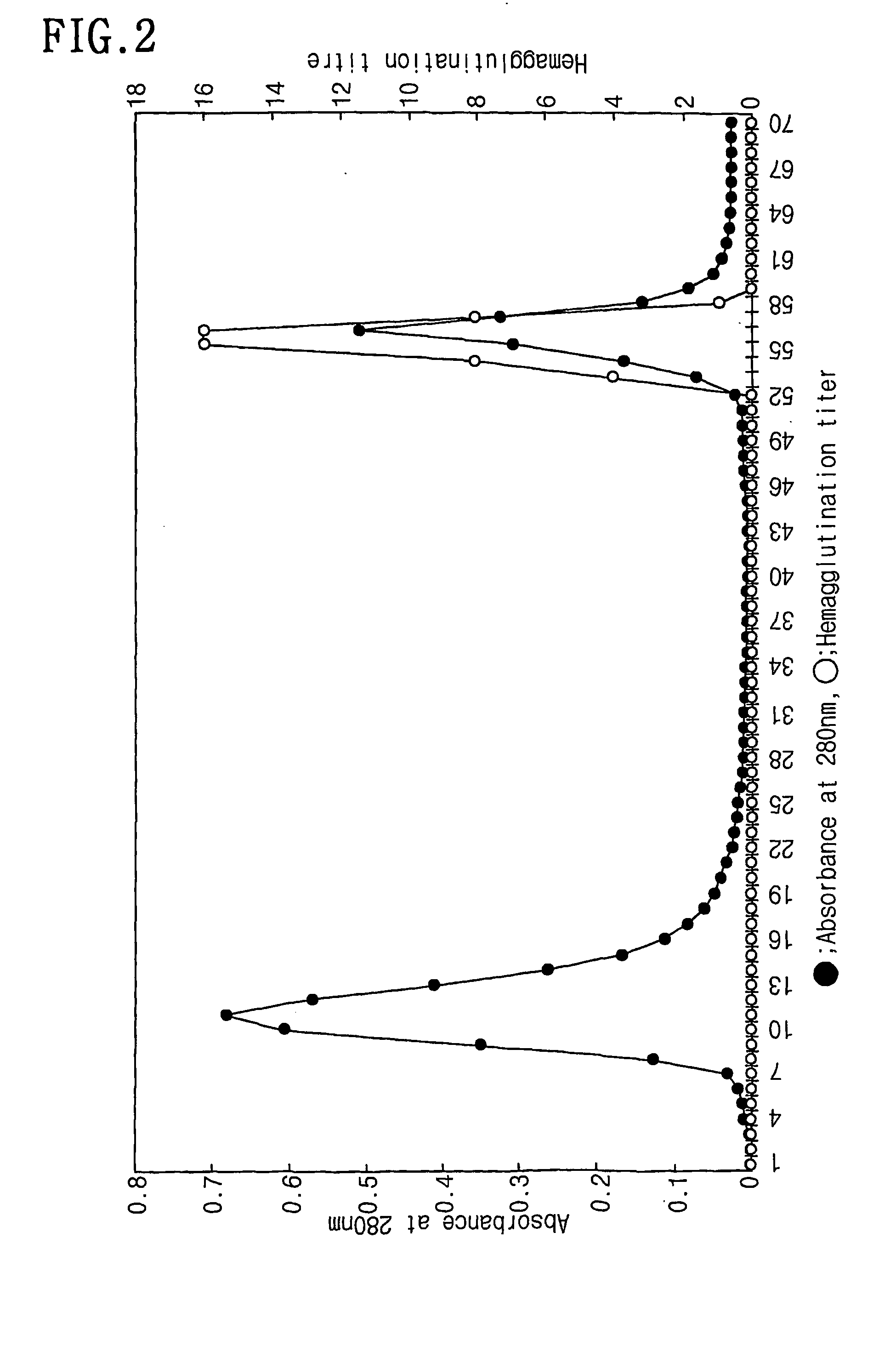
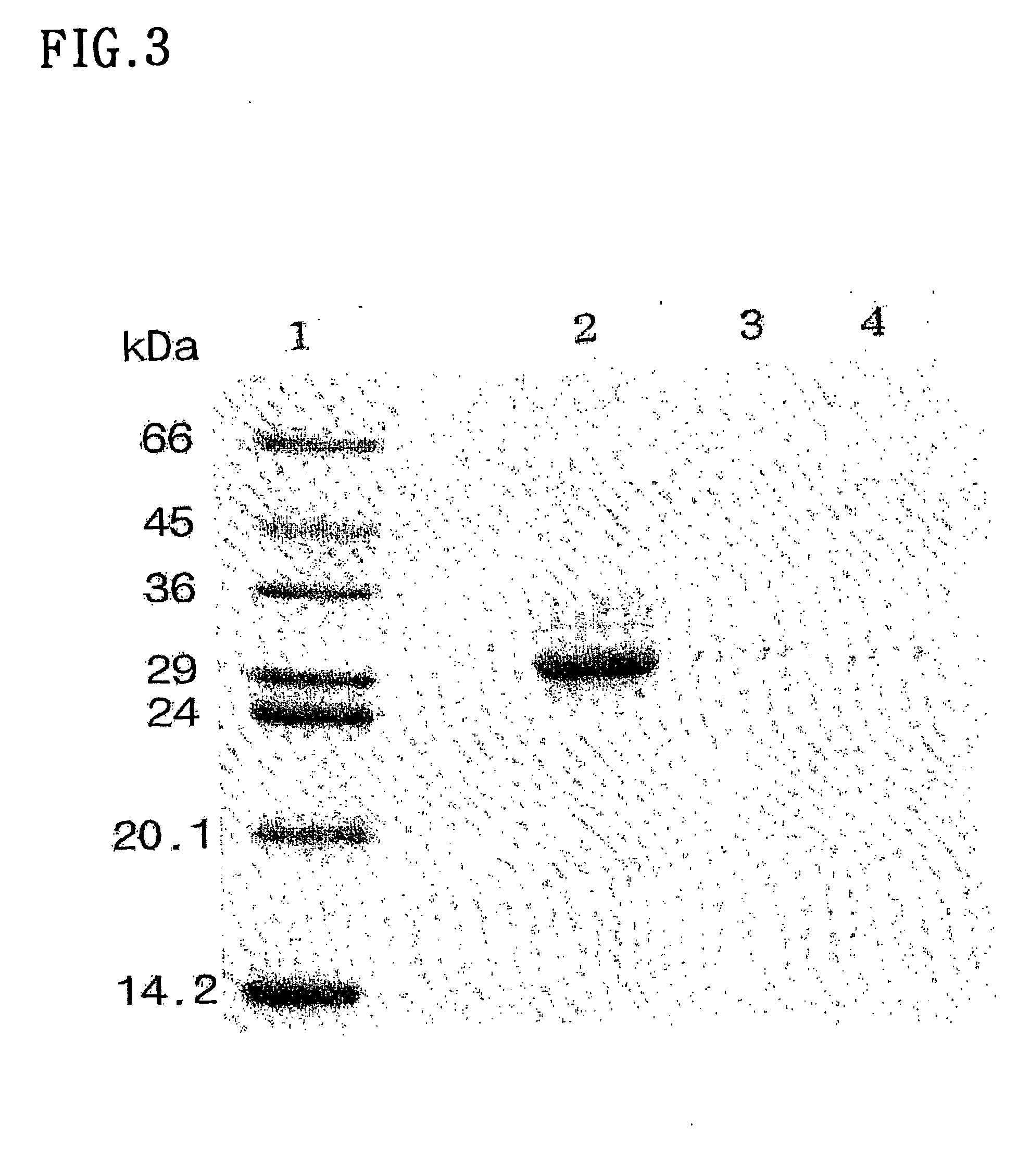
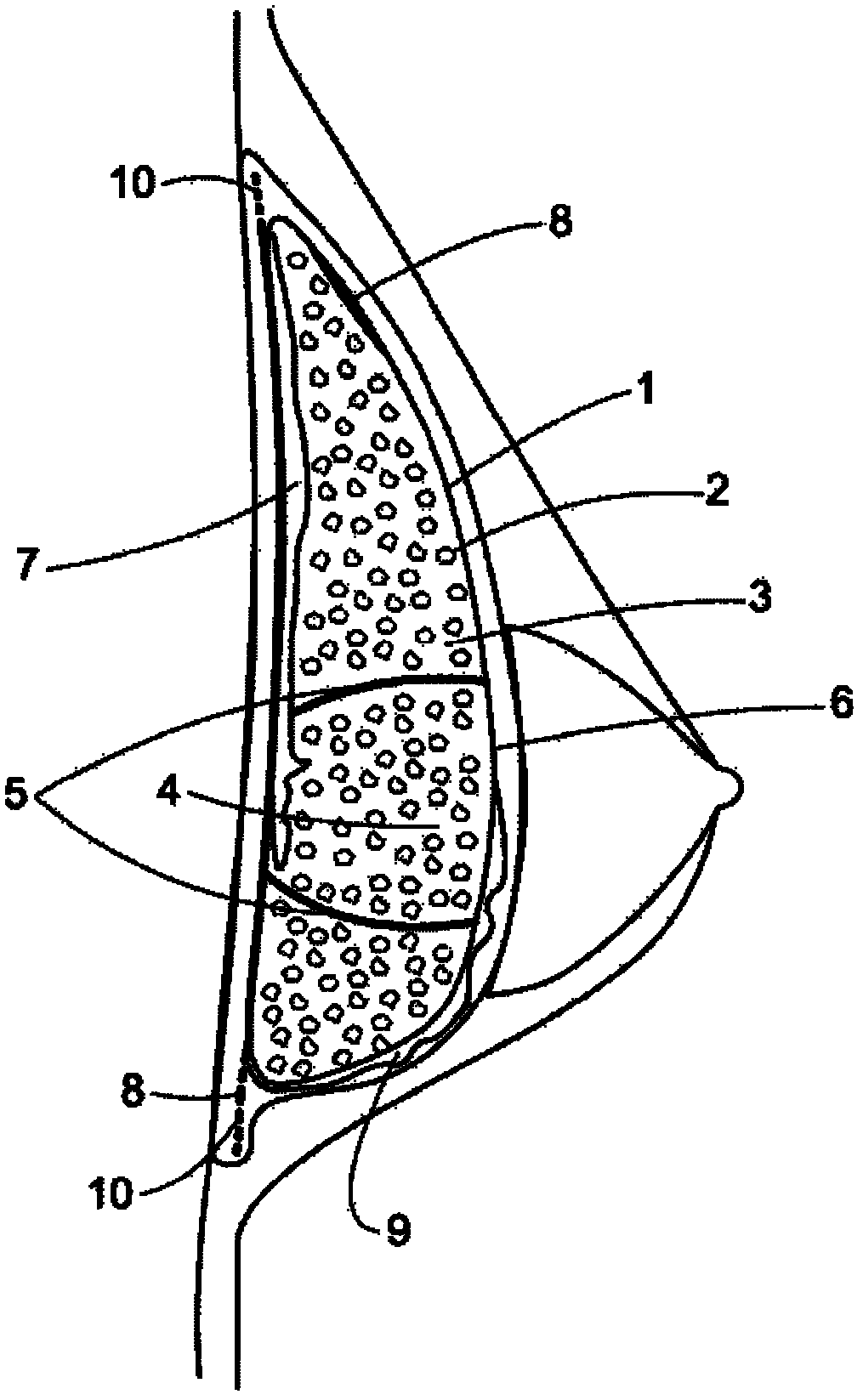
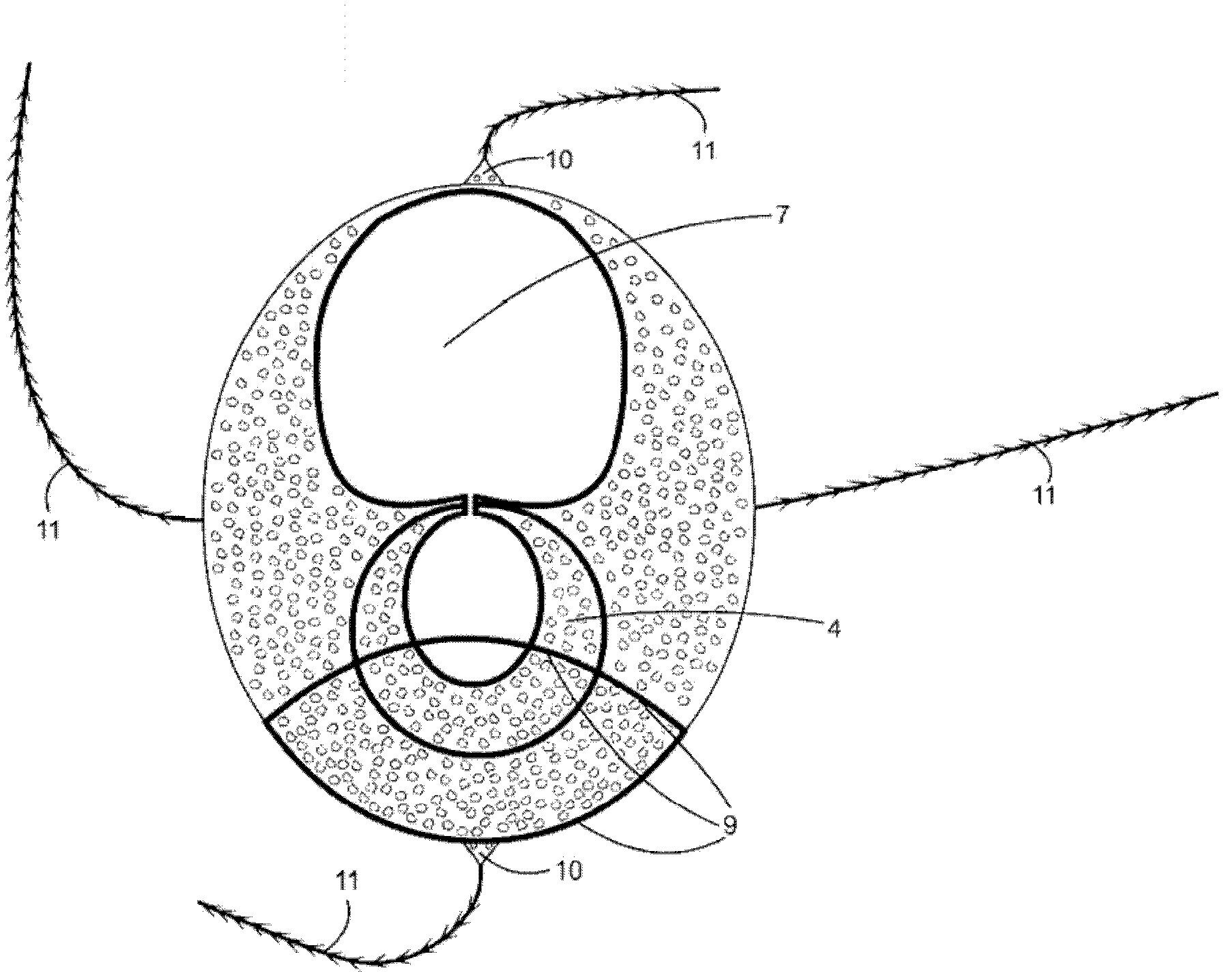
Patents
Literature
90results about How to "Return to normal activities" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
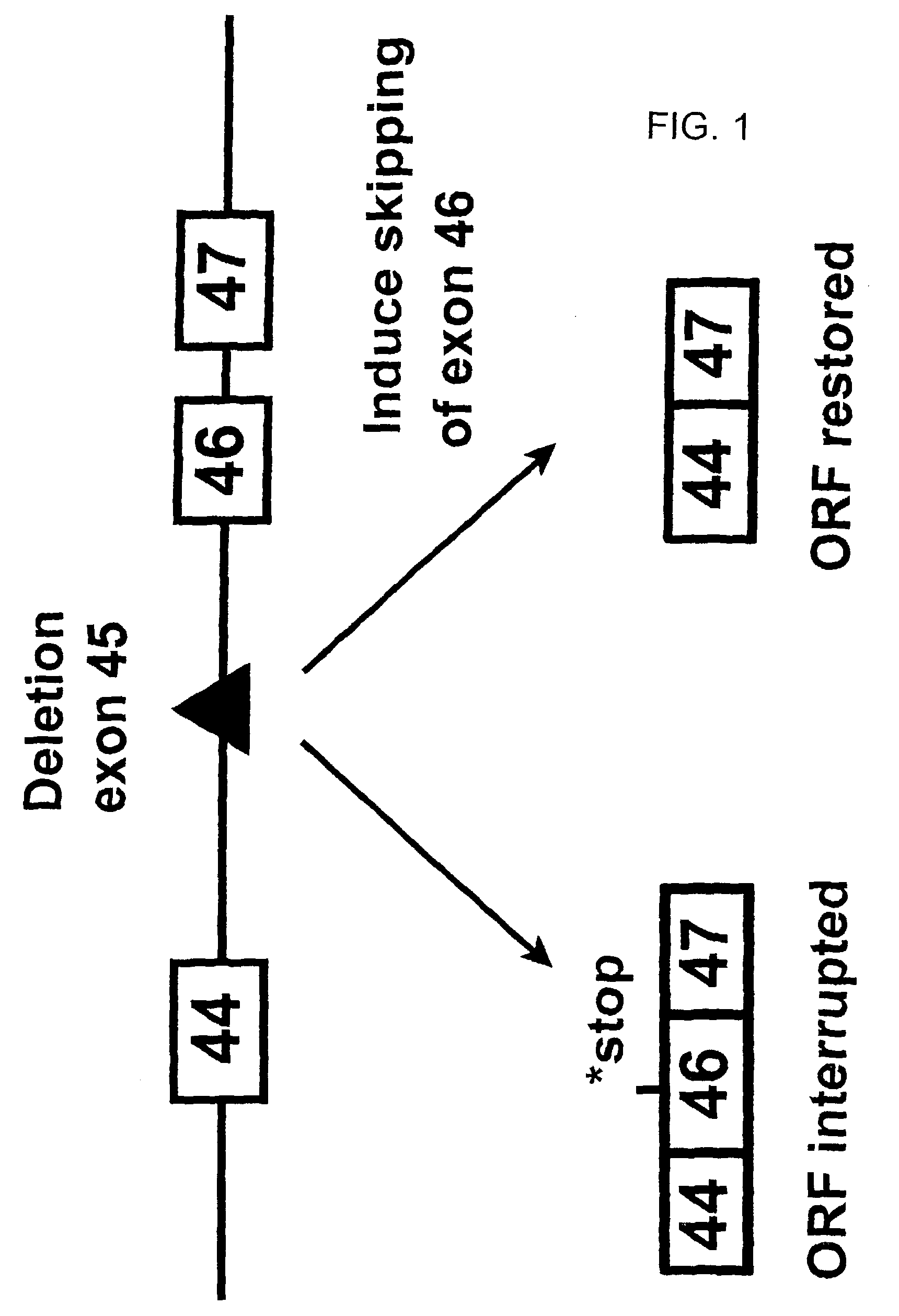
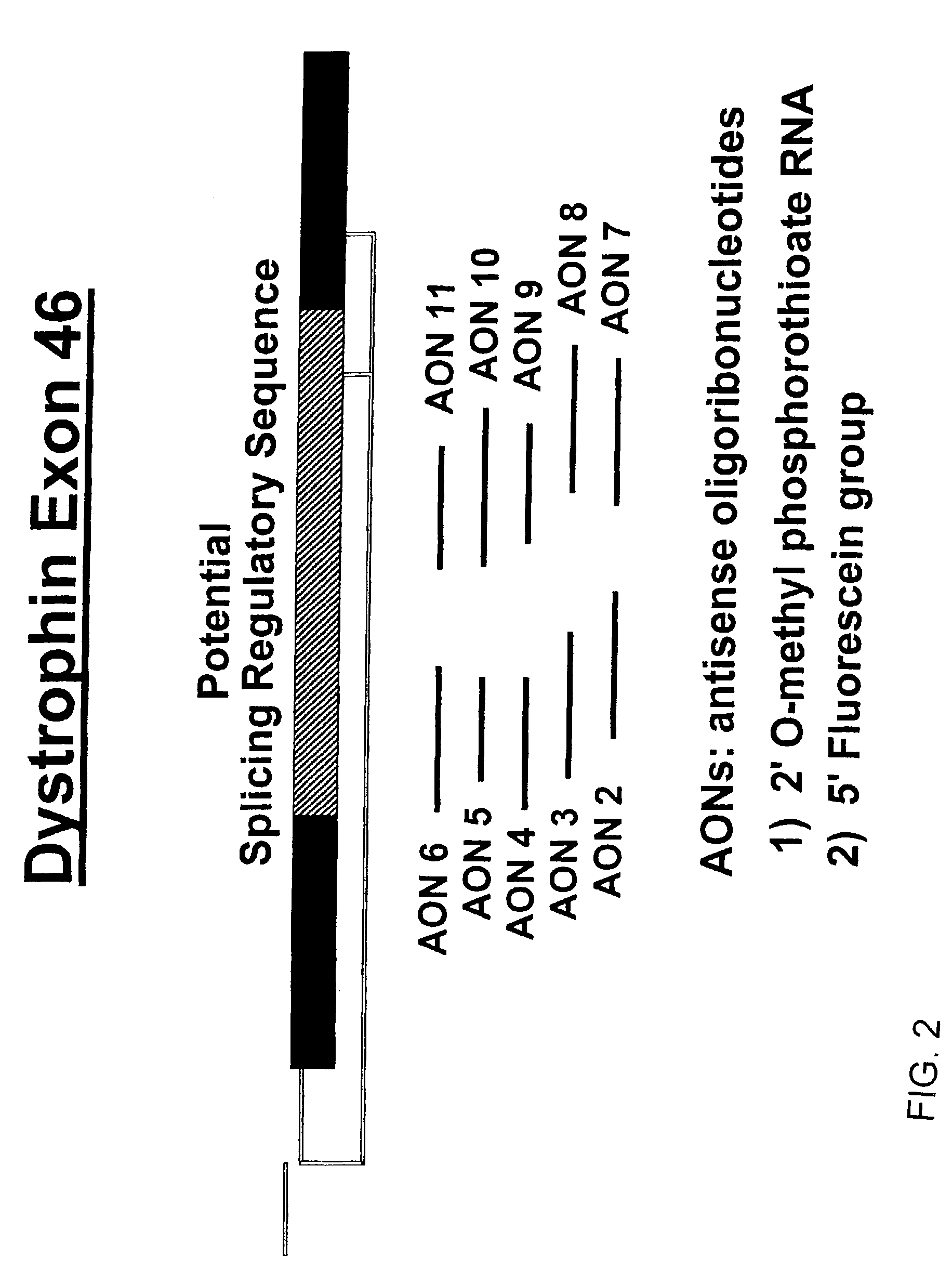
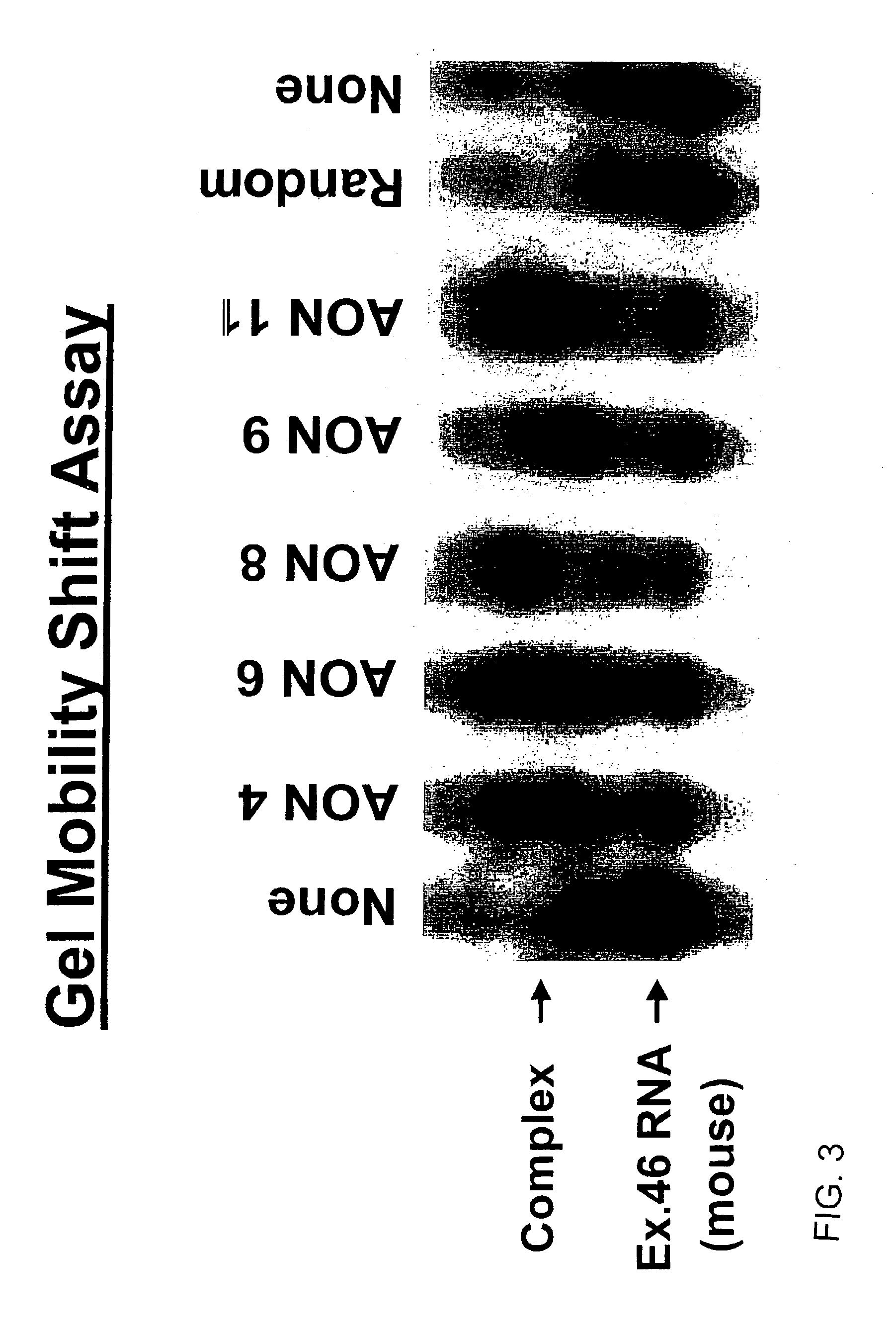
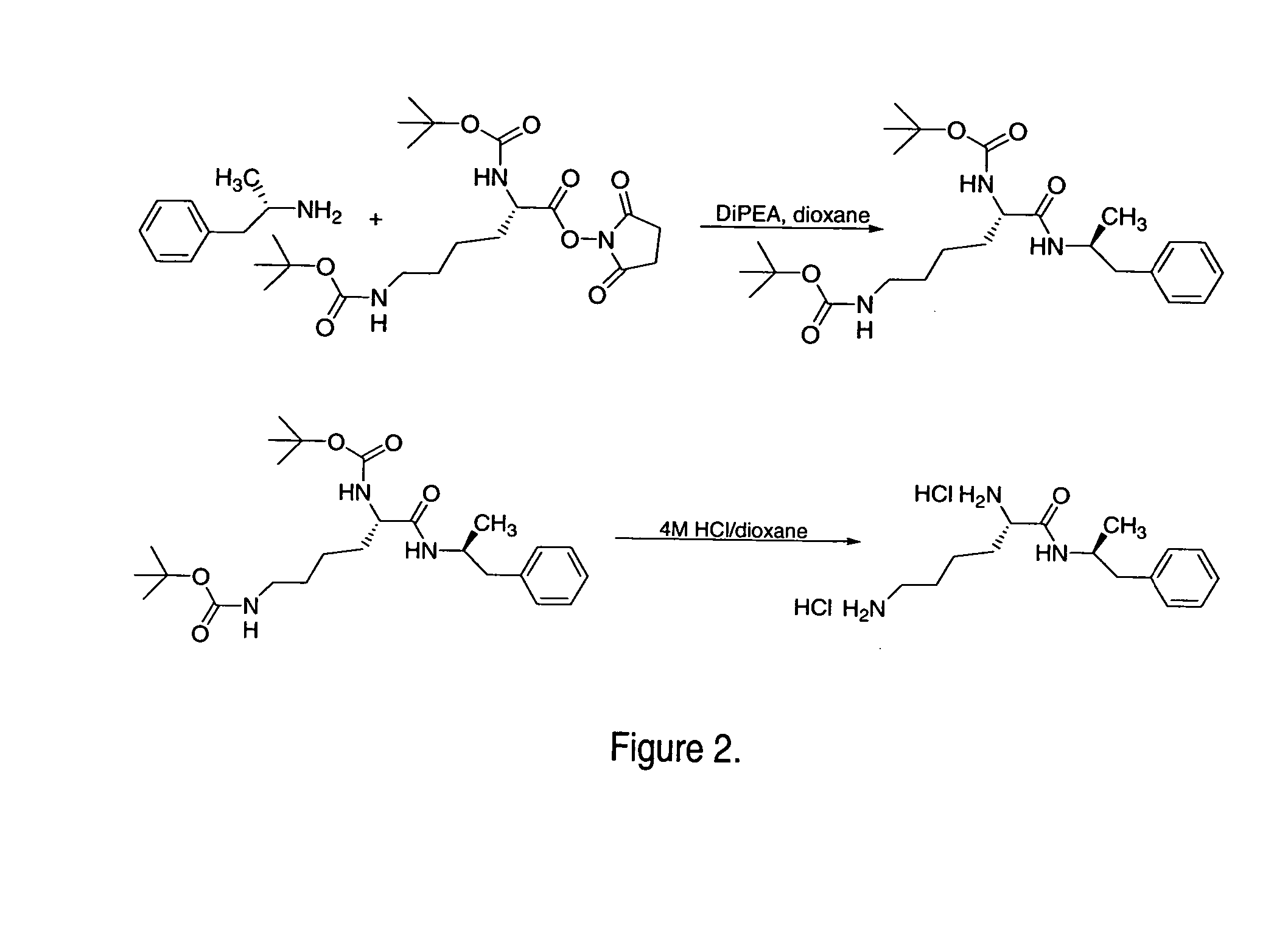
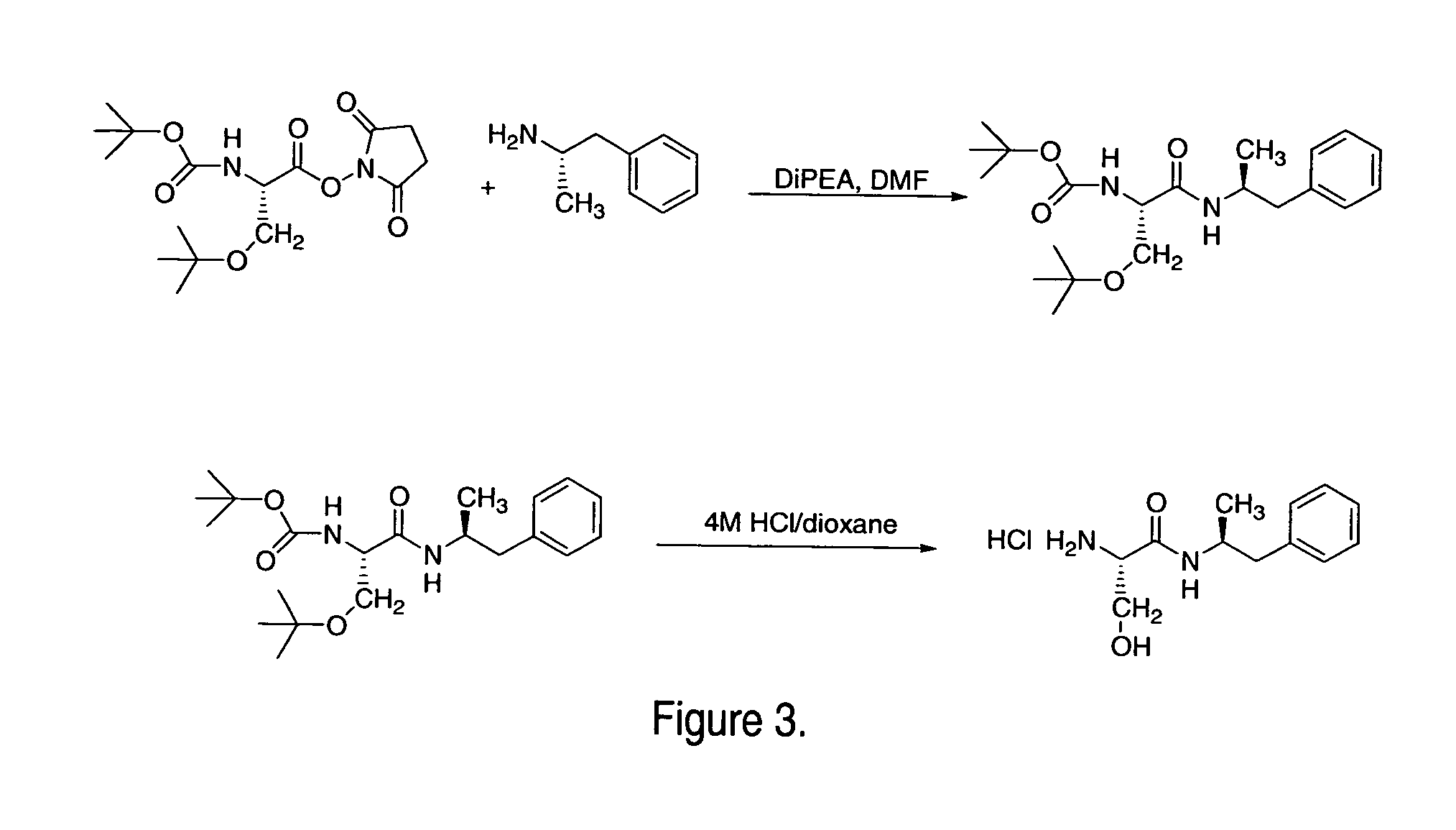
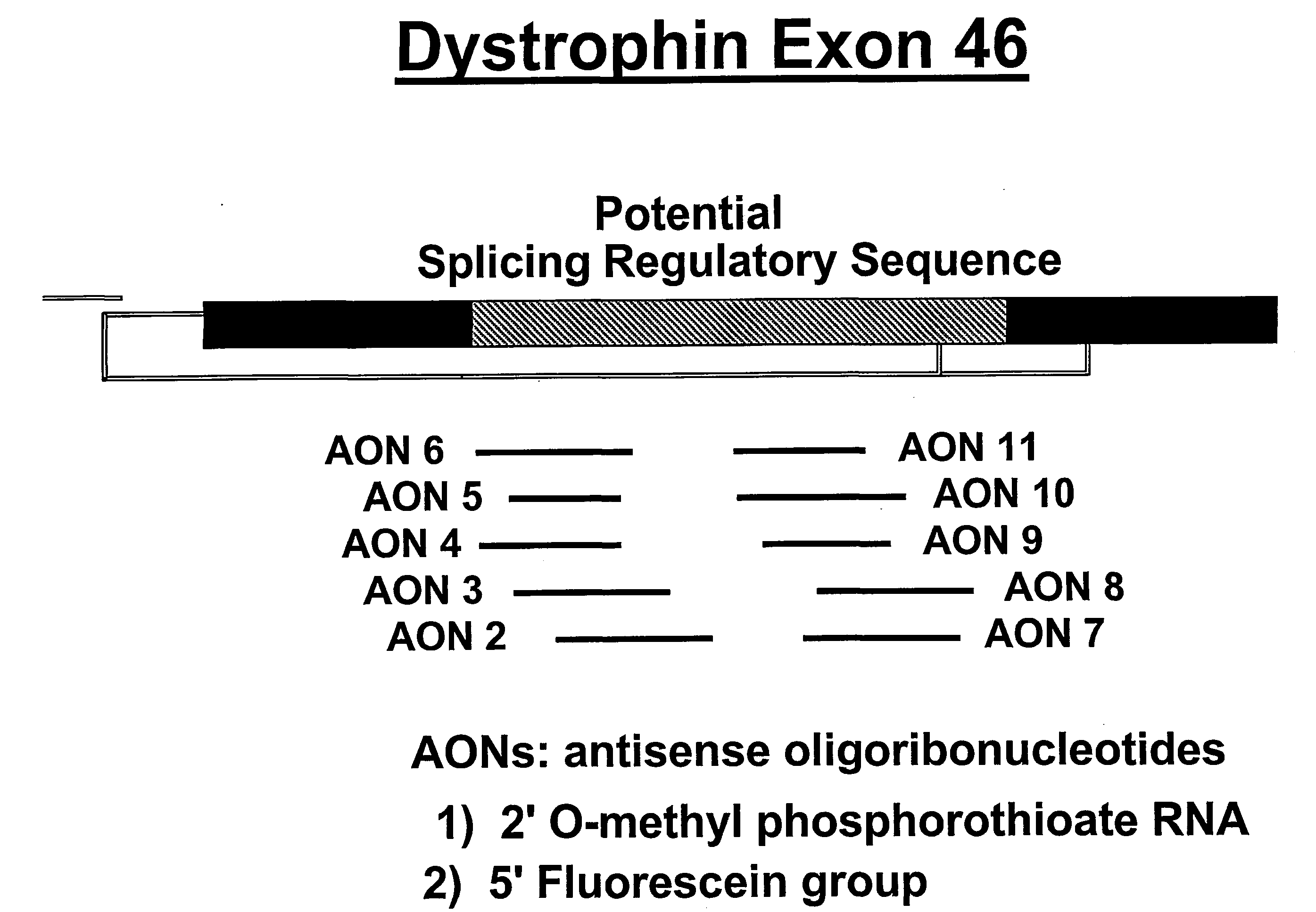
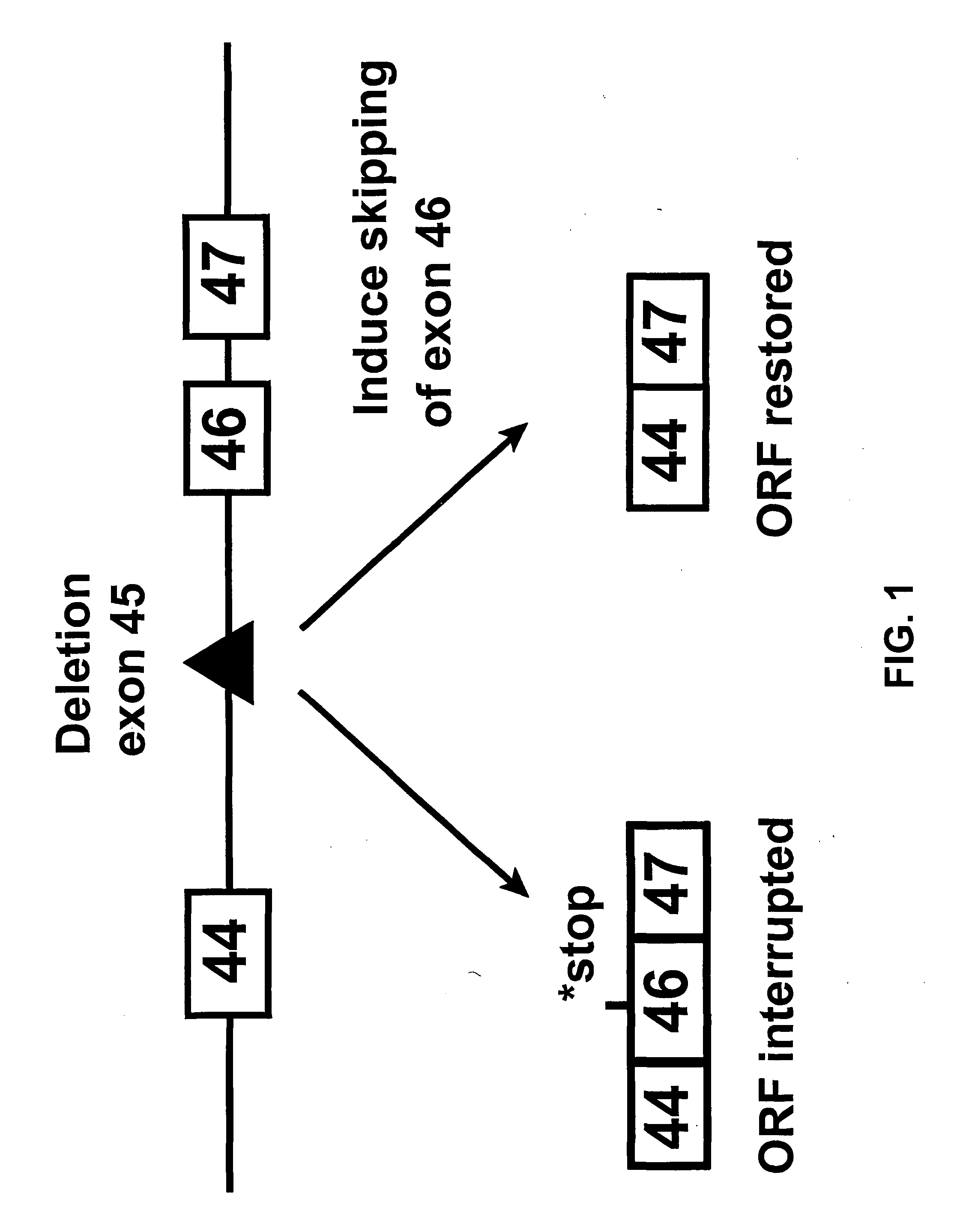
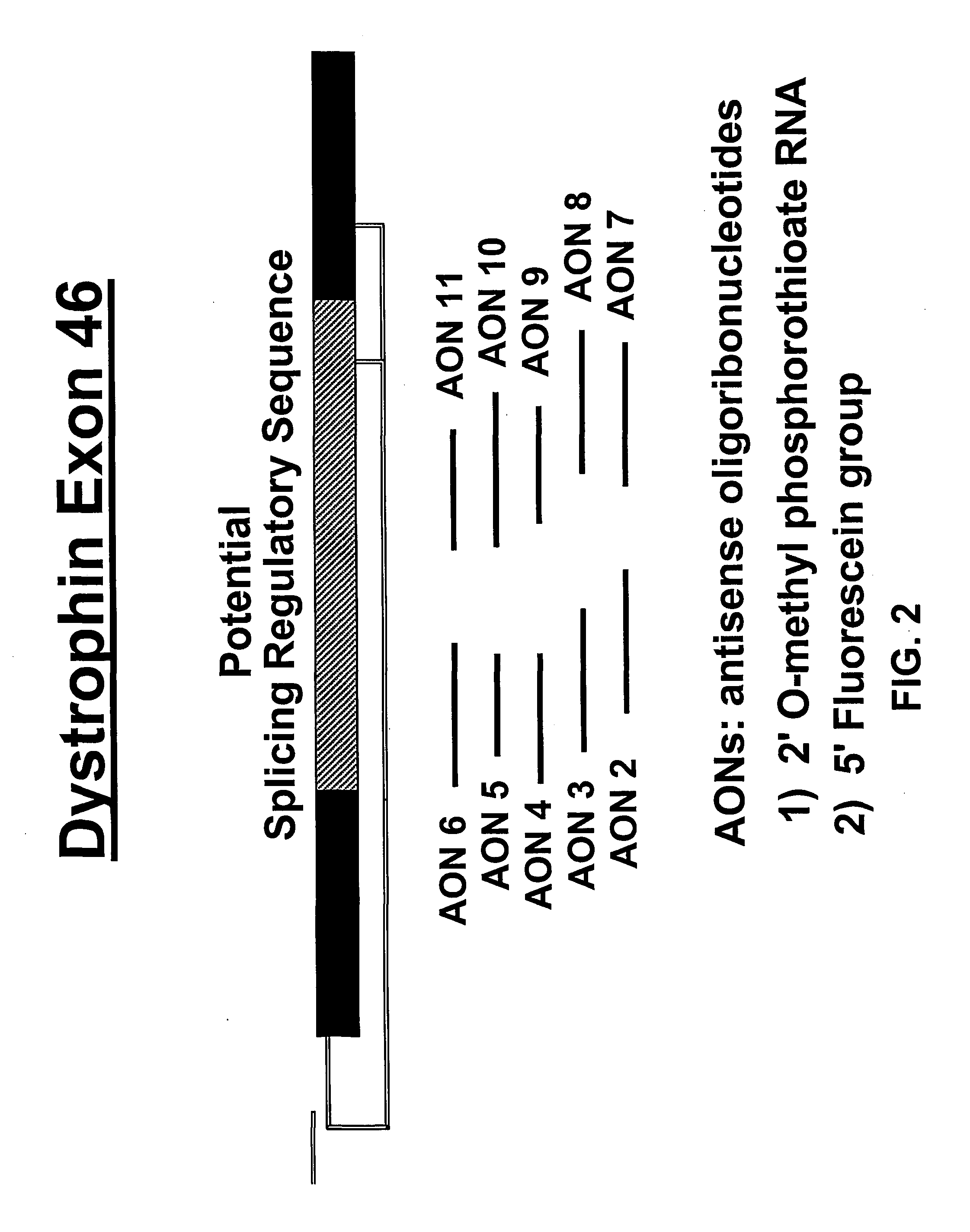
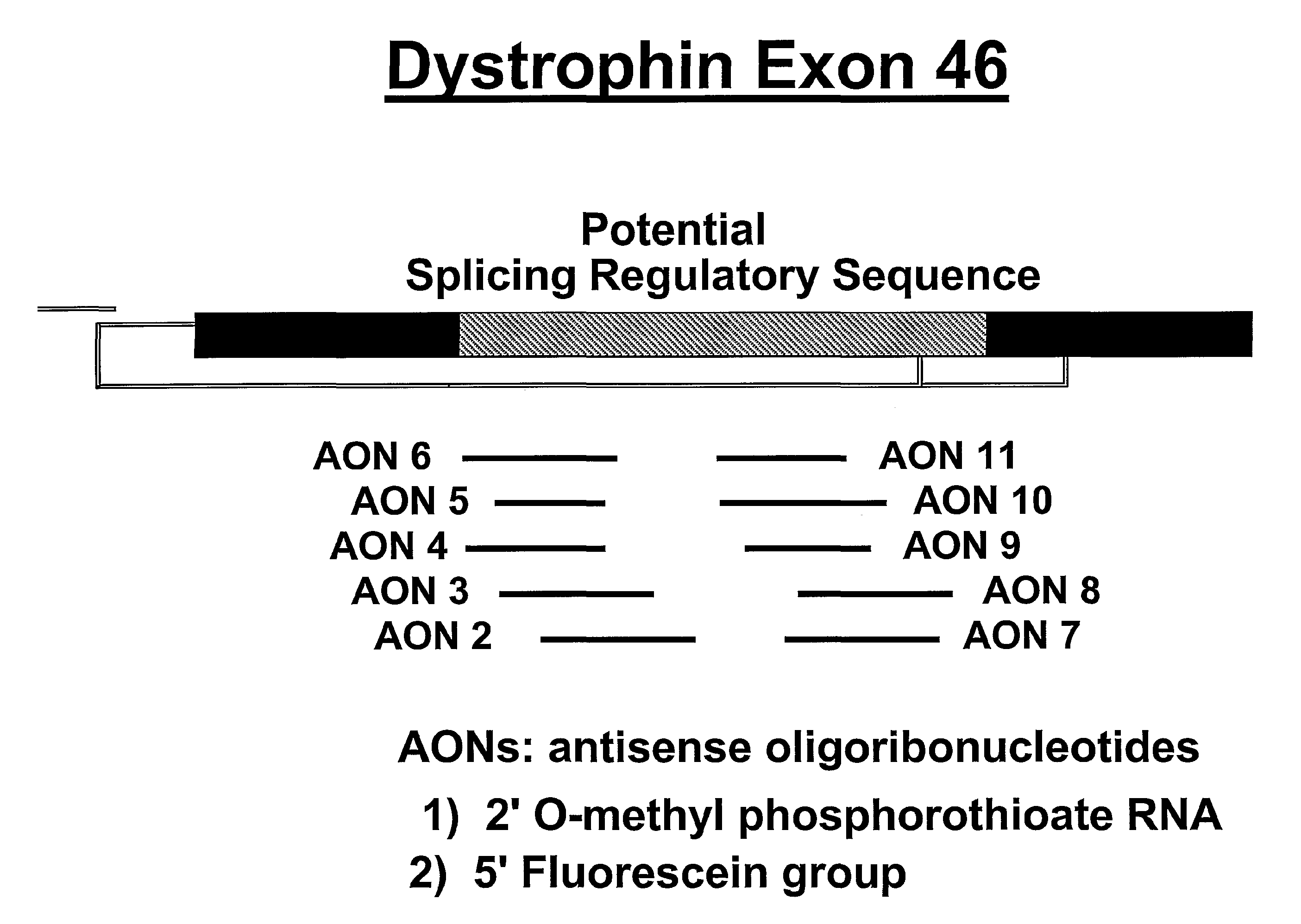
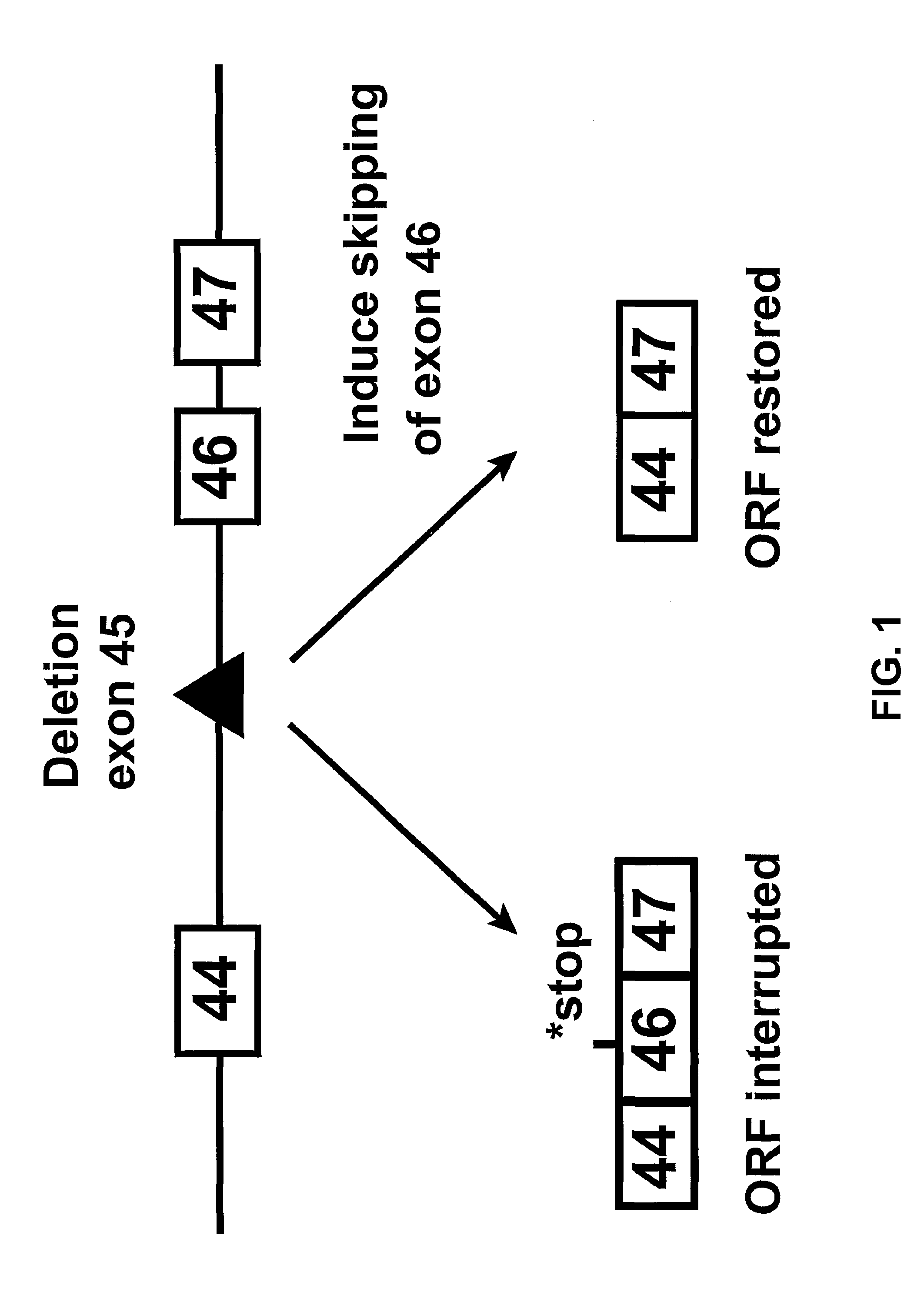
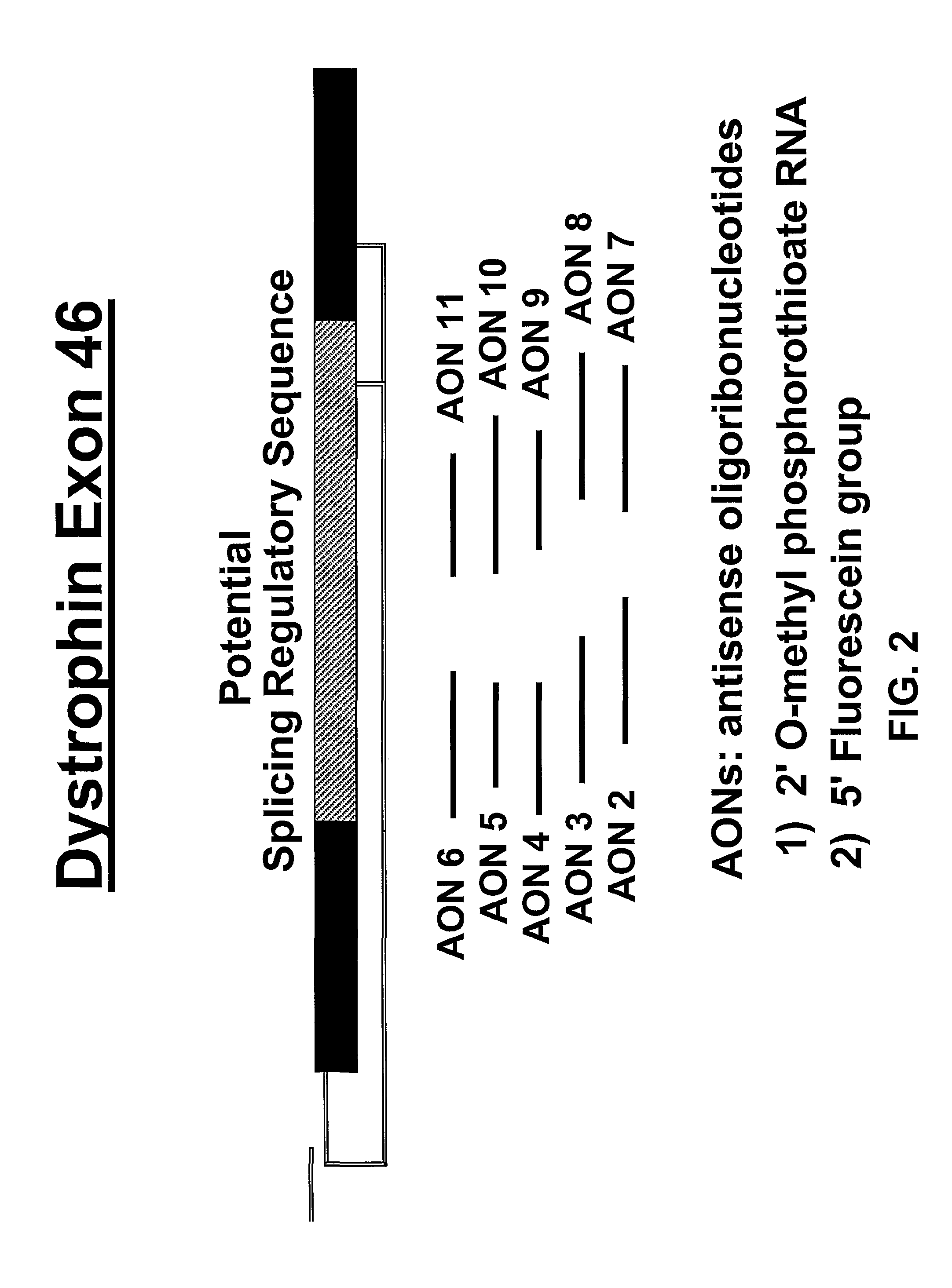
Induction of exon skipping in eukaryotic cells
InactiveUS7973015B2Efficiently maskedPotential for manipulationOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
The present invention provides a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature MRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:LEIDEN ACADEMISCH ZIEKENHUIS
Abuse-resistant amphetamine compounds
InactiveUS20050054561A1Prevents euphoriaLower potentialBiocidePeptide/protein ingredientsChemical MoietyDisease
The invention describes compounds, compositions and methods of using the same comprising a chemical moiety covalently attached to amphetamine. These compounds and compositions are useful for reducing or preventing abuse and overdose of amphetamine. These compounds and compositions find particular use in providing an abuse-resistant alternative treatment for certain disorders, such as attention deficit hyperactivity disorder (ADHD), ADD, narcolepsy, and obesity. Oral bioavailability of amphetamine is maintained at therapeutically useful doses. At higher doses bioavailability is substantially reduced, thereby providing a method of reducing oral abuse liability. Further, compounds and compositions of the invention decrease the bioavailability of amphetamine by parenteral routes, such as intravenous or intranasal administration, further limiting their abuse liability.
Owner:TAKEDA PHARMA CO LTD
Induction of exon skipping in eukaryotic cells
InactiveUS20090228998A1Efficiently maskedPotential for manipulationOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
The present invention provides a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature mRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Induction of exon skipping in eukaryotic cells
InactiveUS20080209581A1Reduce productionReduce amountOrganic active ingredientsSplicing alterationPrecursor mRNARecognition sequence
Described is a method for at least in part decreasing the production of an aberrant protein in a cell, the cell comprising pre-mRNA comprising exons coding for the protein, by inducing so-called exon skipping in the cell. Exon-skipping results in mature mRNA that does not contain the skipped exon, which leads to an altered product of the exon codes for amino acids. Exon skipping is performed by providing a cell with an agent capable of specifically inhibiting an exon inclusion signal, for instance, an exon recognition sequence, of the exon. The exon inclusion signal can be interfered with by a nucleic acid comprising complementarity to a part of the exon. The nucleic acid, which is also herewith provided, can be used for the preparation of a medicament, for instance, for the treatment of an inherited disease.
Owner:ACADEMISCH ZIEKENHUIS BIJ DE UNIV VAN AMSTERDAM ACADEMISCH MEDISCH CENT
Focal noninvasive stimulation of the sensory cortex of a subject with cerebral palsy
InactiveUS20110270345A1Restore levelRegain healthInternal electrodesMagnetotherapy using coils/electromagnetsCerebral paralysisNon invasive
Disclosed are methods and related devices for use with subjects with cerebral palsy or periventricular leukomalacia. In preferred embodiments, diffusion tensor imaging (DTI) is used to identify neural areas and transcranial magnetic stimulation (TMS) is used to stimulate neural pathways.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
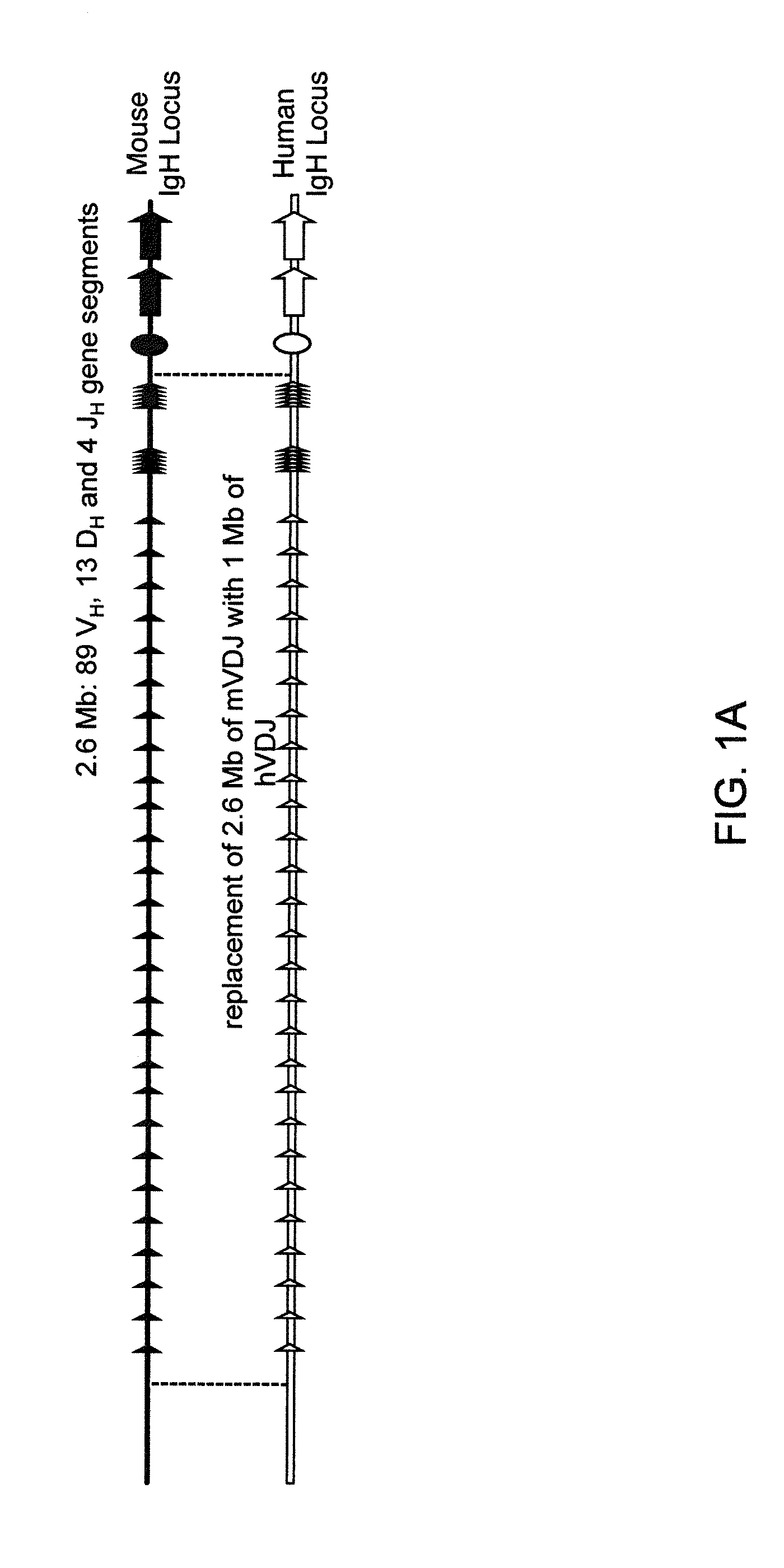
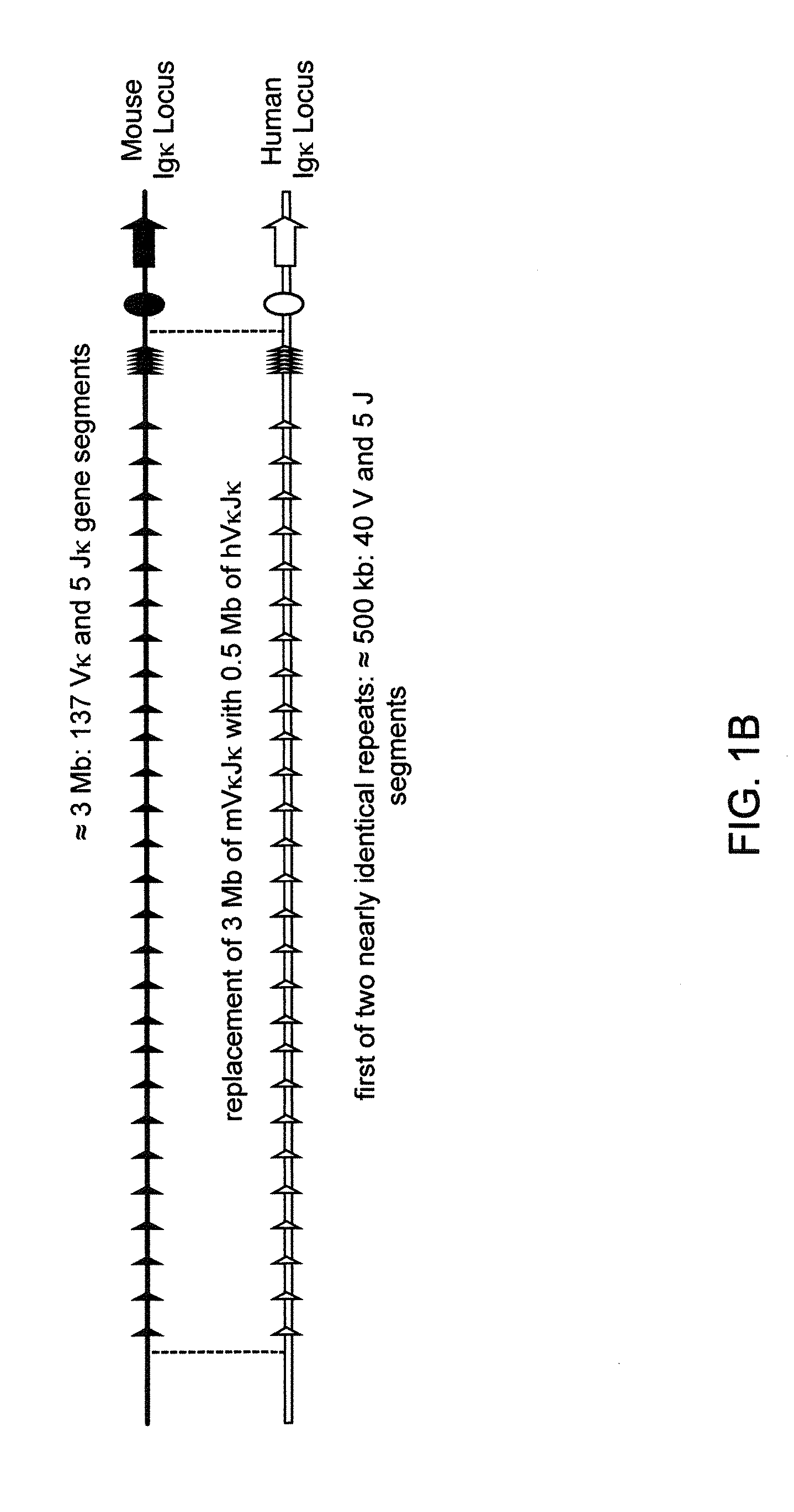
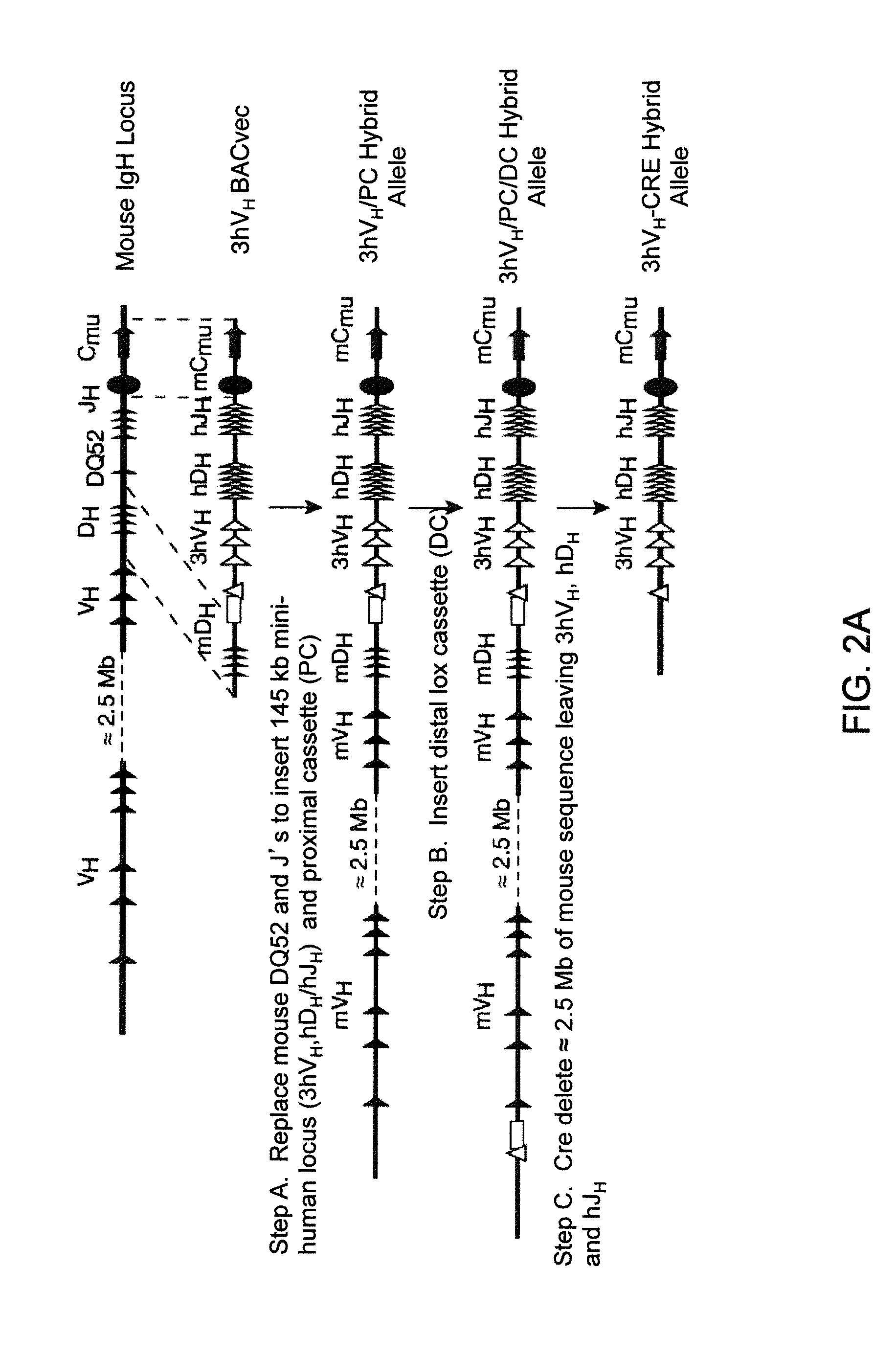
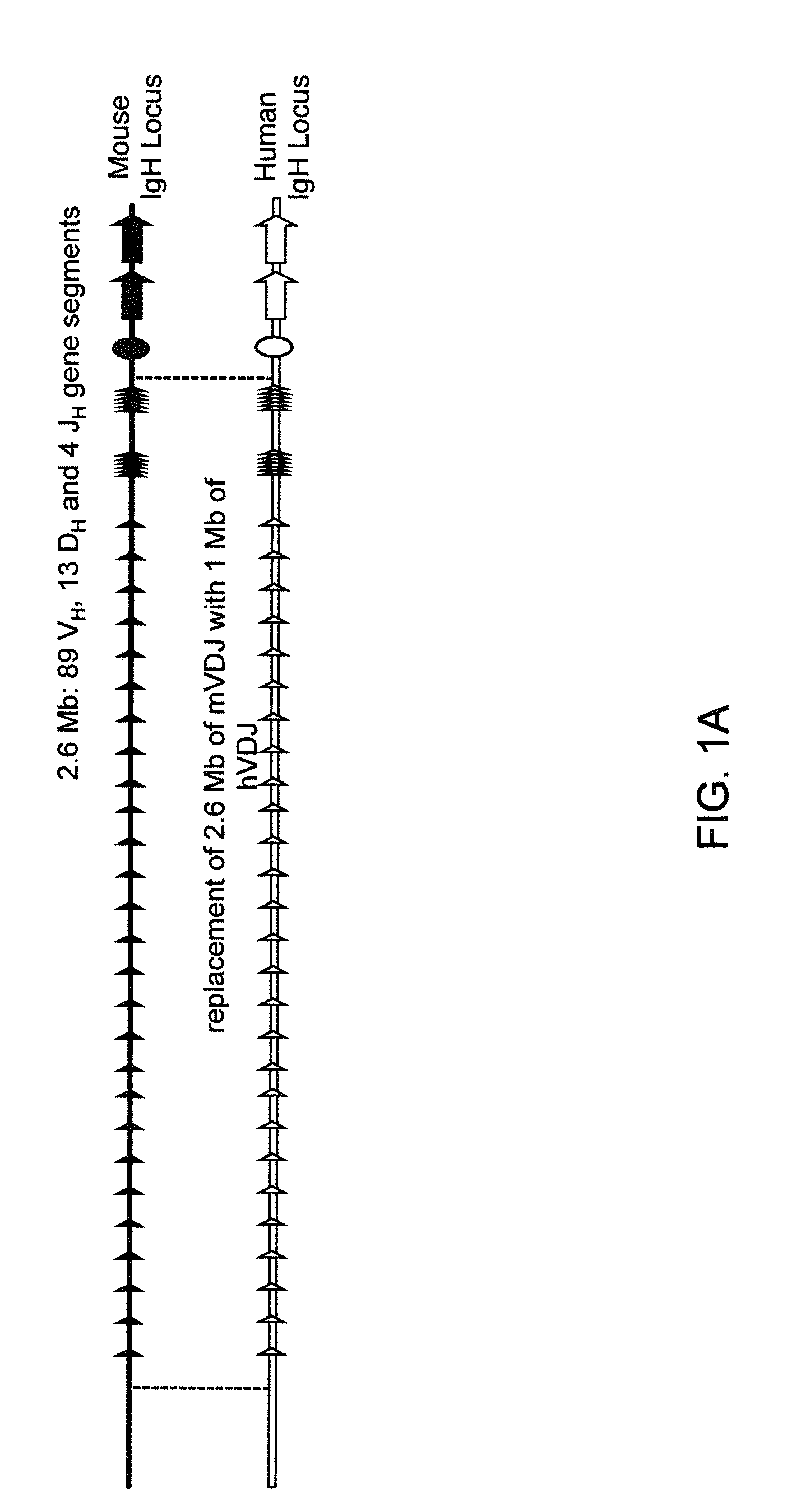
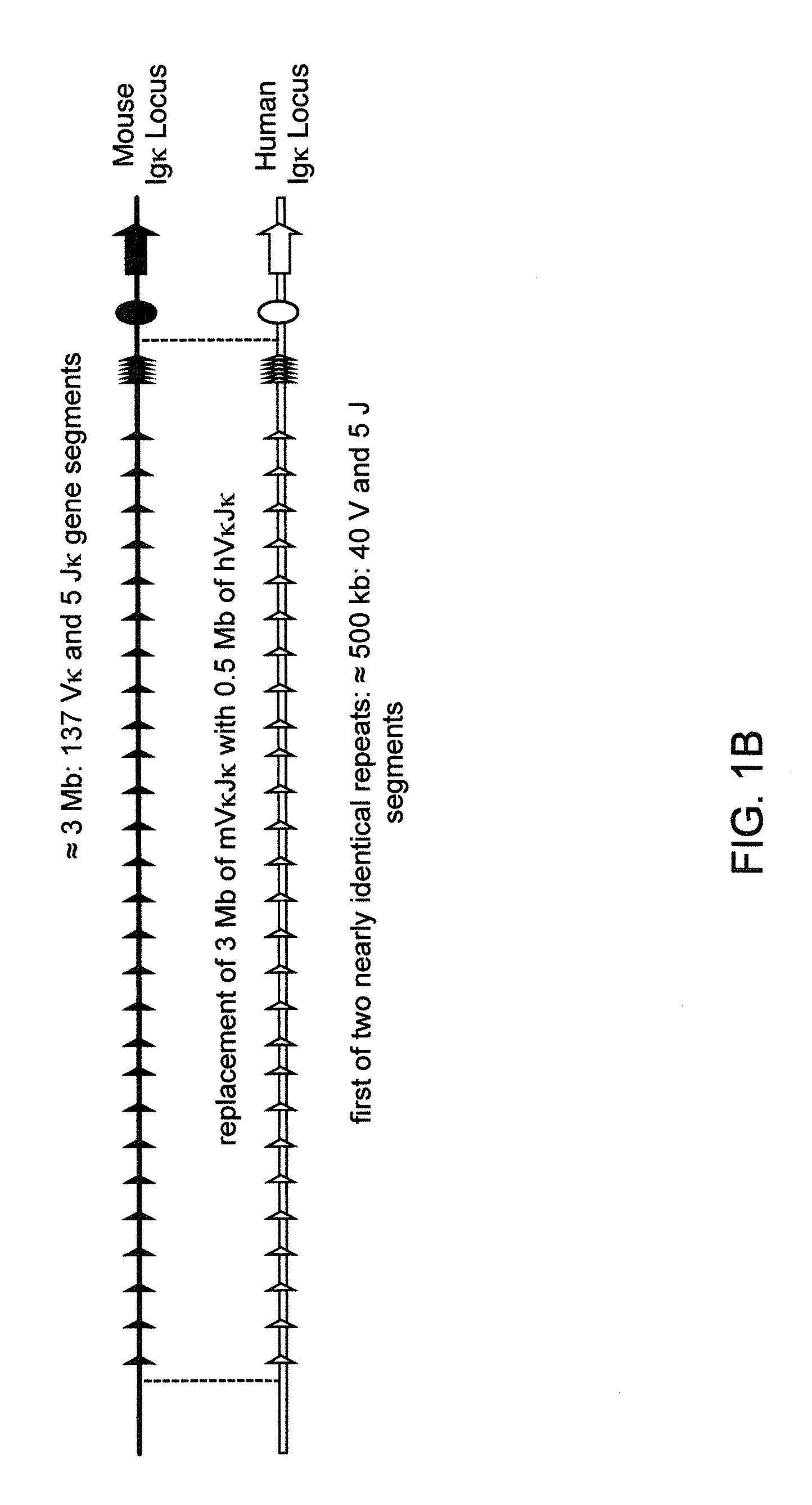
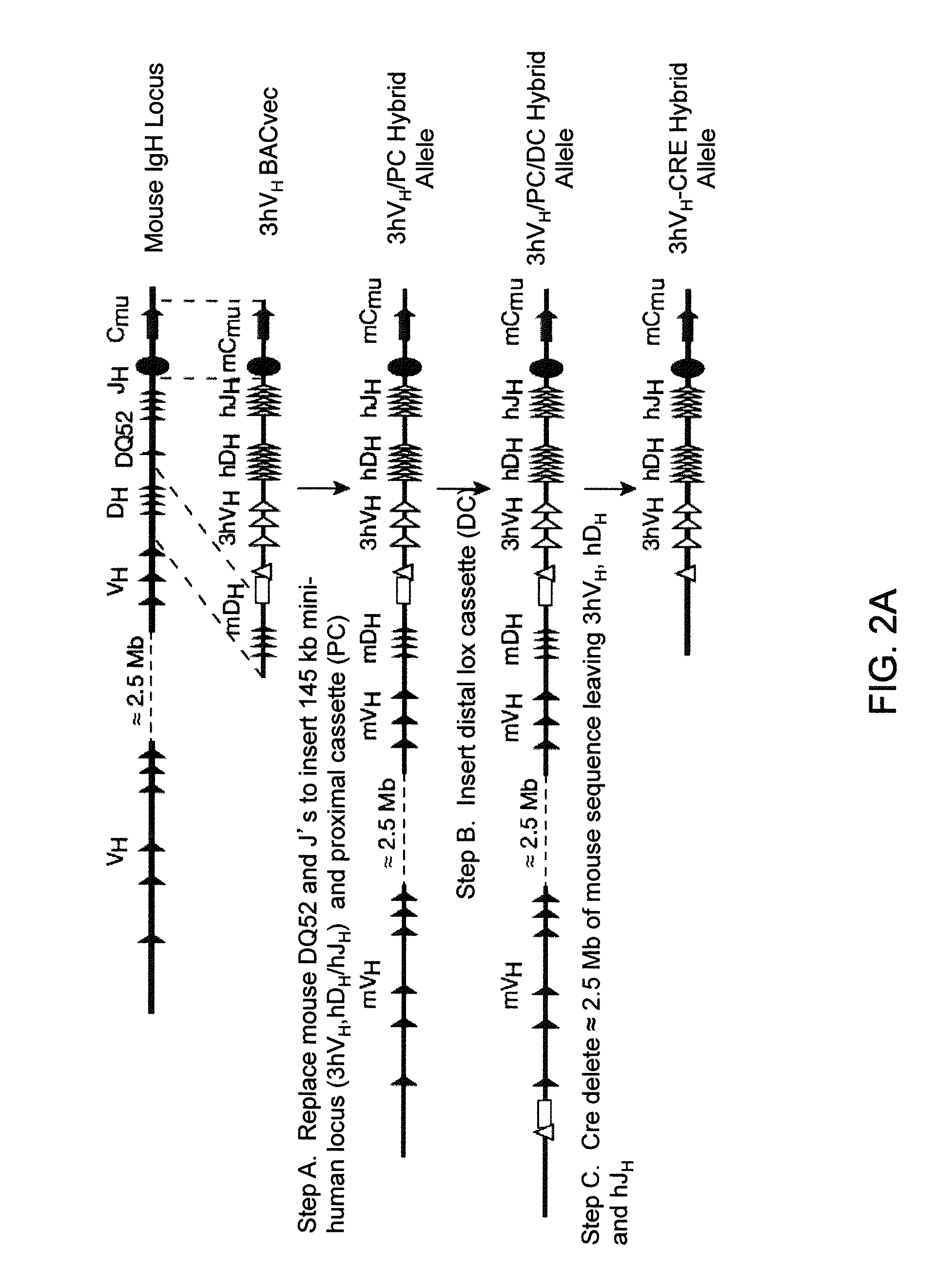
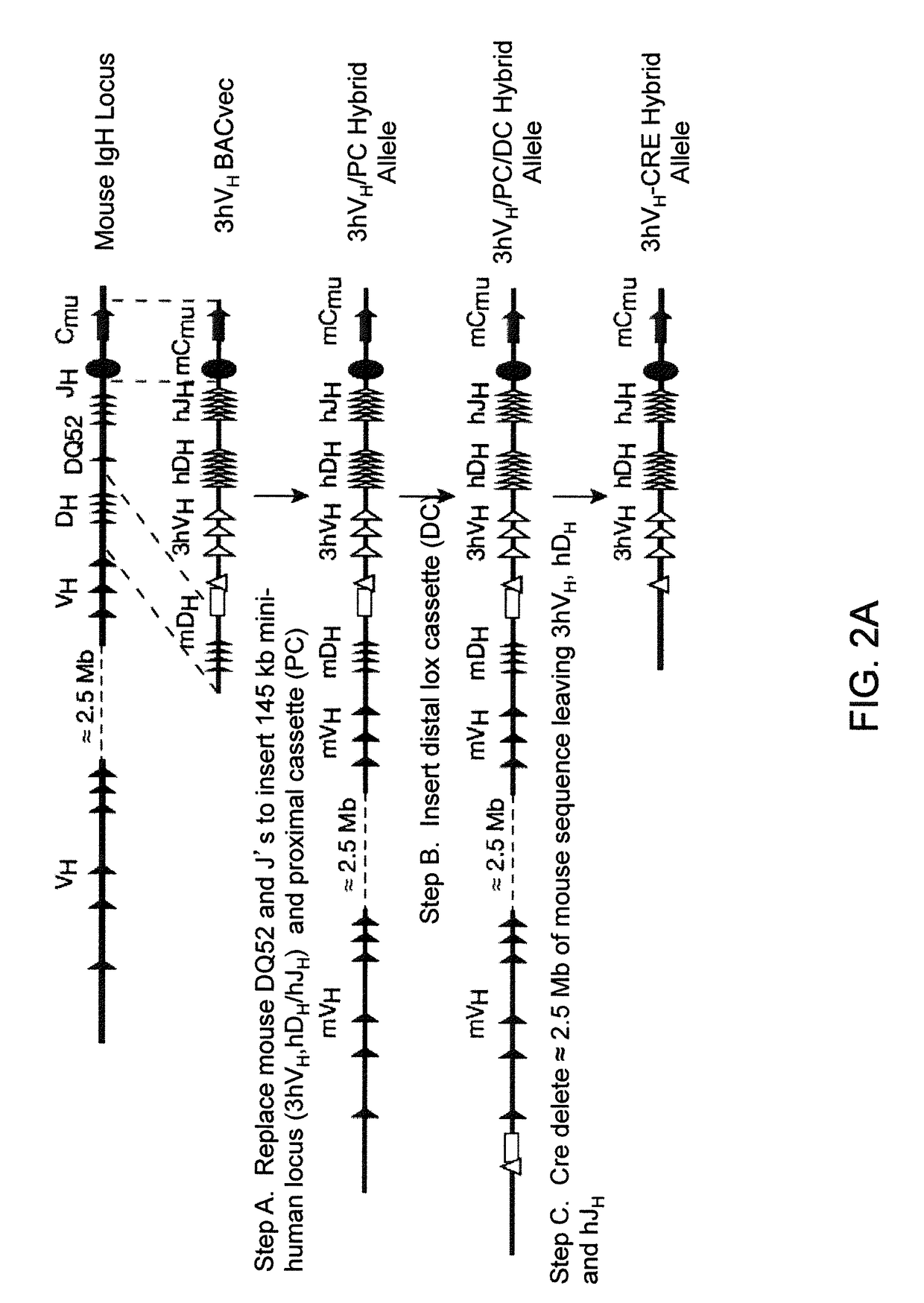
Humanized Light Chain Mice
ActiveUS20130160153A1Reduced fertilityImprove fertilityAnimal cellsNucleic acid vectorHuman immunoglobulinsGenetic Materials
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a mouse, wherein the non-human animals express a human immunoglobulin heavy chain variable domain and a cognate human immunoglobulin λ light chain variable domain.
Owner:REGENERON PHARM INC
Humanized light chain mice
ActiveUS20140017228A1Reduces and eliminates ADAM activityImprove fertilityNucleic acid vectorImmunoglobulinsHuman immunoglobulinsGenetic Materials
Non-human animals, tissues, cells, and genetic material are provided that comprise a modification of an endogenous non-human heavy chain immunoglobulin sequence and that comprise an ADAM6 activity functional in a mouse, wherein the non-human animals express a human immunoglobulin heavy chain variable domain and a cognate human immunoglobulin λ light chain variable domain.
Owner:REGENERON PHARM INC
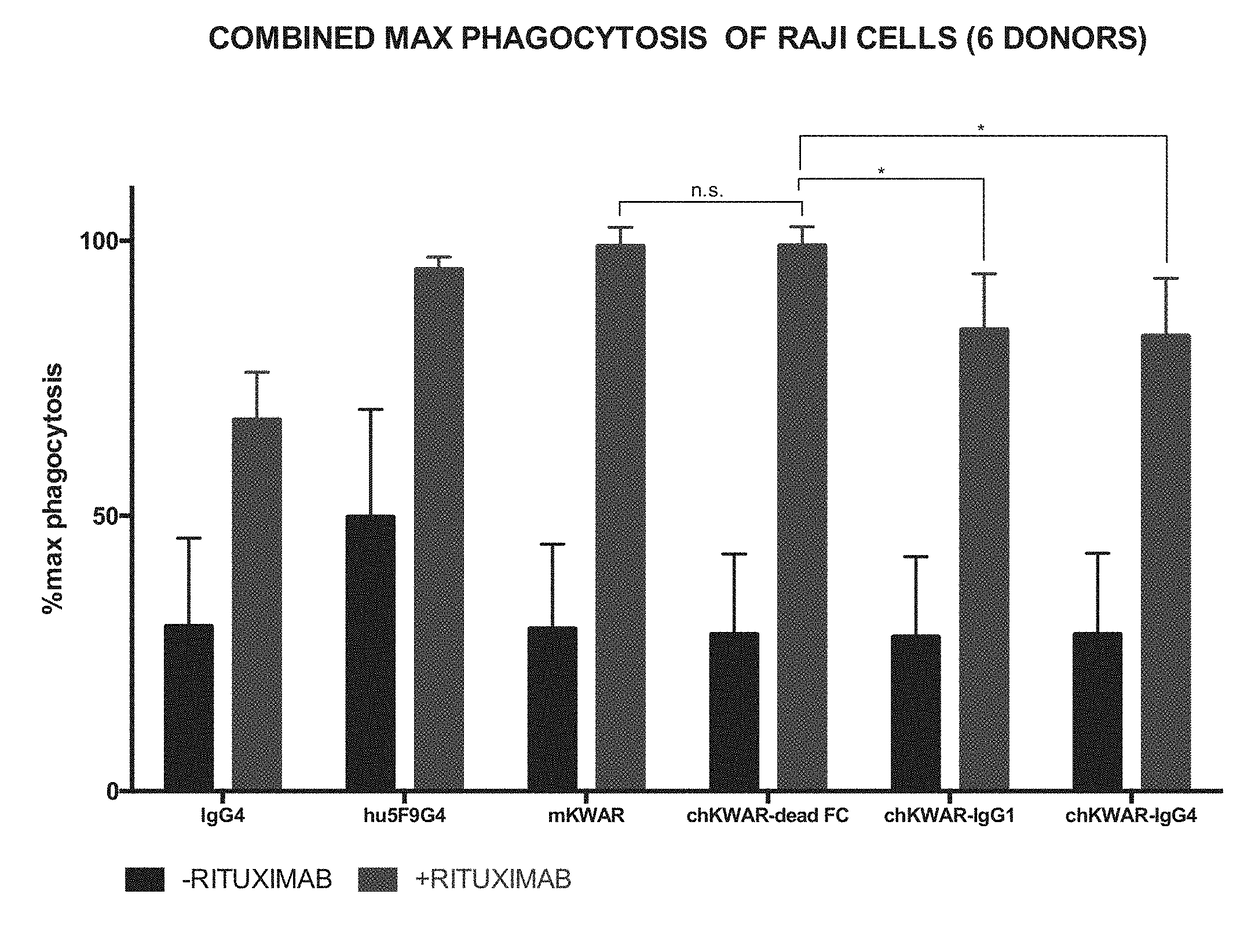
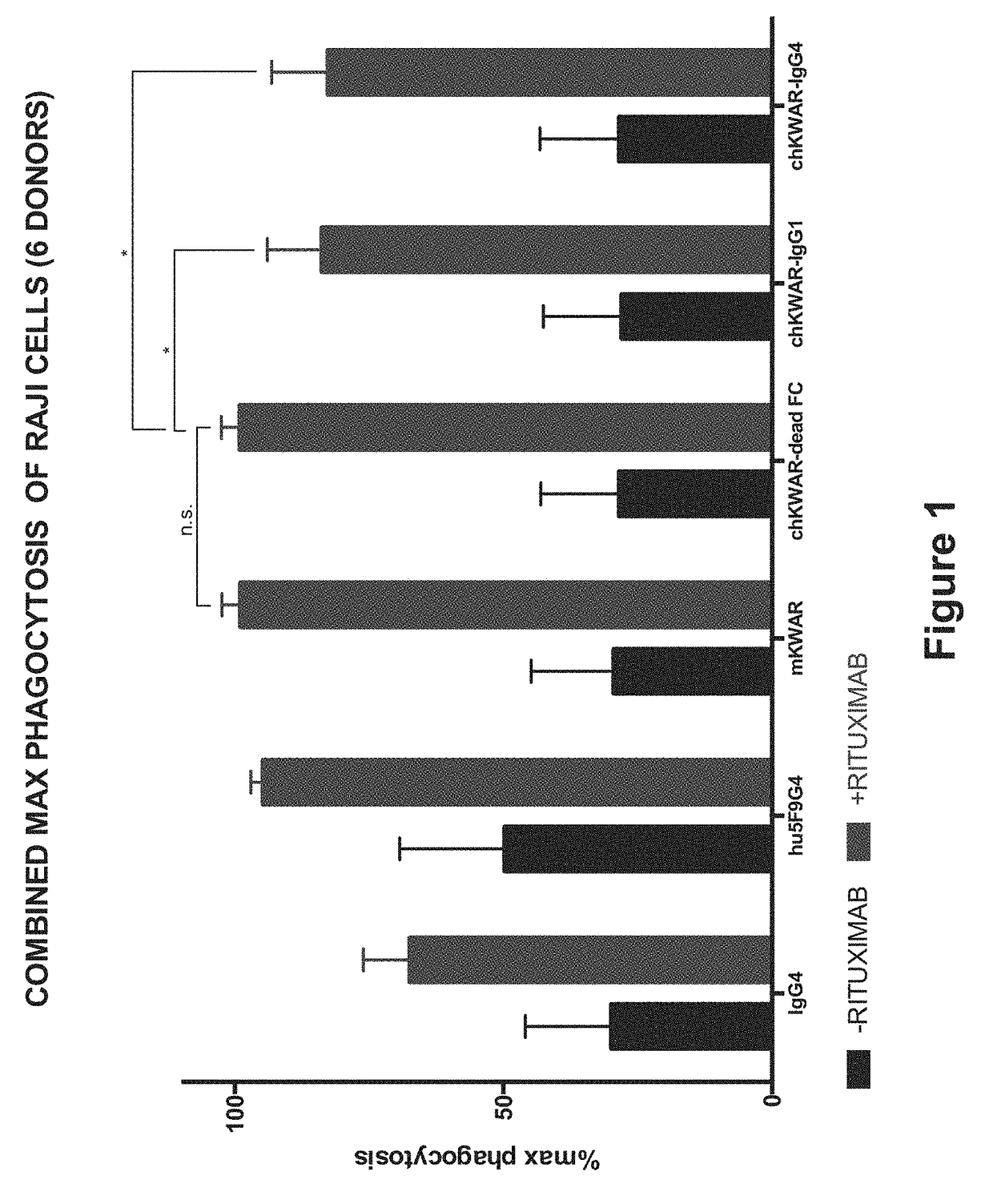
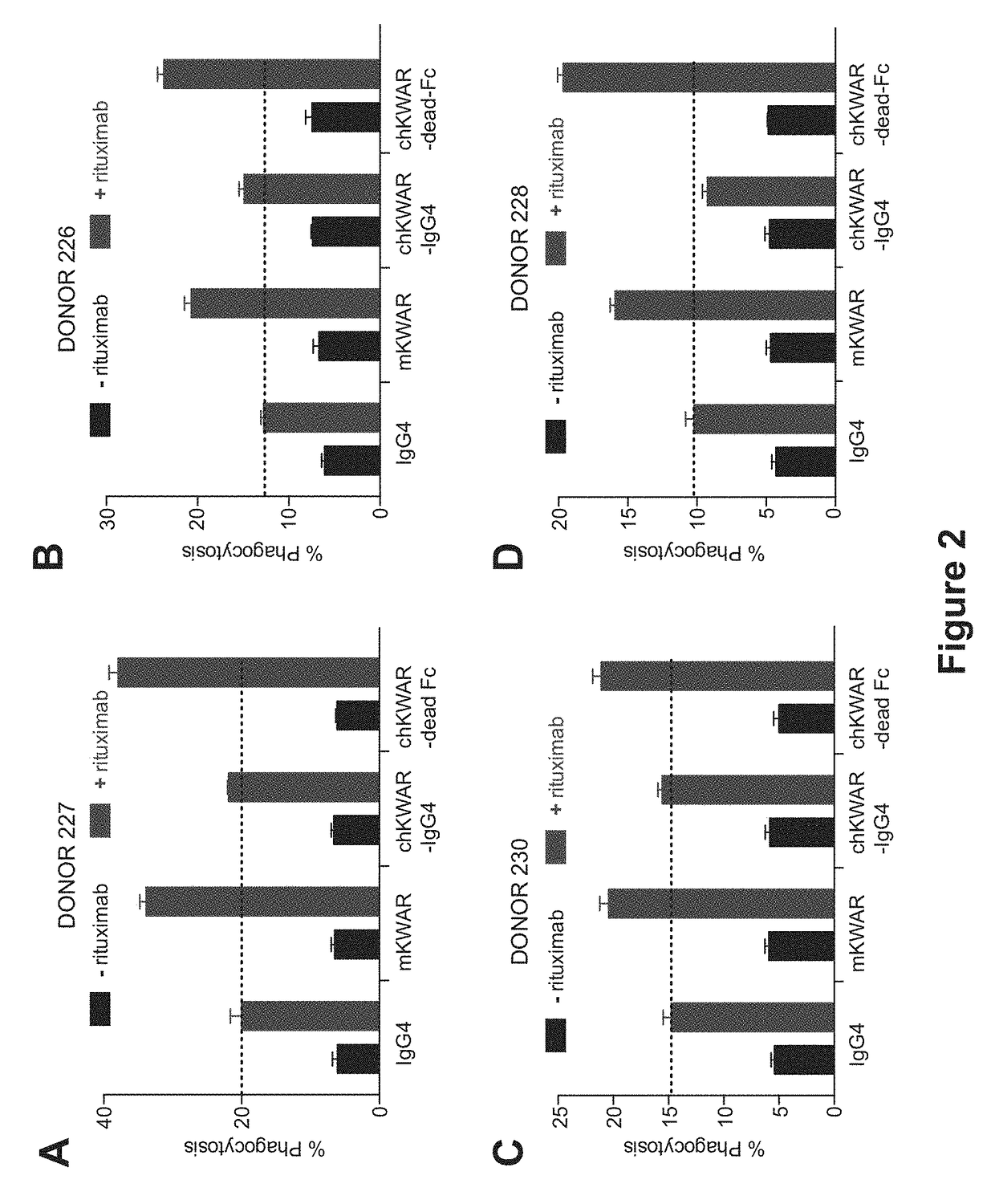
Disrupting FC Receptor Engagement on Macrophages Enhances Efficacy of Anti-SIRPalpha Antibody Therapy
ActiveUS20180037652A1Reduce it effectivenessRestore activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDrugFc receptor
Anti-SIRPα antibodies, including multi-specific anti-SIRPα antibodies, are provided, as are related compositions and methods. The antibodies of the disclosure bind to SIRPα and can block the interaction of CD47 on one cell with SIRPα on a phagocytic cell. The subject anti-SIRPα antibodies find use in various therapeutic methods. Embodiments of the disclosure include isolated antibodies and derivatives and fragments thereof, pharmaceutical formulations comprising one or more of the anti-SIRPα antibodies; and cell lines that produce the antibodies. Also provided are amino acid sequences of exemplary anti-SIRPα antibodies.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Recombinant human naglu protein and uses thereof
ActiveUS20130095092A1Increase in α-N-acetylglucosaminidase activityReduction of substrate levelOrganic active ingredientsNervous disorderPhosphorylationADAMTS Proteins
The present invention provides compositions comprising an isolated mixture of recombinant human NaGlu proteins in which a substantial amount of the NaGlu proteins in the mixture has increased levels of phosphorylated mannose that confer the proteins to be efficiently internalized into human cells. The present invention also provides methods of producing such mixture of NaGlu proteins, vectors used in transgenesis and expression, host cells harboring such vectors, and methods of isolating and purifying the mixture of NaGlu proteins. The invention further provides methods of treating NaGlu associated diseases.
Owner:ALEXION PHARMA INC
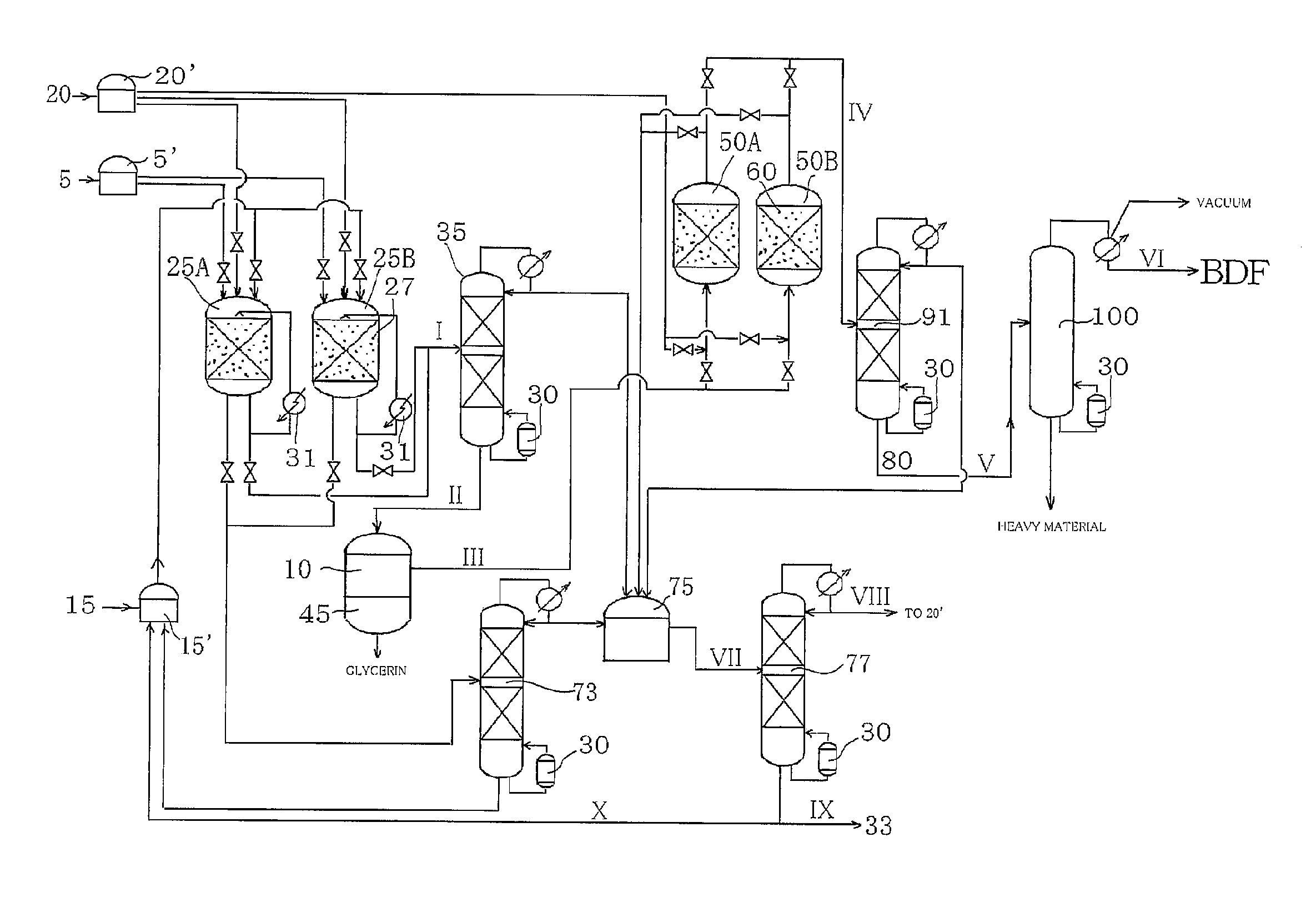
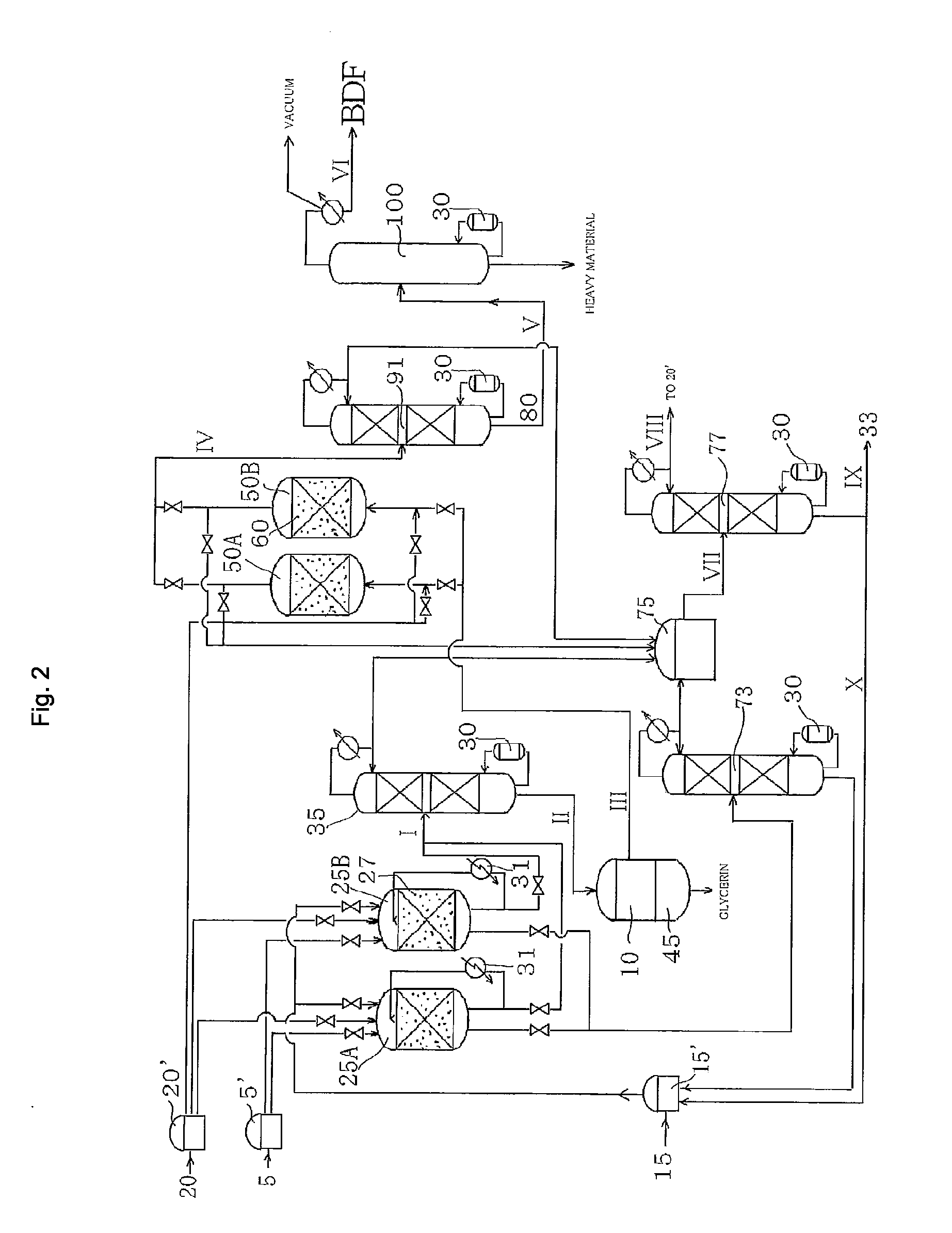
Method for producing fatty acid monoesterified product using solid acid catalyst
InactiveUS20100305346A1Production of a monoglyceride produced as a by-product can be suppressedProlong lifeFatty acid esterificationOrganic compound preparationMonoglycerideAlcohol
The present invention provides a method for producing a fatty acid monoester product by reacting an animal oil and / or a vegetable oil with an alcohol in the presence of a sulfonic acid group-introduced amorphous carbon catalyst and water. By this method, the production of a fatty acid monoglyceride, which is difficult to separate from the fatty acid monoester, can be suppressed, and the fatty acid monoester can be efficiently produced.Also, the present invention provides a method for producing a fatty acid monoester product by performing the transesterification reaction of an animal oil and / or a vegetable oil with an alcohol, or the esterification reaction of a fatty acid with an alcohol, in the presence of a sulfonic acid group-introduced amorphous carbon catalyst washed with water. By this method, the life of the catalyst can be extended, and the production cost can be reduced.
Owner:TOKYO INST OF TECH
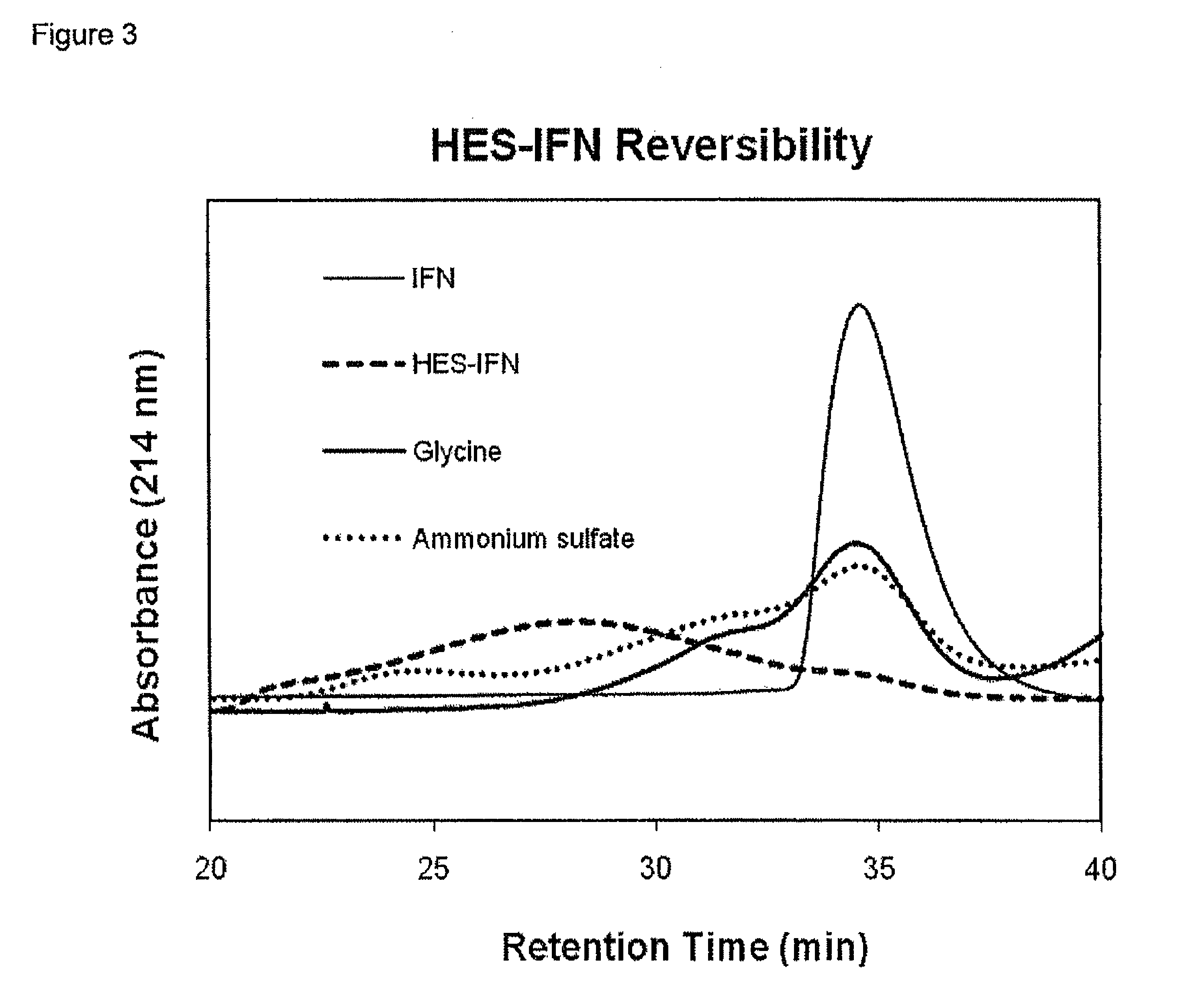
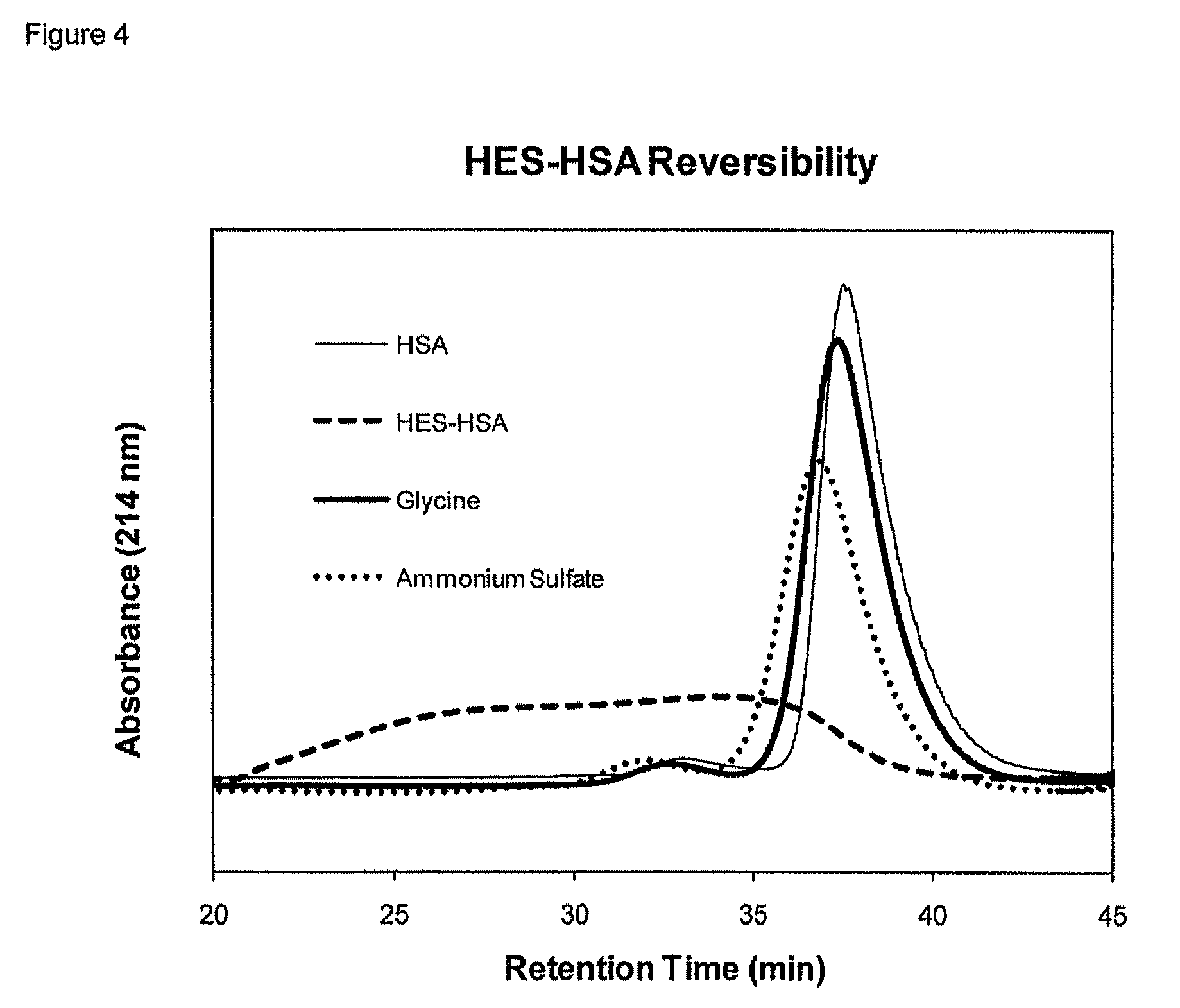
Polysaccharide-protein conjugates reversibly coupled via imine bonds
InactiveUS8840877B2Minimize the numberReduced activityHydrolasesPeptide/protein ingredientsProtein compositionPolyol
Method for preparing an oxidized polysaccharide-protein composition, by (a) oxidizing a polysaccharide with an oxidizing agent to form an oxidized polysaccharide where less than 20% of the oxidized units are comprised of alpha-hydroxy aldehyde units, (b) reacting the oxidized polysaccharide with a protein to form a composition comprising an oxidized polysaccharide-protein conjugate, and (c) maintaining the oxidized polysaccharide-protein conjugate composition by placing it in an environment where the temperature is less than 8° C. The oxidized polysaccharide and the protein are conjugated via one or more imine bonds, the oxidized polysaccharide-protein composition is soluble in aqueous solvent, and the composition is capable of releasing the protein.
Owner:THERAPURE BIOPHARMA INC
Supported catalysts for the fixation of carbon dioxide into aliphatic polycarbonates and a process for preparing the same
InactiveUS20030134740A1High catalytic efficiencyHigh molecular weightOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsGlutaric acidCarboxylic acid
The present invention provides a process for preparing supported zinc dicarboxylate catalysts with high activity for the copolymerization of carbon dioxide and epoxides by supporting zinc dicarboxylate on silica support. The zinc dicarboxylate may be synthesized from zinc oxide and dicarboxylic acid such as succinic acid, glutaric acid, adipic acid, pimelic acid and suberic acid. The silica support can be selected from the group consisting of aerosil, silica gel for chromatography or reagent grade silicon dioxide. The supporting process is performed in a planetary ball grinder under vacuum.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Method for reactivating ruthenium catalyst
InactiveUS6077983AEffective activityLess activityOther chemical processesOrganic compound preparationOrganic compoundHydrogen partial pressure
PCT No. PCT / JP96 / 03026 Sec. 371 Date Apr. 30, 1998 Sec. 102(e) Date Apr. 30, 1998 PCT Filed Oct. 18, 1996 PCT Pub. No. WO97 / 16249 PCT Pub. Date May 9, 1997A method for recovering the activity of a ruthenium catalyst which comprises a step of bringing a ruthenium catalyst decreased in activity by its use in hydrogenation of an unsaturated organic compound into contact with oxygen in a liquid phase, and a step of maintaining the catalyst at a hydrogen partial pressure lower than that at the hydrogenation and a temperature not lower than a temperature lower by 50 DEG C. than the hydrogenation temperature.
Owner:ASAHI KASEI KK
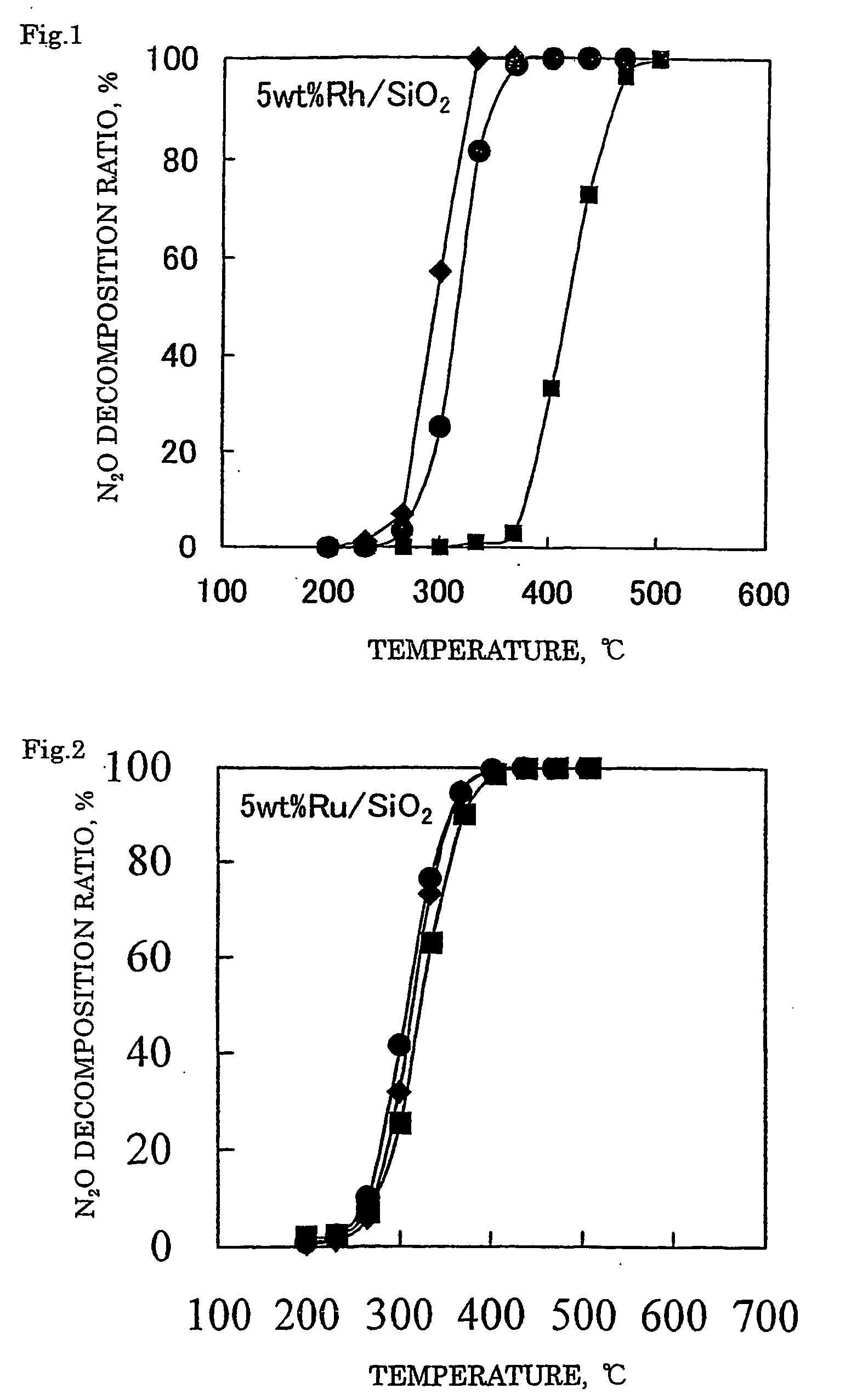
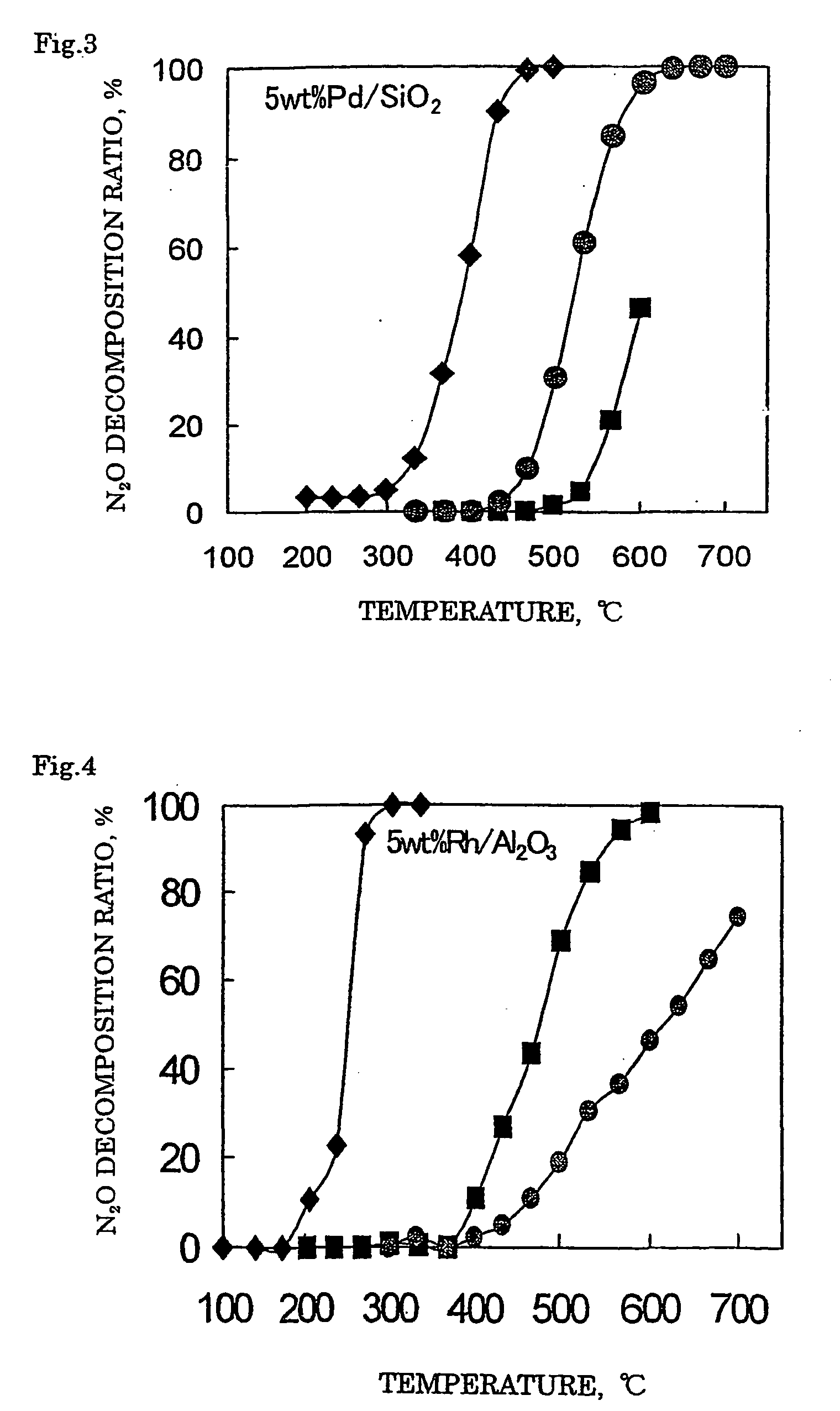
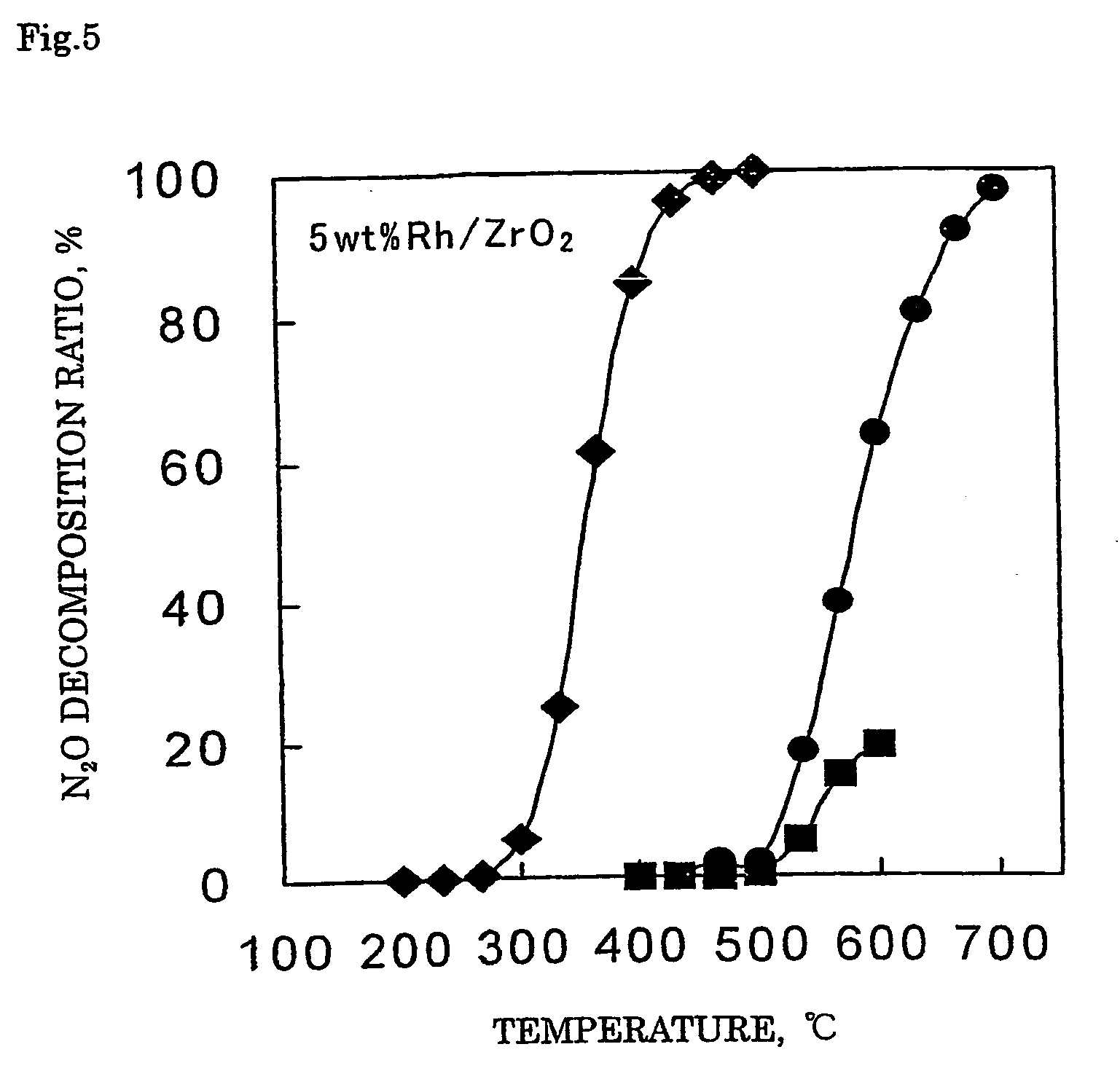
Decomposition catalyst for nitrous oxide, process for producing the same and process for decomposing nitrous oxide
InactiveUS20060008401A1Recovered activityReduce the amount requiredRespiratorsNitrous oxide captureDecompositionZinc
A method for decomposing nitrous oxide comprises contacting a catalyst for decomposing nitrous oxide with a nitrous oxide-containing gas at 200 to 600° C. The catalyst comprises a support and supported thereon at least one noble metal selected from rhodium, ruthenium and palladium. The support comprises silica or silica alumina. At least one metal selected from zinc, iron and manganese can be supported on the support.
Owner:SHOWA DENKO KK
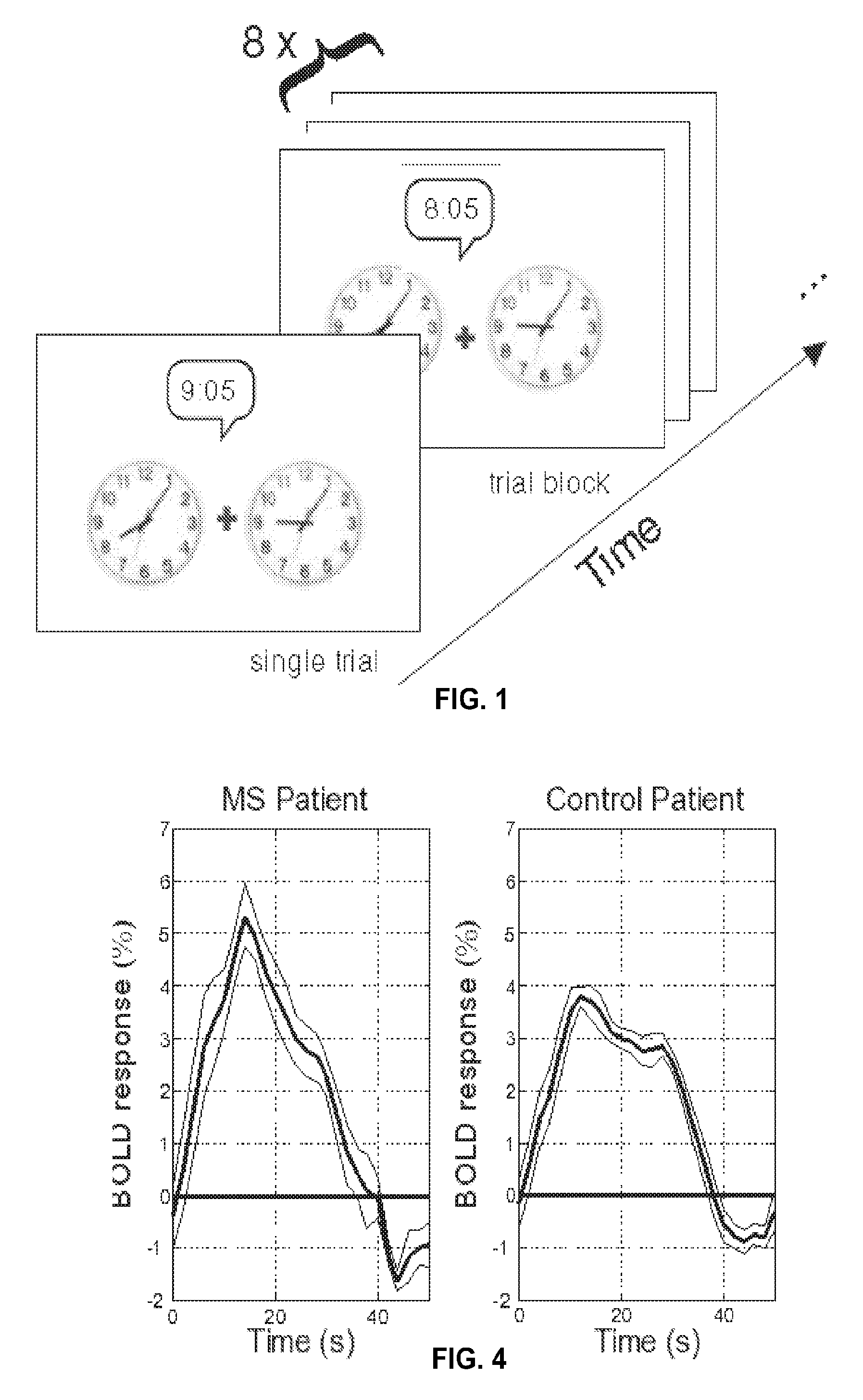
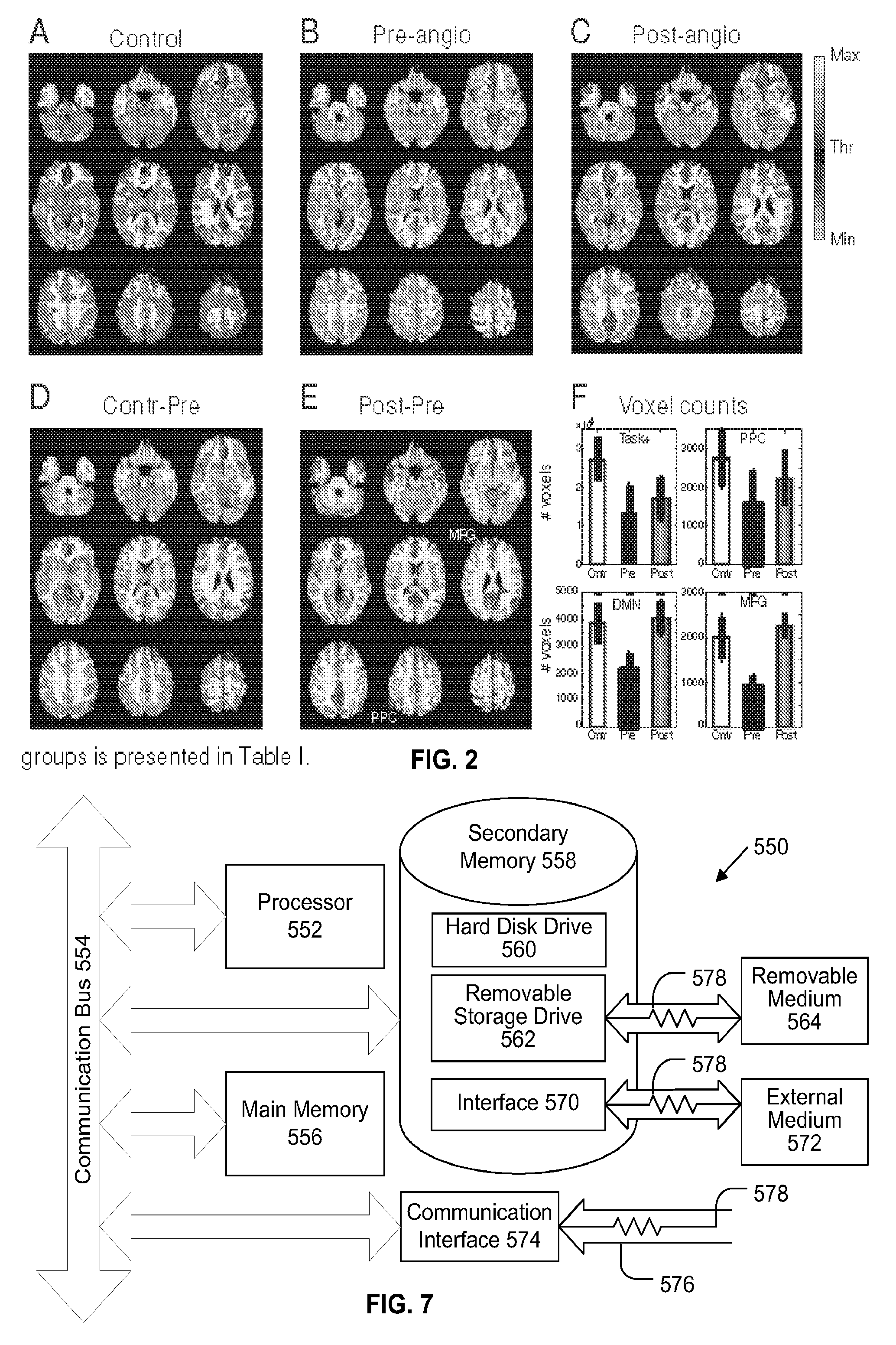
Method to Diagnose and Measure Vascular Drainage Insufficiency in the Central Nervous System
InactiveUS20120277572A1Increased post-stimulus undershootReturn to normal activitiesSensorsBlood flow measurementDiseaseBiological activation
Neurodegenerative diseases, such as multiple sclerosis, may be caused or aggravated by insufficient venous draining from the central nervous system. Functional MRI measures the surge of blood flow into localized regions of cerebral cortex in response to activation of those regions by performing visual, auditory or executive tasks. These fMRI measurements are based on blood-oxygen-level dependence. The resulting fMRI / BOLD data is converted to hemodynamic response data and analyzed to determine any abnormality in the hemodynamic response data. Vascular drainage insufficiency is identified in the presence of abnormal hemodynamic response data. Abnormal hemodynamic response data can be determined by a negative trough in a graph of the HDR data or by the duration, depth, or area of the negative trough.
Owner:HUBBARD DAVID R
Reversible attachment of a membrane active polymer to a polynucleotide
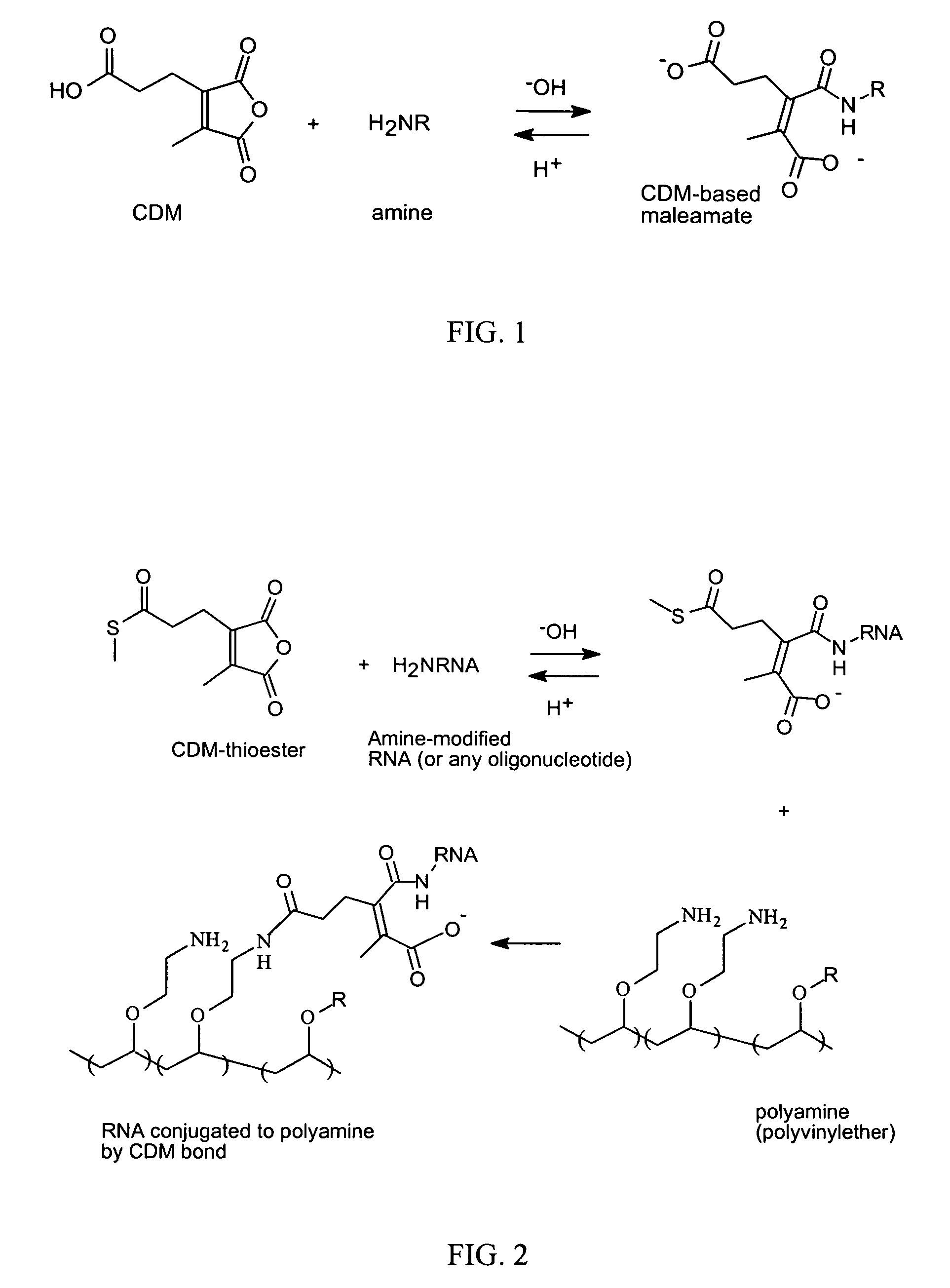
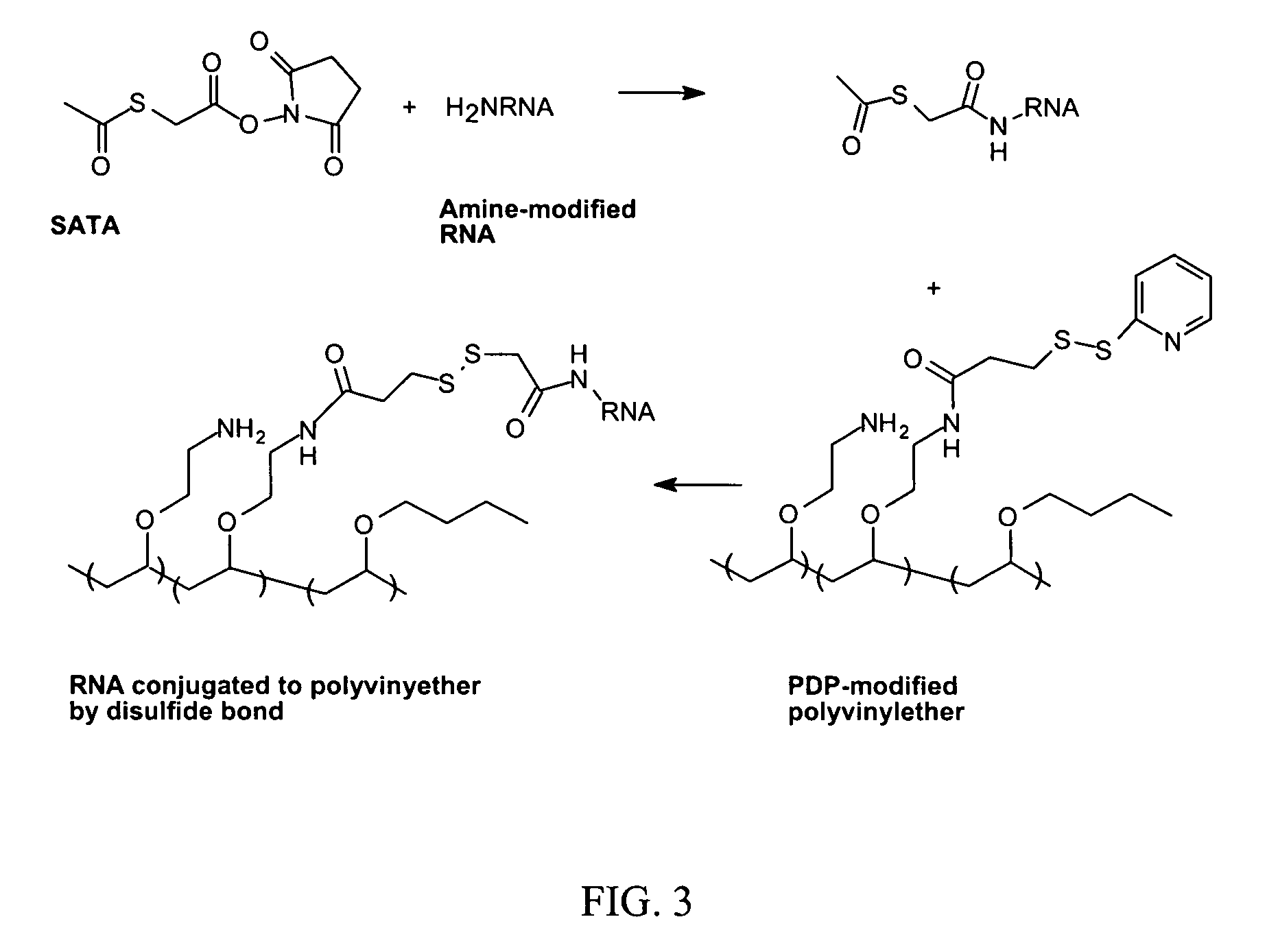
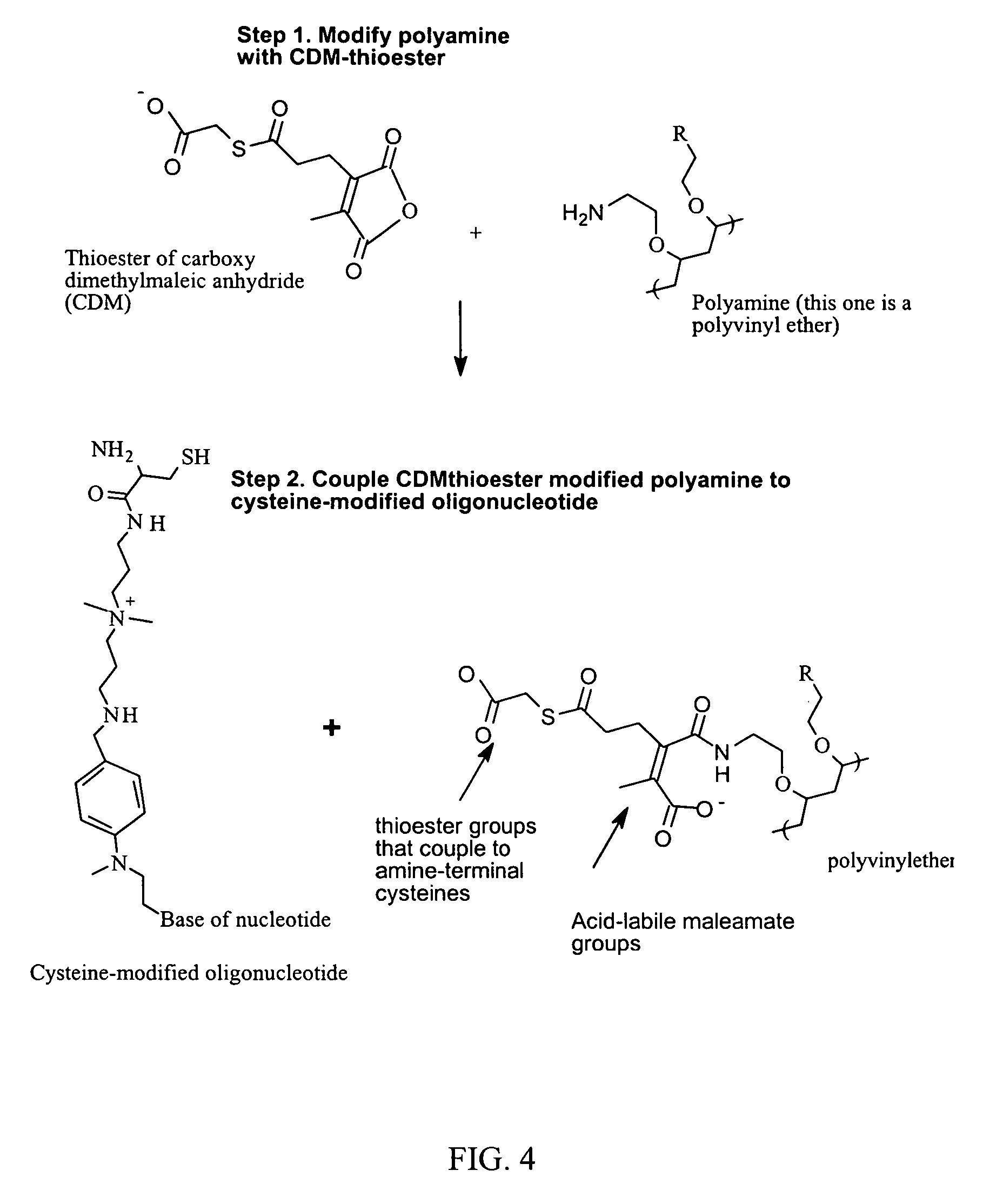
ActiveUS7816337B2Reduce membrane activityReduces membrane activityBiocideSpecial deliveryToxicityNucleotide
Described is a process for delivering a biologically active compound to a cell by reversibly linking the compound to a membrane active polymer. In particular, polymer-polynucleotide conjugates are described. Methods for reversibly modifying the polymers to decrease cellular toxicity and improve efficacy are provided.
Owner:ARROWHEAD MADISON
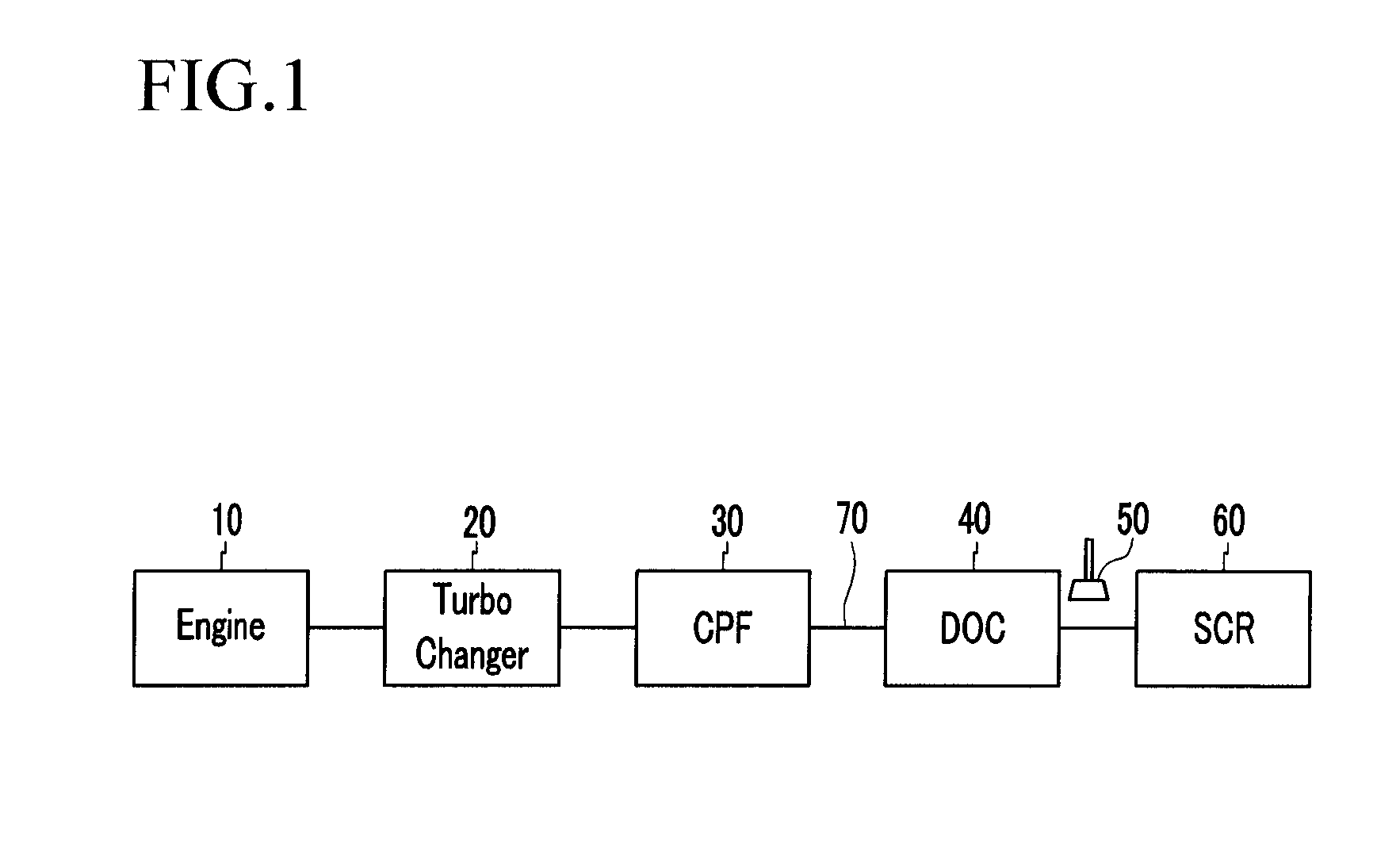
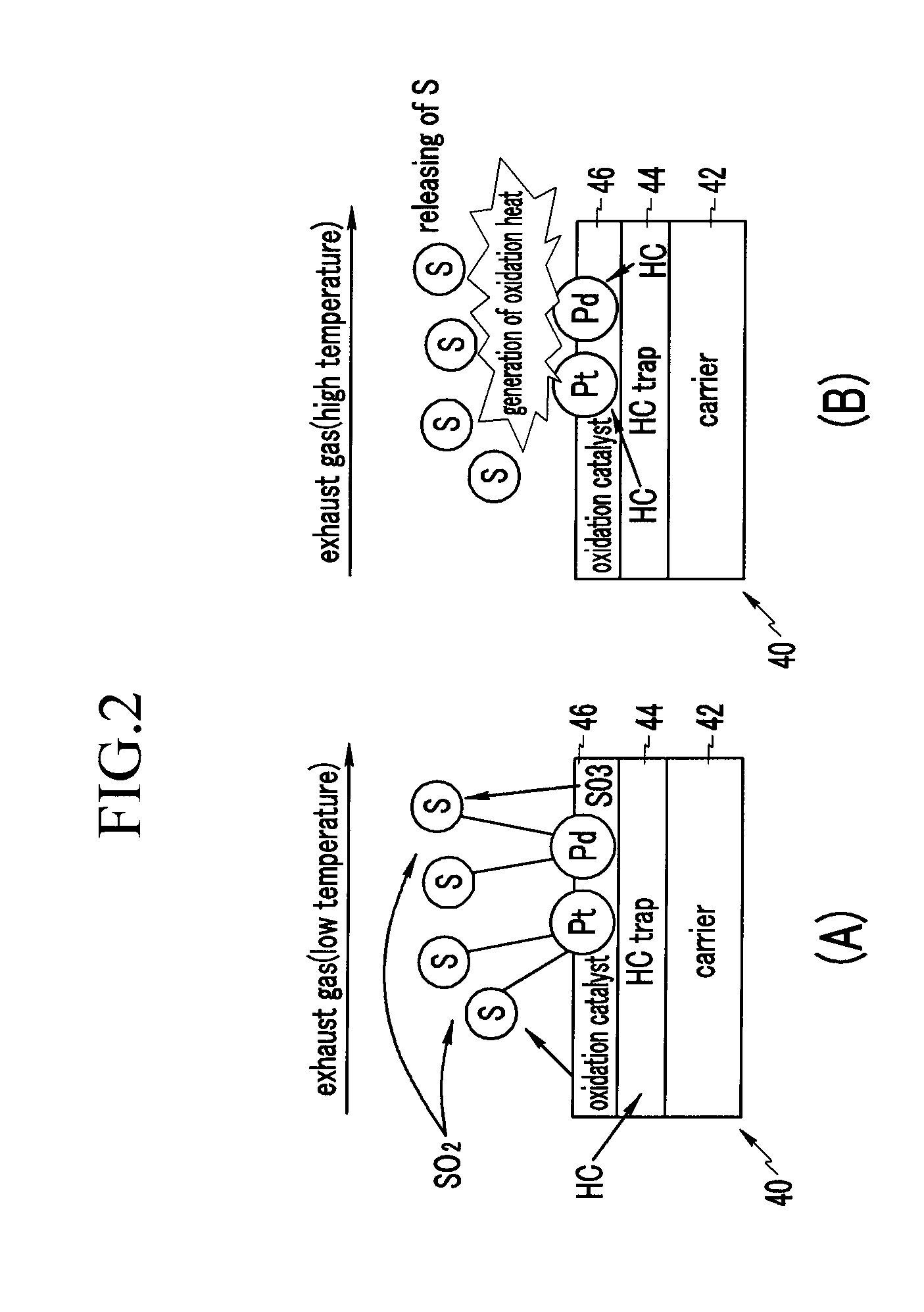
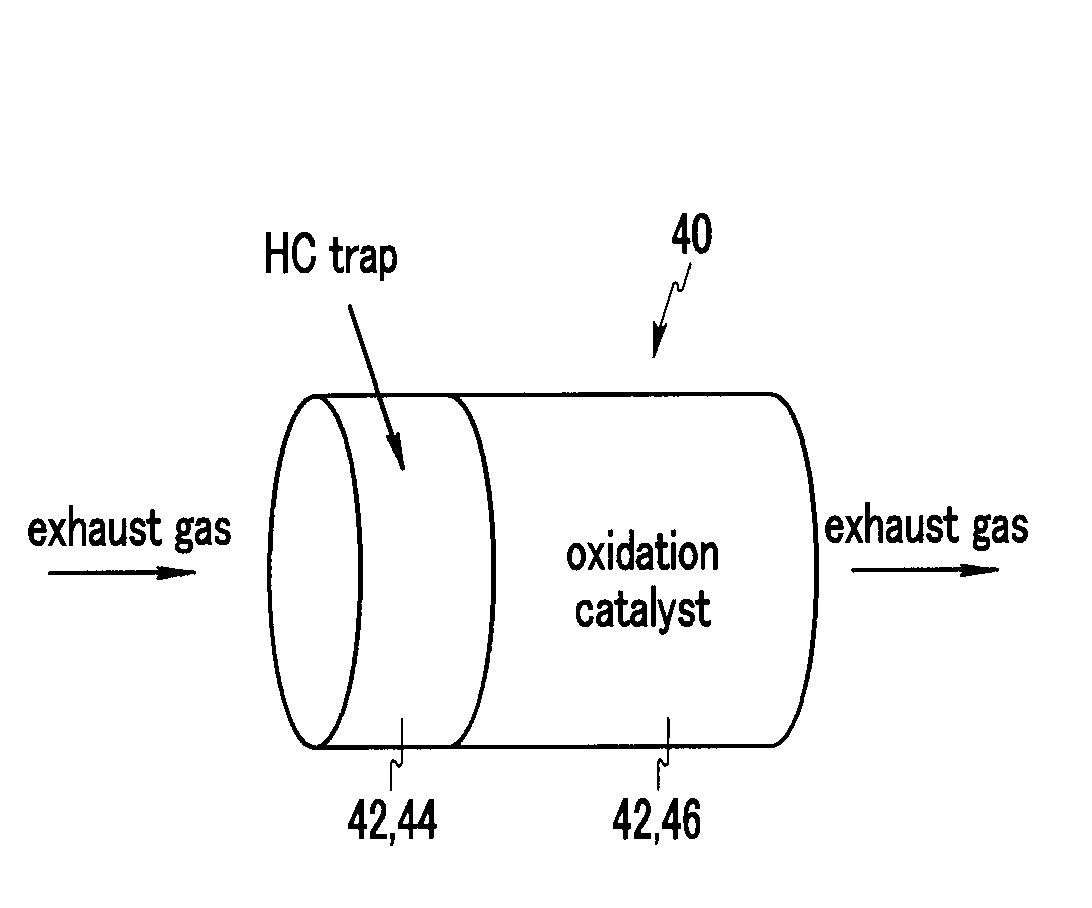
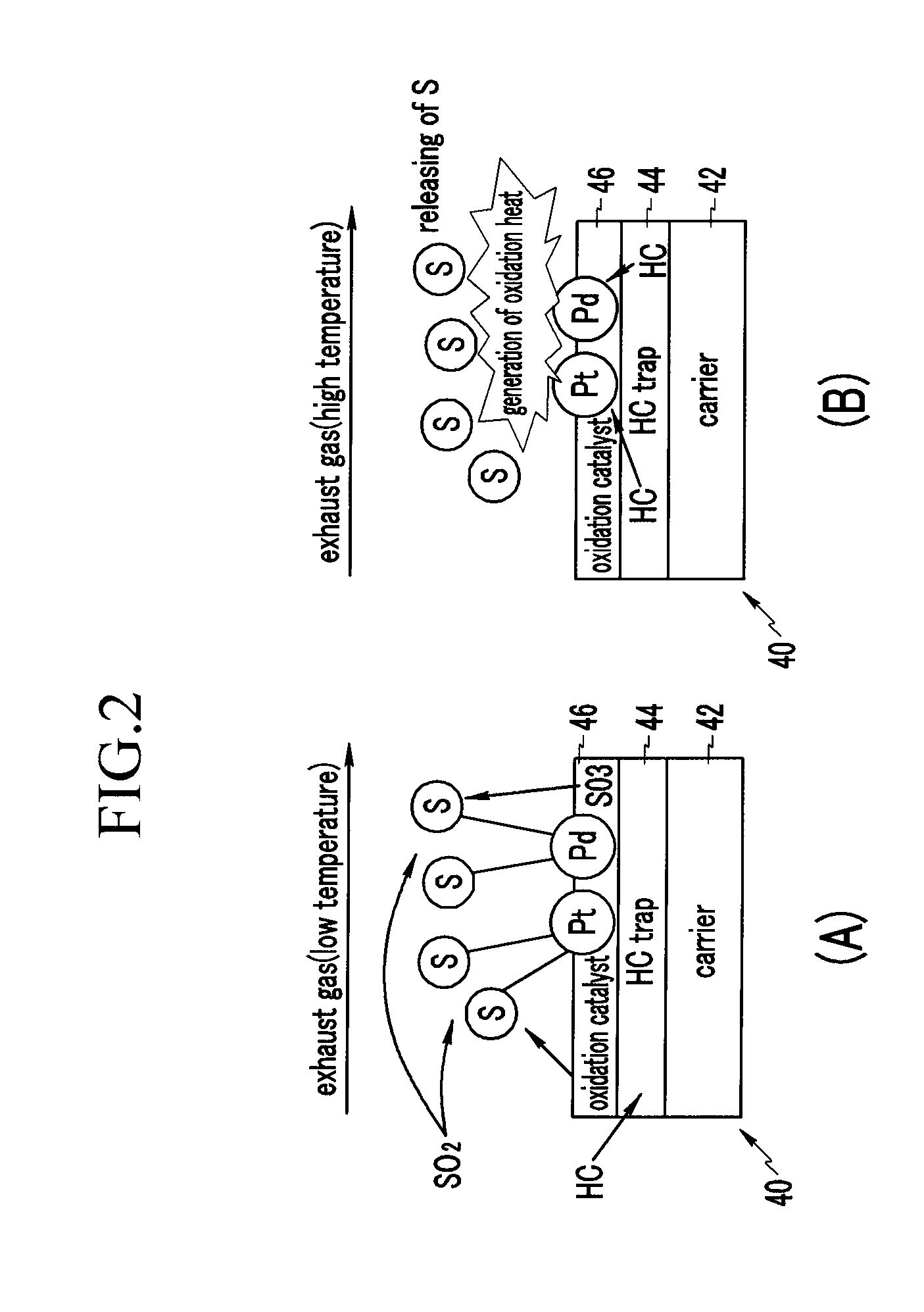
Diesel Oxidation Catalyst and Exhaust System Provided with the Same
InactiveUS20100126150A1Return to normal activitiesCombination devicesInternal combustion piston enginesExhaust fumesExhaust gas emissions
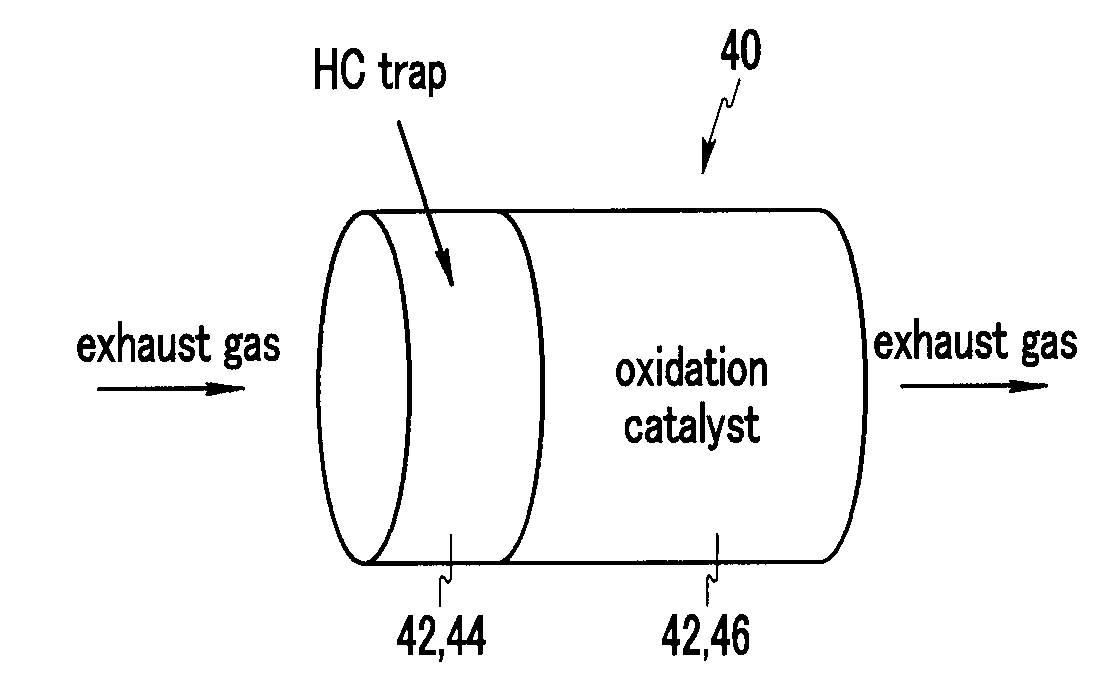
A diesel oxidation catalyst mounted on an exhaust pipe that exhausts an exhaust gas generated in an engine to the exterior may include a first portion having a hydrocarbon trap (HC trap) coated thereon, the HC trap absorbing or releasing a hydrocarbon (HC) depending on whether or not a predetermined condition is satisfied, and a second portion having an oxidation catalyst coated thereon, the oxidation catalyst oxidizing the hydrocarbon (HC) and a carbon monoxide (CO) in the exhaust gas, wherein the second portion performs oxidation reaction with the HC released from the first portion and releases sulphur absorbed at the oxidation catalyst by using oxidation heat generated in the oxidation reaction thereof.
Owner:HYUNDAI MOTOR CO LTD +1
Methods and Compositions for Treating Status Epilepticus and Seizures Causing Status Epilepticus
InactiveUS20110230473A1Improve survivabilityPreventing and inhibiting and reducing seizureBiocideNervous disorderNR1 NMDA receptorNMDA receptor
Disclosed herein are methods, kits and compositions for treating, preventing, inhibiting, or reducing a seizure, status epilepticus, neuropathogenesis or a neuropathology caused by overstimulation of the NMDA receptor pathway and / or exposure to an OP compound.
Owner:GORDON RICHARD K +5
Alpha1 proteinase inhibitor peptides methods and use
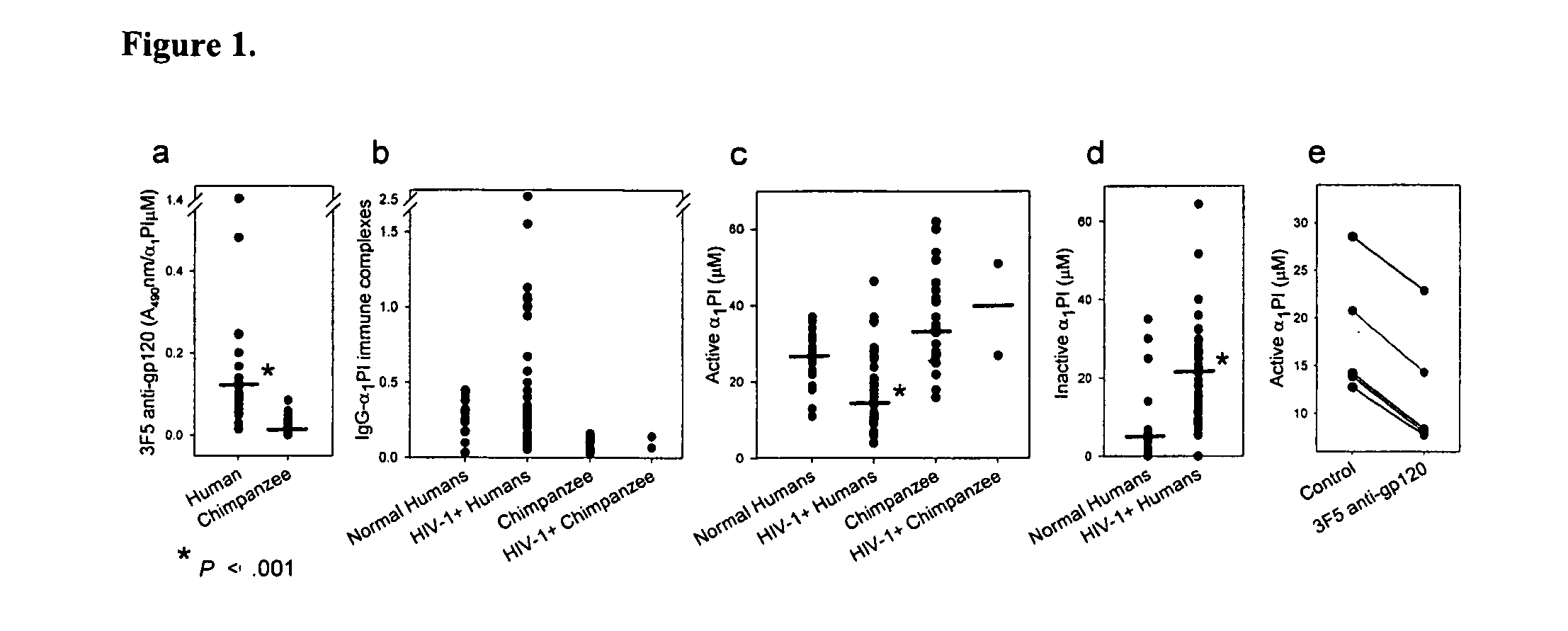
InactiveUS20100029558A1Increasing CD lymphocyte renewalReturn to normal activitiesCompound screeningAnimal cellsDiseaseAlpha1-proteinase inhibitor
The invention is directed to the use of peptides that can bind and block the interaction of α1 proteinase inhibitor (α1PI) and one or more molecules, for example antibodies to HIV-1 envelope proteins. The invention features methods of activating α1PI in a cell, methods of treating or preventing a disease or disorder in a subject, for example HIV-1 or AIDS. The invention also features pharmaceutical compositions comprising one or more peptides that block the interaction of α1α1PI and one or more molecules. Also included in the invention are kits.
Owner:BRISTOW CYNTHIA L
Diesel oxidation catalyst and exhaust system provided with the same
InactiveUS8168125B2Return to normal activitiesCombination devicesInternal combustion piston enginesExhaust gas emissionsExhaust pipe
A diesel oxidation catalyst mounted on an exhaust pipe that exhausts an exhaust gas generated in an engine to the exterior may include a first portion having a hydrocarbon trap (HC trap) coated thereon, the HC trap absorbing or releasing a hydrocarbon (HC) depending on whether or not a predetermined condition is satisfied, and a second portion having an oxidation catalyst coated thereon, the oxidation catalyst oxidizing the hydrocarbon (HC) and a carbon monoxide (CO) in the exhaust gas, wherein the second portion performs oxidation reaction with the HC released from the first portion and releases sulphur absorbed at the oxidation catalyst by using oxidation heat generated in the oxidation reaction thereof.
Owner:HYUNDAI MOTOR CO LTD +1
Preservatives
ActiveUS20150289503A1Return to normal activitiesRecovery functionAntibacterial agentsPowder deliveryActive agentPreservative
There is described a composition comprising a microparticle component; optionally an encapsulated active agent; and a preservative amount of one or more terpenes.
Owner:EDEN RESPLC
Apparatus for the stimulation of neural networks
ActiveUS8538547B2Return to normal activitiesSpinal electrodesArtificial respirationElectricityNerve network
Owner:NEMOTEC UG HAFTUNGSBESCHRANKT
Humanized light chain mice
ActiveUS9622459B2Reduces and eliminates ADAM activityImprove fertilityHybrid immunoglobulinsHydrolasesHuman immunoglobulinsGenetic Materials
Owner:REGENERON PHARM INC
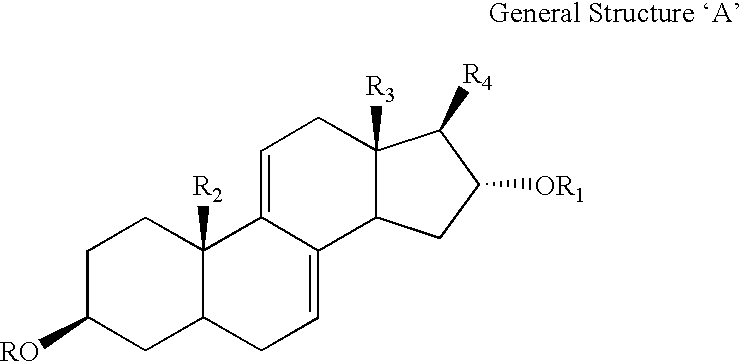
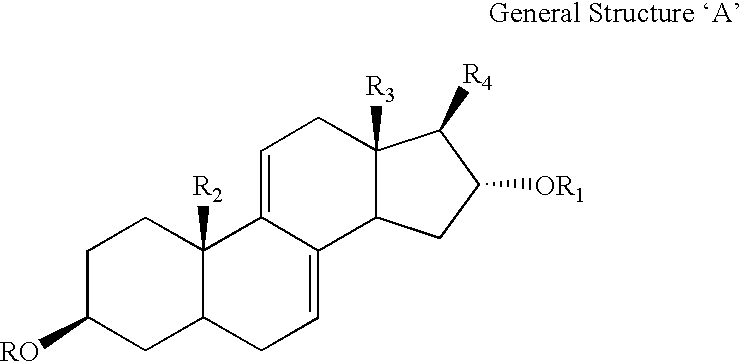
Medicament for the treatment of diabetes
InactiveUS6531461B1Effective treatmentReturn to normal activitiesOrganic active ingredientsEndocrine system disorderDiabetes mellitusToxic dose
A series of compounds of general structure I and metal salts thereof. These compounds are useful in the treatment of diabetes mellitus and associated conditions when administered in an effective non-toxic dose in the form of a pharmaceutically acceptable composition resulting in cell regeneration.wherein for example,R=H, R1=H, R2=Me, R3=Meand R4=
Owner:NELSON LOUIS OBYO OBYO
Fuel cell apparatus
A fuel cell apparatus includes a fuel cell generating electric power, and including a fuel electrode which includes an anode catalyst, which is disposed in one side of an electrolyte membrane, which is supplied with liquid fuel, and which discharges gas generated by a chemical reaction accelerated by the anode catalyst, and an oxidizing agent electrode which includes a cathode catalyst, which is disposed in the other side of the electrolyte membrane, and which is supplied with air, and a control unit controlling a load applied to the fuel cell. The control unit increases the load in at least one of two cases, one case being when electric power generated by the fuel cell lowers below a predetermined reference value and another case being at predetermined time intervals, and stops the increase of the load after elapsing a predetermined time period from the start of the increase of the load.
Owner:KK TOSHIBA
Lectin protein prepared from maackia fauriei, process for preparing the same and the use thereof
InactiveUS20050084903A1Return to normal activitiesSaccharide peptide ingredientsDepsipeptidesMelanomaCancer cell
The present invention relates to a lectin protein, designated MFA isolated and purified from the bark of the Korean legume Maackia fauriei, process for preparing the same and the use thereof. This protein can be used as reagents in the study of carbohydrate binding proteins as well as to examine the distribution of N-acetylneuraminic acid in cancer cells owing to its capability that specifically recognizes N-acetylneuraminic acid which plays important structural and functional roles in the expressions of various cells or oligosaccharide terminal residue of glycoconjugates, and, in addition, used as an anti-cancer drug in view of its anti-proliferation effect against various cancers such as breast cancer, melanoma, hepatoma, etc.
Owner:CHUNG ANG UNIV IND ACADEMIC COOP FOUND
Chinese patent medicine for treating gouty
InactiveCN102366566ANo painReturn to normal activitiesSkeletal disorderPlant ingredientsSide effectCurative effect
The invention provides a Chinese patent medicine for treating gouty, which comprises the following components in part by weight: 28-32 parts of mulberry branch, 9-15 parts of radix achyranthis bidentatae, 12-18 parts of caulis lonicerae, 9-15 parts of phellodendron, 28 - 32 parts of coix seed, 9-15 parts of erythrina indica lam, 9-15 parts of clematis chinensis, 7-11 parts of Chinese atractylodes, 18-22 parts of licorice, and 8-12 parts of large-leaved gentian. Compared with the prior art, aiming at the inherent pathogenic factor of the gouty, the Chinese patent medicine carries out syndrome differentiation and treatment, has quick curative efficacy, low cost, no toxic or side effects, accurate curative effects, and obvious effects, and addresses both the symptoms and root causes.
Owner:CHENGDU LYUDI TECH
Recombinant human naglu protein and uses thereof
InactiveUS20140255383A1Reducing potential risk of immunogenicityHigh activityOrganic active ingredientsNervous disorderPhosphorylationADAMTS Proteins
The present invention provides compositions comprising an isolated mixture of recombinant human NaGlu proteins in which a substantial amount of the NaGlu proteins in the mixture has increased levels of phosphorylated mannose that confer the proteins to be efficiently internalized into human cells. The present invention also provides methods of producing such mixture of NaGlu proteins, vectors used in transgenesis and expression, host cells harboring such vectors, and methods of isolating and purifying the mixture of NaGlu proteins. The invention further provides methods of treating NaGlu associated diseases.
Owner:SYNAGEVA BIOPHARMA CORP
Silicon implant with expandable and/or interactive compartments, optionally coated with a ricinus communis and/or hydroxylapatite polyurethane foam, with attachment flaps or strings
InactiveCN102325507AReturn to daily activitiesQuality improvementMammary implantsCosmetic implantsAreolaButtocks
The invention relates to an implant for augmenting or reconstructing breasts, buttocks, thighs and calves, having independent expandable and interactive compartments that can be filled during manufacture or during or after surgery, with an external silicone membrane (1) internally and / or externally coated with a Ricinus communis polyurethane foam (14) covered with hydroxylapatite microcrystals (13) or nanocrystals (13) incorporated into the same membrane, with an inner space divided into compartments (3) that are or can be filled with three-dimensional geometric structures (2) immersed in cohesive silicone (3) or another gel, liquid, air or gas, with a chamber for the projection of the body (4) or of the lower pole of the breast, having a conical structure within the main chamber in order to project the latter. The inner and outer chambers are independently filled and have different volumes, and since they are interactive, they allow any necessary adjustments of the implant through filling valves (8), in order to project the region (6) of the areola and nipple, the body or other region of the breast, correcting ptosis, assymetry, congenital thoracic hypertrophy in the mammal or other region. Flaps (10) or strings (11) for immediate attachment ensure safe and early mobility, faster return to normal activity, and a secure final placement of the implant; they avoid displacement of the implant and can be previously removed by the surgeon.
Owner:乔斯・玛利亚・德米兰达
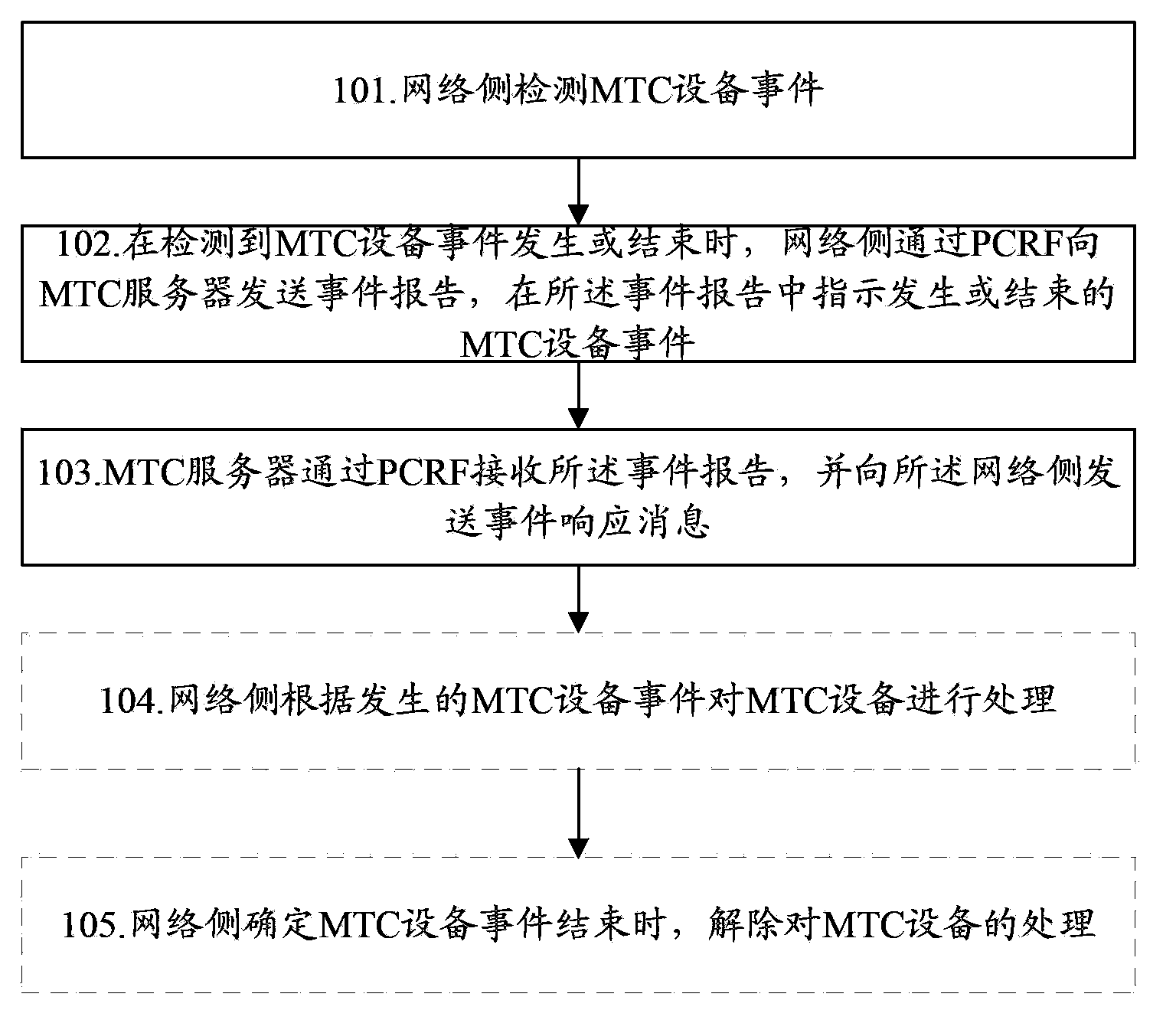
Method and system for monitoring machine type communication device events, and network side
InactiveCN103781015AReturn to normal activitiesAssess restrictionTelephonic communicationCommunication deviceReal-time computing
The invention discloses a method for monitoring machine type communication device events. The method comprises the following steps: a network side detects a machine type communication (MTC) device event; and the network side sends an event report to a MTC server through a PCRF after detecting that the MTC device event happens or ends, and indicates the happened or ended MTC device event in the event report. The invention further discloses a system for monitoring machine type communication device events and a network side. According to the scheme of the invention, the network side can report a MTC device event to the MTC server, processes a MTC device according to the happened MTC device event, and restores normal operation of the MTC device after detecting that the happened MTC device event ends.
Owner:ZTE CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com