a solid dosage format
InactiveUS20210252132A1SsRNA viruses positive-senseHydroxy compound active ingredientsMonosodium glutamatePolyvinyl alcohol
A composition for stabilising a vaccine in a solid dosage format is provided wherein the composition comprises an antioxidant, such as glutathione, a monosaccharide or disaccharide sugar, such as trehalose, a polyol sugar, such as sorbitol, one or more salts, such as magnesium chloride and sodium glutamate, and a vaccine. The composition may also comprise an aqueous soluble polymer, such as polyvinyl alcohol (PVA). A preferred composition comprises 40 mM glutathione, 20% w / v trehalose, 3% w / v sorbitol, 5% w / v PVA, 3% w / v magnesium chloride and 3% w / v sodium glutamate. Also provided is a method of stabilising a vaccine in a solid dosage format, the method comprising drying the stabilising composition to provide the vaccine in the solid dosage format. The composition and method may be used to stabilise any suitable vaccine, such as poliovirus or adenovirus, in a solid dosage format, such as microneedle patches or wafers.
Owner:UNIV COLLEGE CORK NAT UNIV OF IRELAND CORK
Multivalent immunogenic composition
ActiveCN103394082BImprove securitySave the number of seedsBacterial antigen ingredientsViral antigen ingredientsHemagglutininTetanus toxoids
The invention provides a multivalent immunogenic composition, which includes an inactivated hepatitis A antigen and an inactivated poliovirus. The composition also can further include over one or two of a purified pertussis antigen, diphtheria toxoid, tetanus toxoid, filamentous hemagglutinin, Haemophilus influenzae type b polysaccharide, Neisseria meningitidis capsular polysaccharide, a hepatitis B virus antigen, enterovirus 71 and a coxsackievirus A16 antigen, and a physiologically acceptable carrier. The composition involved in the invention is employed to immunize the inoculated population in the form of a bivalent vaccine or more combined vaccines. Without reducing the immune effects of each immunizing antigen, the inoculation number of times can be reduced at the same time, and the time and human resources can also be saved.
Owner:SINOVAC BIOTECH
Antibodies specific to human poliovirus receptor (PVR)
ActiveUS10906987B2Return to normal activitiesStrong specificitySenses disorderAntipyreticTIGITSpecific antibody
The present invention provides monoclonal antibodies that recognize polio virus receptor (PVR) and inhibit its binding to T cell immunoreceptor with Ig and ITIM domains (TIGIT). The present invention further provides pharmaceutical compositions comprising the antibodies and methods for their use in cancer immunotherapy, treating infections and in diagnosis.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD +1
Gene chip and reagent box for detecting food-borne virus
InactiveCN101328505BGuaranteed reproducibilityGuaranteed accuracyMicrobiological testing/measurementAgainst vector-borne diseasesFood borneRotavirus RNA
The invention relates to a gene chip for detecting food-home virus and a kit, belonging to the inspection field. The surface of a solid phase carrier is fixed with a plurality of detection probes and quality control contrast probes, wherein, the detection probes consist of the probes for detecting hepatitis A virus, human astrovirus, norwalk virus G1, norwalk virus G2, rotavirus, polio virus 1, polio virus 2 and polio virus 3; and the quality control contrast probes consist of a spotting positive quality control probe, a chip hybridization positive quality control probe and a chip negative quality control probe. The gene chip and the kit have the advantages of: (1) high throughout: five common viruses are integrated and detected simultaneously, and the practicability is strong; (2) rapidness: the detection time is only 4 hours; (3) specificity: the false positive caused by the cross reaction is avoided; (4) flexibility: the detection flexibility of the chip is 10<8> virus particles per gram of the tissue sample, which is higher than that of RT-PCR; and (5) good repetitiveness and stable results. The gene chip and the kit can be widely applied to the food safety inspection of the inspections system.
Owner:PEOPLES REPUBLIC OF CHINA BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com