Vaccine for inducing an improved immune reaction
A vaccine and antigen technology, applied in the field of immune response vaccine composition, can solve the problem of negligible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0084] Hereinafter, the present invention will be described in more detail through examples. These embodiments are only to describe the present invention in more detail. According to the gist of the present invention, the scope of protection claimed by the present invention is not limited to these embodiments, which is obvious to those of ordinary skill in the technical field of the present invention .
[0085] 【Example】
Embodiment 1
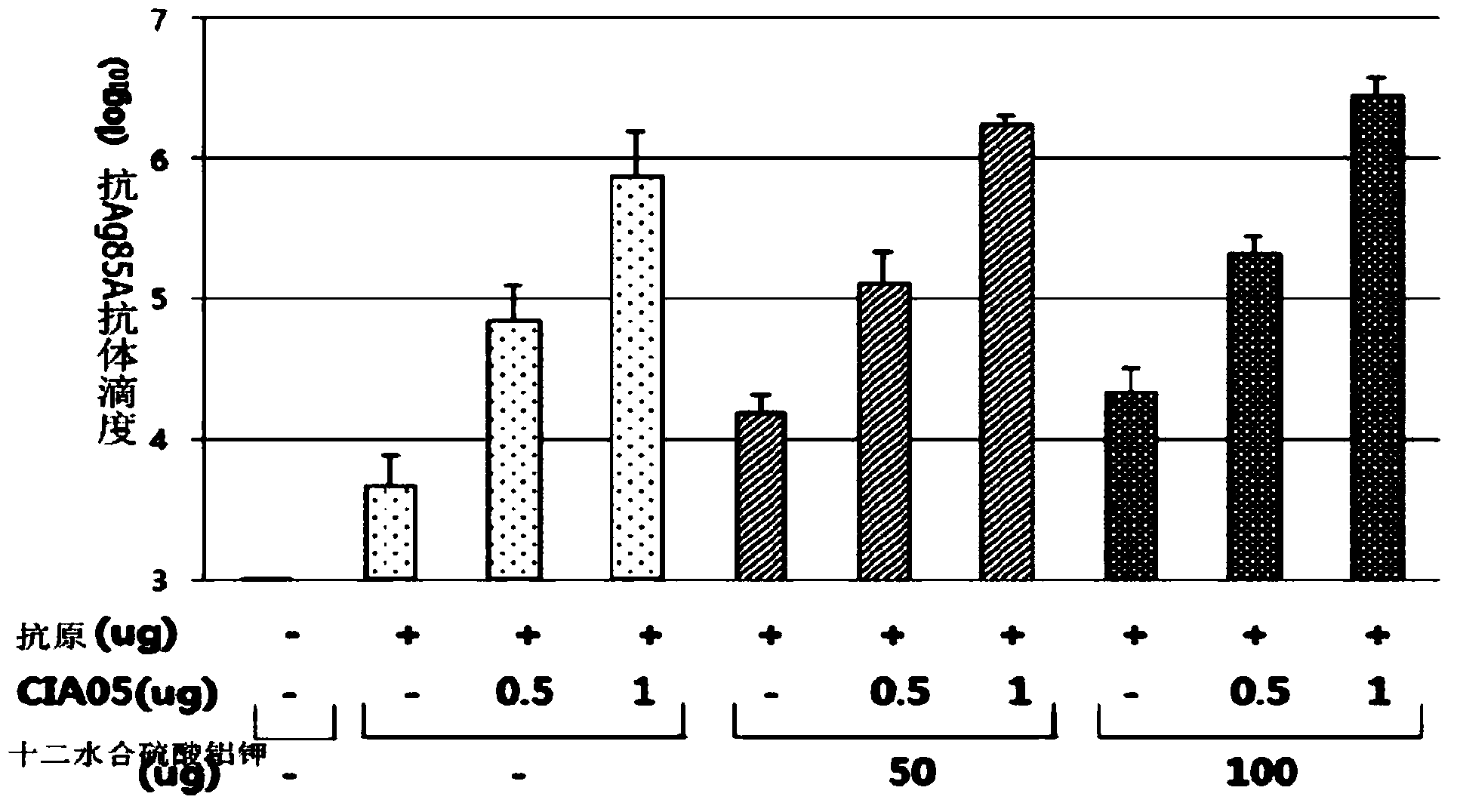
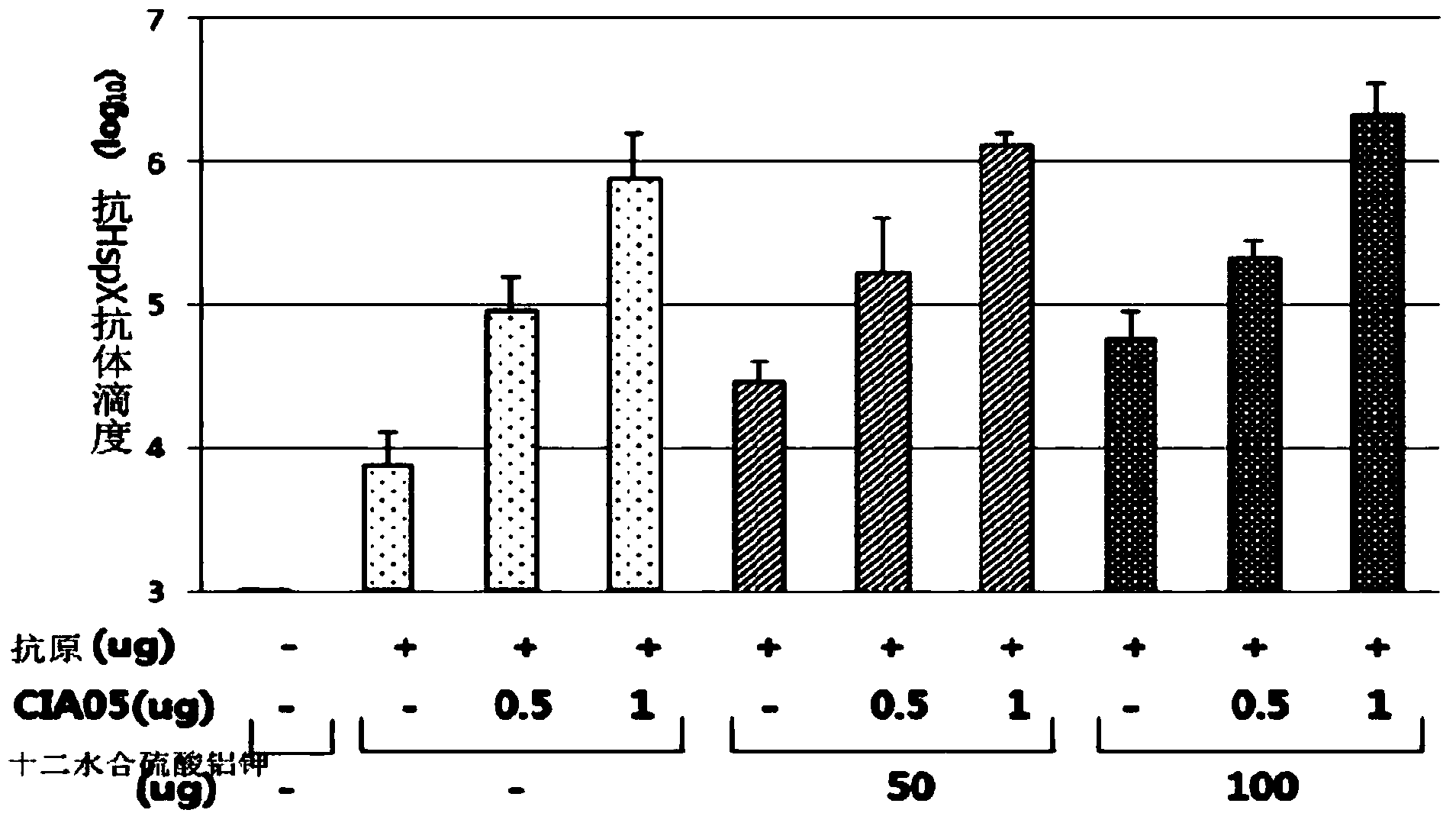
[0086] [Example 1: Production of very short lipooligosaccharide (LOS) CIA05 as a new immune adjuvant]
[0087]The present inventors have discovered a lipopolysaccharide strain (E. coli EG0021) having a very short sugar chain length of lipooligosaccharide from Escherichia coli living in the intestinal tract of healthy humans, and published on May 2, 2002 , entrusted this strain to the depository organization - Korea Microorganism Storage Center, and obtained the deposit number KCCM10374 (reference: Korean Patent Grant No. 0456681; WO2004 / 039413; Korean Patent Grant No. 0740237; WO2006 / 121232). Purification of lipopolysaccharide from the above strains was performed according to the methods disclosed in Korean Patent Grant No. 0456681; WO2004 / 039413; Korean Patent Grant No. 0740237; WO2006 / 121232. The molecular weight of the purified lipopolysaccharide was measured by MALDI-MASS (Shimadz Corporation, Axima-LNR V2.3.5 (Mode Liner, Power: 106)). As a result of the measurement, it w...
Embodiment 2
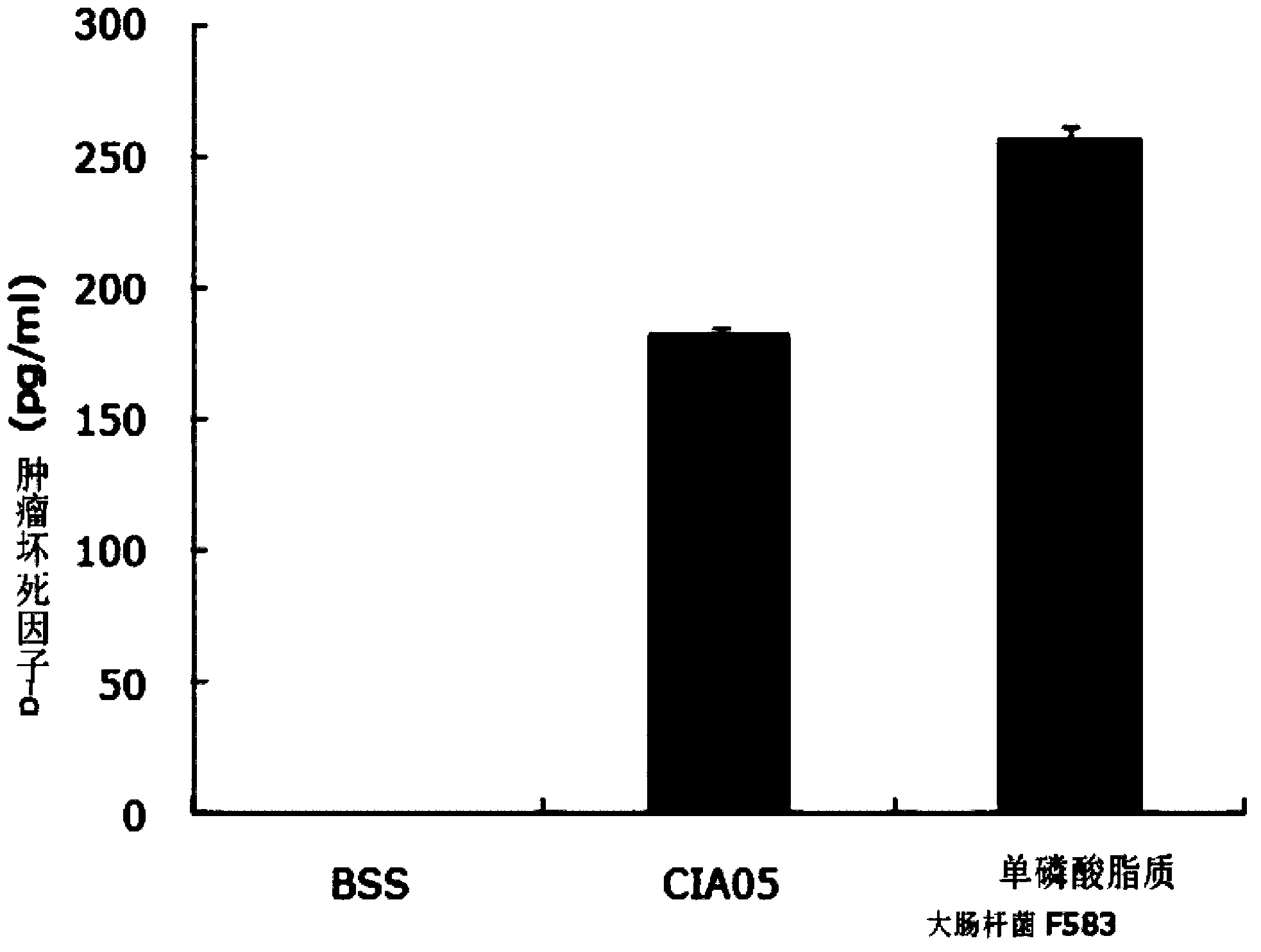
[0088] [Example 2: Comparative experiment on toxicity between the new immune adjuvant CIA05 and the existing immune adjuvant monophospholipid A]
[0089] The toxicity of CIA05 prepared in the above examples used as immune adjuvant in the vaccine of the present invention and the monophospholipid A used in the existing cervical cancer vaccine was compared and analyzed. Receive blood from healthy people to isolate peripheral blood mononuclear cells (PBMC, Peripheral Blood Mononuclear Cell), in 24-well culture dishes with 5×10 5 The above-mentioned peripheral blood mononuclear cells were cultured at a concentration of cell / ml. At this time, a culture solution obtained by mixing 10% fetal bovine serum (FBS, fetal calf serum; Gibco) with RPMI1640 (Gibco) was used, and the volume of each well was divided into 1 ml. The prepared Petri dishes were individually processed under the following conditions. 1) Negative control group: Balanced salt solution (BBS, Balanced salt solution) 100...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com