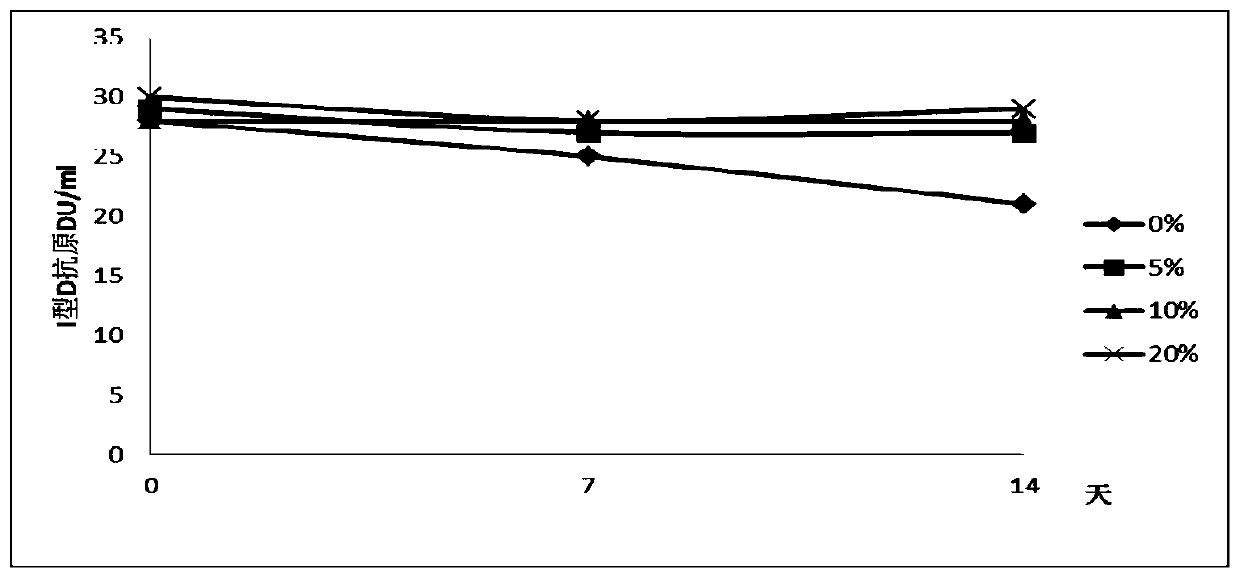
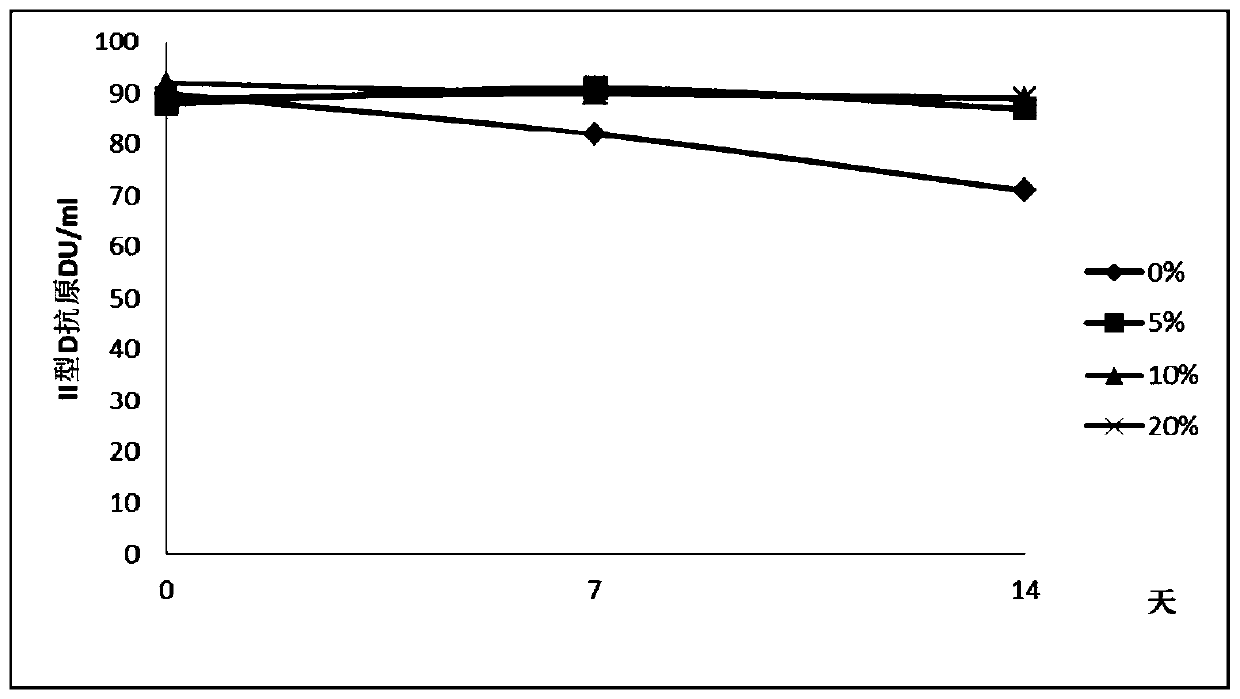
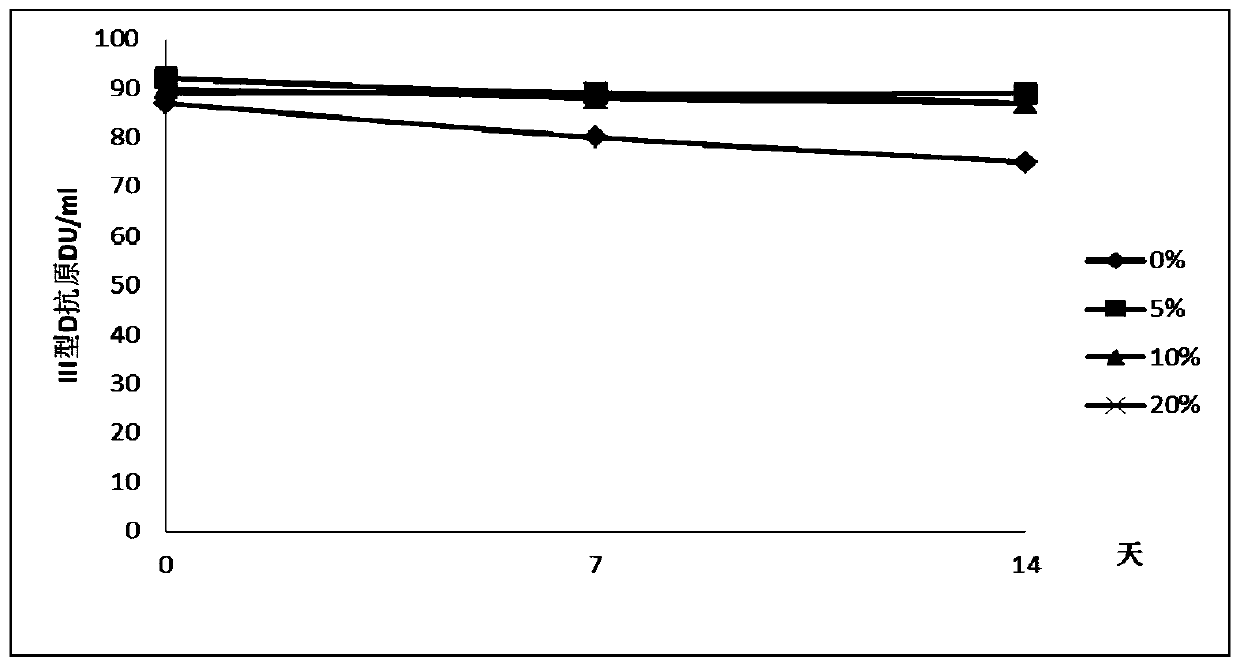
Liquid vacuum composition and application thereof
A liquid vaccine and composition technology, applied in the field of vaccines, can solve the problems of rebound of pertussis incidence, cumbersome reconstitution process, drop in vaccination rate, etc., so as to avoid wrong or missed vaccination, avoid reconstitution process, and reduce workload. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] This embodiment provides a liquid vaccine preparation, which contains the following components per person-time dosage:
[0047] Pertussis toxin 8μg, filamentous hemagglutinin 8μg, pertactin 2.5μg, diphtheria toxoid 2Lf, tetanus toxoid 5Lf, inactivated poliovirus type I stock solution 7.5DU, inactivated poliovirus type II Virus stock solution 22.5DU, inactivated type III poliovirus stock solution 22.5DU, group A meningitis polysaccharide-protein conjugate 10 μg, group C meningitis polysaccharide-protein conjugate 10 μg, Haemophilus influenzae type b polysaccharide-protein conjugate 10μg, aluminum content 0.6mg / ml, appropriate amount of sodium chloride aqueous solution, and phosphate buffer solution to adjust the pH value to 5.8-7.2. An appropriate amount of stabilizers, such as M199 medium and amino acids, can also be added to the vaccine.
[0048] Mix the combined vaccine well before use for intramuscular injection.
[0049] The liquid vaccine formulation provided in ...
Embodiment 2
[0051] This embodiment provides a liquid vaccine preparation, which contains the following components per person-time dosage:
[0052] Pertussis toxin 8 μg, filamentous hemagglutinin 8 μg, pertactin 2.5 μg, diphtheria toxoid 2Lf, tetanus toxoid 5Lf, inactivated poliovirus type I stock solution 15DU, inactivated poliovirus type II Stock solution 45DU, inactivated type III poliovirus stock solution 45DU, group A meningitis polysaccharide-protein conjugate 15 μg, group C meningitis polysaccharide-protein conjugate 15 μg, Haemophilus influenzae type b polysaccharide-protein conjugate 15 μg, The aluminum content is 0.8 mg / ml, an appropriate amount of sodium chloride aqueous solution, and a phosphate buffer solution to adjust the pH value to 5.8-7.2. An appropriate amount of stabilizers, such as M199 medium and amino acids, can also be added to the vaccine.
[0053] Mix the combined vaccine well before use for intramuscular injection.
[0054] The liquid vaccine formulation provid...
Embodiment 3
[0056] This embodiment provides a liquid vaccine preparation, which contains the following components per person-time dosage:
[0057] Pertussis toxin 25μg, filamentous hemagglutinin 25μg, pertactin 8μg, diphtheria toxoid 12.5Lf, tetanus toxoid 3.5Lf, inactivated poliovirus type I stock solution 15DU, inactivated poliovirus type II Virus stock solution 45DU, inactivated type III poliovirus stock solution 45DU, group A meningitis polysaccharide-protein conjugate 10 μg, group C meningitis polysaccharide-protein conjugate 10 μg, Haemophilus influenzae type b polysaccharide-protein conjugate 10 μg , aluminum content 0.8mg / ml, appropriate amount of sodium chloride aqueous solution, and phosphate buffer solution to adjust the pH value to 5.8-7.2. An appropriate amount of stabilizers, such as M199 medium and amino acids, can also be added to the vaccine.
[0058] Mix the combined vaccine well before use for intramuscular injection.
[0059] The liquid vaccine preparation provided in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com