Interfusion protein between diphtheria toxin and GM CSF mutant, coded gene and application
A technology of GM-CSF and diphtheria toxin, which is applied to the fusion protein of diphtheria toxin and GM-CSF mutant and its encoding gene and application field, can solve the problems of low expression amount, unfavorable production, large resistance to research and development, etc. The effect of increasing the amount of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
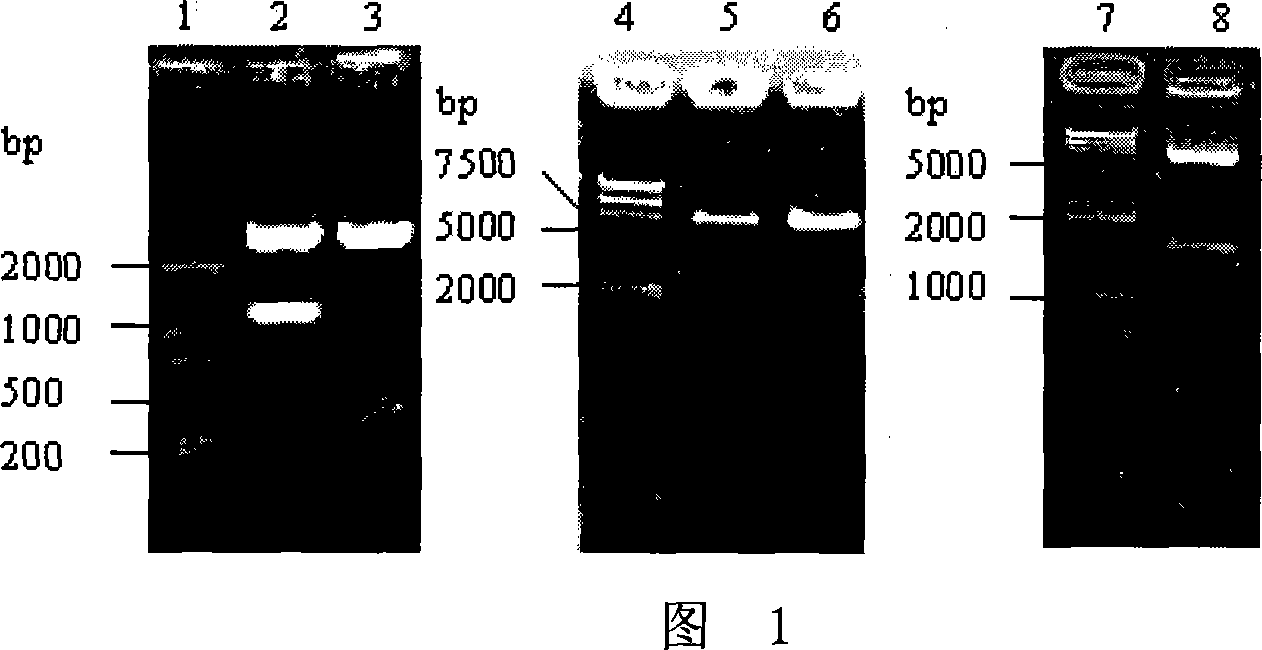
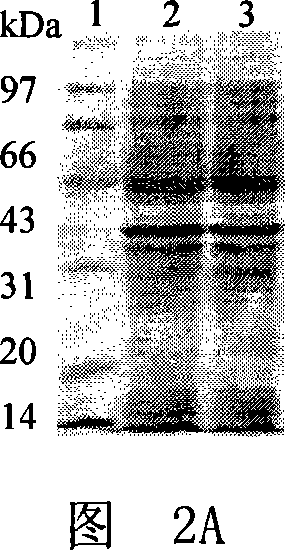
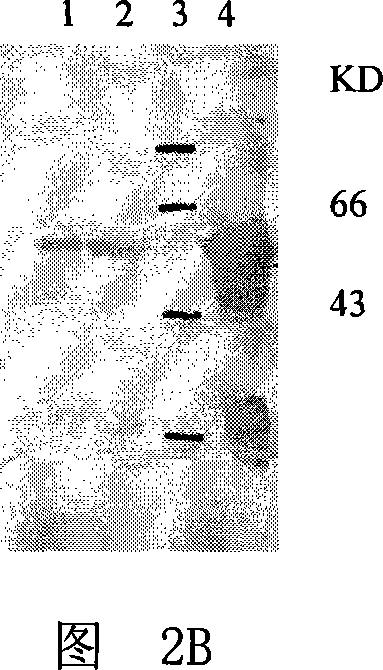
[0045] Embodiment 1, fusion protein DT of diphtheria toxin and GM-CSF mutant 386 -Expression of GMCSF 123GVT
[0046] 1. The fusion protein DT of diphtheria toxin and GM-CSF 386 -Expression of GMCSF
[0047] 1. DT 386 - Construction of GMCSF expression plasmid
[0048] The ORF of the human GM-CSF gene composed of E. coli preferred codons (the gene is called GM-CSFm) was artificially synthesized and inserted into the EcoR V site of pcDNAII (Invitrogen, V40020) to obtain the recombinant vector pcDNAII-GMCSFm. Wherein, the nucleotide sequence of the ORF of GM-CSFm is sequence 3 in the sequence listing (sequence 3 consists of 390 nucleotides).
[0049] Using pcDNAII-GMCSFm as a template, primer GFS with SphI restriction site and primer GFP14 with BamH I restriction site were used for PCR amplification. The length of the PCR product is about 400bp, and the blunt end connection is inserted into the EcoR V site of the pcDNAII plasmid. The results of Sph I and Bam HI double enzy...
Embodiment 2
[0102] Example 2, DT 386 - Activity assay of GMCSF 123GVT
[0103] In order to observe the effect of immunotoxin on killing tumor cells in vivo, HL60 cells were inoculated into mice to grow into tumor masses, and then tumor single cells were prepared for the determination of cytotoxic activity. NOD / SCID mice aged 6-8 weeks (purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences) were injected with HL60 cells (5×10 6each mouse), about 4 weeks later a human leukemia model was formed, and some mice developed tumors. Tumor-bearing NOD / SCID mice were taken to peel off the tumor body, placed in 10% FCS RPMI 1640 culture medium, shredded fully, and passed through a 400-mesh sieve. Centrifuge the cell suspension to 4,000rpm to stop, discard the supernatant, and wash 3 times with RPMI 1640 culture medium. The cell pellet was suspended in RPMI 1640 medium with 10% FCS. Take part of the tumor single cell suspension or HL60 cells, and incubate with m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com