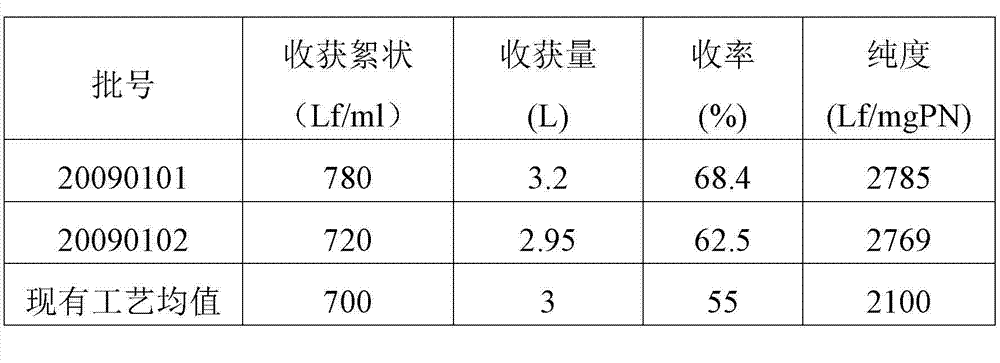
Method for preparing tetanus toxoid vaccine
A tetanus toxoid and tetanus technology, applied in the direction of bacterial antigen components, antibacterial drugs, etc., can solve the problems of inconvenient refining operation, process that does not meet the requirements of GMP, and easy blockage of filter plates, so as to reduce antigen damage and shorten preparation Time, reduce the effect of channel clogging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
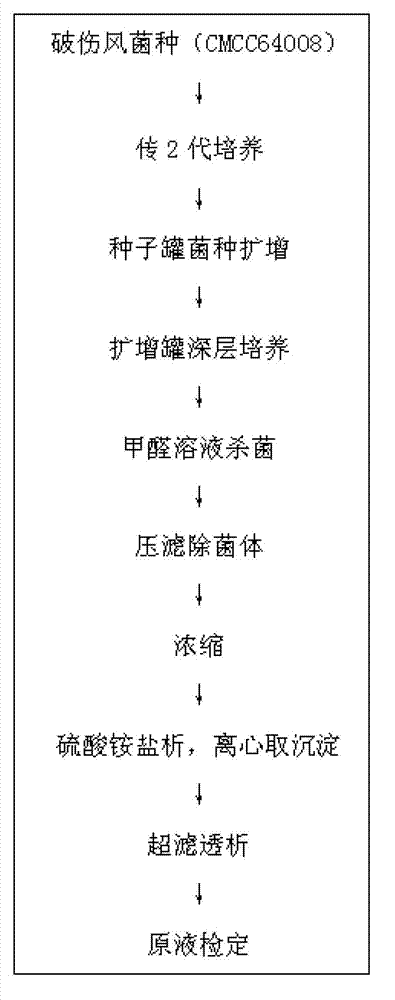
[0016] A preparation method of tetanus toxoid vaccine, comprising the following steps:
[0017] 1) Tetanus toxoid culture
[0018] The bacterial classification adopted is Clostridium tetani, derived from China Food and Drug Control Research Institute, and the bacterial number is CMCC64008, which is used after passing the test. Means to ferment the strain, cultivate it in a seed tank at 34-36°C for 40 hours, the size of the seed tank is 50L, and then transfer it to a large tank for cultivation, adopt a 1000L fermentation system, culture at a temperature of 34-36°C, and cultivate 67 The culture was stopped after 1 hour. After the fermentation is completed, add formaldehyde solution until the final concentration of formaldehyde is 0.35% (V / V), and keep warm for 30 minutes at 30~35°C.
[0019] 2) Separation of bacterial liquid
[0020] Take 50L of tetanus toxoid culture solution (73Lf / ml), with NaHCO 3 Adjust the pH to 6.9, and press filter with a plate and frame filter (model...
Embodiment 2
[0035] A preparation method of tetanus toxoid vaccine, comprising the following steps:
[0036] 1) Tetanus toxoid culture
[0037] The culture method is the same as in Example 1.
[0038] 2) Separation of bacterial liquid
[0039] Get 50L tetanus toxoid culture fluid (68Lf / ml), with NaHCO 3 Adjust the pH to 7.5, and use a filter press to remove bacteria under the condition that the pressure is not greater than 0.1Mp.
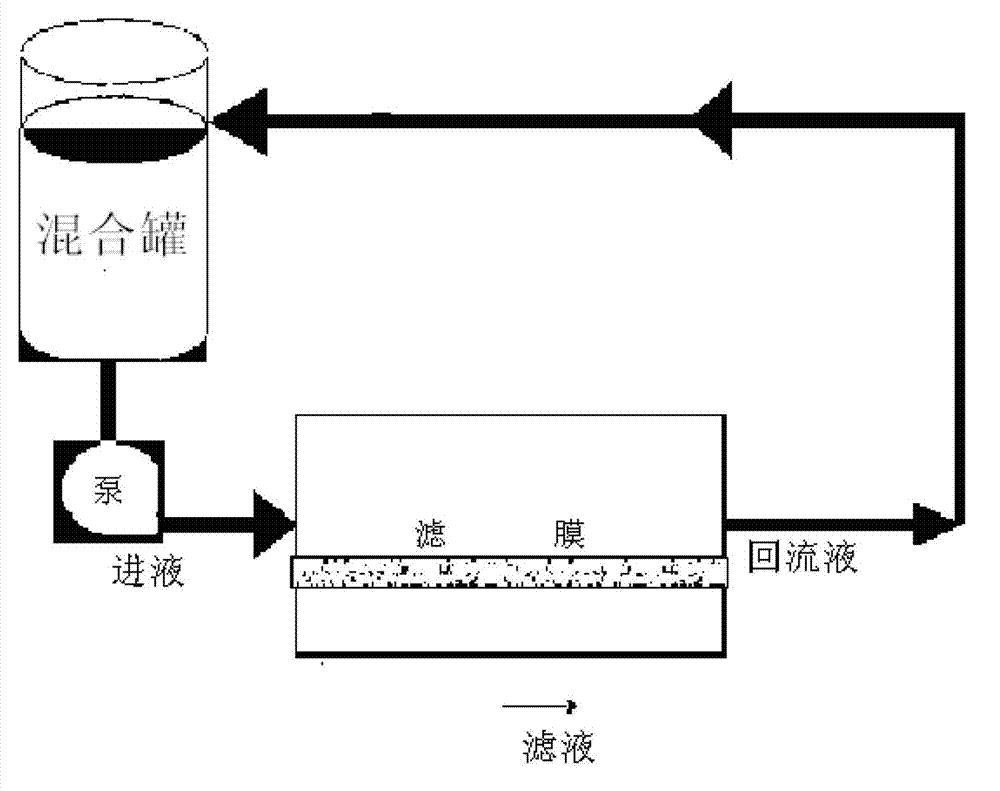
[0040] 3) Concentration by ultrafiltration
[0041] The filtrate is collected, and the filtrate is concentrated by tangential flow ultrafiltration with an ultrafiltration system of a 40KD ultrafiltration membrane. The steps of the ultrafiltration concentration are:
[0042] Connect the ultrafiltration system and the refining tank, connect the inlet of the ultrafiltration system, the return port and the waste liquid port respectively, and open the ultrafiltration system;
[0043] Confirm that all the valves of the system are open, adjust the frequency conver...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com