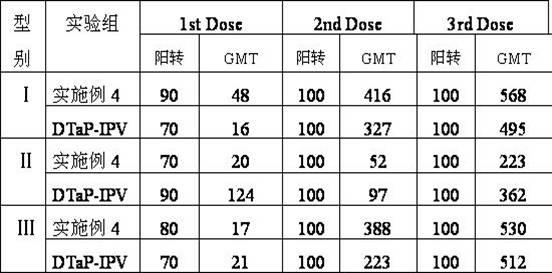
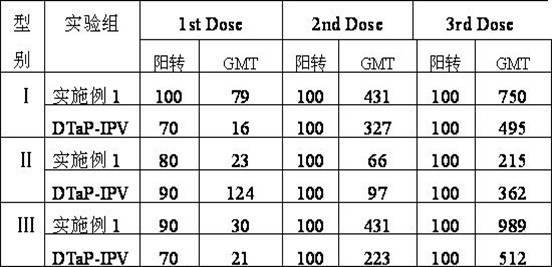
Combined vaccine for adsorbing Diphtheria, tetanus and acellular pertussis-Sabin inactivated poliovirus and preparation thereof
A cell-free DTP and poliomyelitis technology, applied in the field of medical biology, can solve the problems of increasing the chance of cross-infection, unfavorable planned immunization, and affecting the vaccination rate, so as to reduce the number of vaccinations, prevent various target diseases, and improve the The effect of vaccination rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1) Phase I pertussis CS strain (bacteria number 58003-3), Clostridium tetani (bacteria number 64008) and diphtheria PW8 strain (bacteria number 38007) purchased from the serum laboratory of China National Institutes for Food and Drug Control ), and poliovirus type I SabinSO+2, type II SabinSO+2, and type III PfizerSOR+1 purchased from the World Health Organization (WHO), respectively, according to the requirements of the Pharmacopoeia of the People's Republic of China in 2010, prepared into acellular pertussis stock solution AP, Tetanus toxoid TT, diphtheria toxoid DT, Ⅰ, Ⅱ, Ⅲ Sabin strain poliovirus inactivation solution sIPV Ⅰ, sIPV Ⅱ, sIPV Ⅲ, spare;
[0043] 2) Take the material according to the following formula:
[0044] Acellular pertussis liquid AP 400ugPN
[0045] Tetanus Toxoid TT 700Lf
[0046] Diphtheria Toxoid DT 2500Lf
[0047] Al(OH) 3 154mg
[0048] sIPVI type 6000DU
[0049] sIPV Ⅱ type 7100DU
[0050] sIPV Ⅲ type 9...
Embodiment 2
[0057] 1) same as step 1 of embodiment 1);
[0058] 2) Take the material according to the following formula:
[0059] Acellular pertussis liquid AP 1800ugPN
[0060] Tetanus Toxoid TT 300Lf
[0061] Diphtheria Toxoid DT 1000Lf
[0062] Al(OH) 3 126mg
[0063] sIPV type Ⅰ 6000DU
[0064] sIPV Ⅱ type 5700DU
[0065] sIPV Ⅲ type 9000DU
[0066] NaCl 765mg
[0067] h 2 O 8.6g;
[0068] 3) Aluminum hydroxide adsorption
[0069] According to the ratio of step 2), take 126mgAl(OH) 3 (final concentration of 1.26mg / ml), 935mgNaCl (final concentration of 9.35mg / ml) and 8.6g of water for injection were mixed and then autoclaved; at room temperature and under continuous stirring, 1000Lf of DT (final concentration of 10Lf / ml), 300Lf of TT (final concentration of 3Lf / ml), 1800ugPN of AP (final concentration of 18ugPN / ml), 6000DU of sIPV I (final concentration of 60DU / ml), 5700DU of sIPV II (antigen concentration of 57 DU / ml), 9000 DU of sIPV II...
Embodiment 3
[0071] 1) same as step 1 of embodiment 1);
[0072] 2) Take the material according to the following formula:
[0073] Acellular pertussis liquid AP 1100ugPN
[0074] Tetanus Toxoid TT 500Lf
[0075] Diphtheria Toxoid DT 1800Lf
[0076] Al(OH) 3 140mg
[0077] sIPVI type 4500DU
[0078] sIPVⅡ 6400DU
[0079] sIPVⅢ type 6600DU
[0080] NaCl 850mg
[0081] 2-Phenoxyethanol 300mg
[0082] h 2 O 27.2g;
[0083] 3) Aluminum hydroxide adsorption
[0084] According to the ratio of step 2), take 140mgAl(OH) 3(final concentration of 1.40mg / ml), 850mgNaCl (final concentration of 8.50mg / ml) and 27.2g of water for injection were mixed and then autoclaved; at room temperature and under continuous stirring, 1800Lf of DT (final concentration of 18Lf / ml), 500Lf of TT (final concentration of 5Lf / ml), 1100ugPN of AP (final concentration of 11ugPN / ml), 4500DU of sIPV I (final concentration of 45DU / ml), 6400DU of sIPV II (final concentration of 64DU / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com