Preparation method of group C meningococcal capsular polysaccharide conjugate vaccine
A technology of meningococcal and capsular polysaccharides, which is applied to medical preparations containing active ingredients, carrier-bound antigen/hapten components, antibacterial drugs, etc., which can solve high prices, increase vaccine manufacturing costs, and restrict wide application, etc. problems, achieve good stability, reduce hydrolytic denaturation, and cause little harm to the environment and personnel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
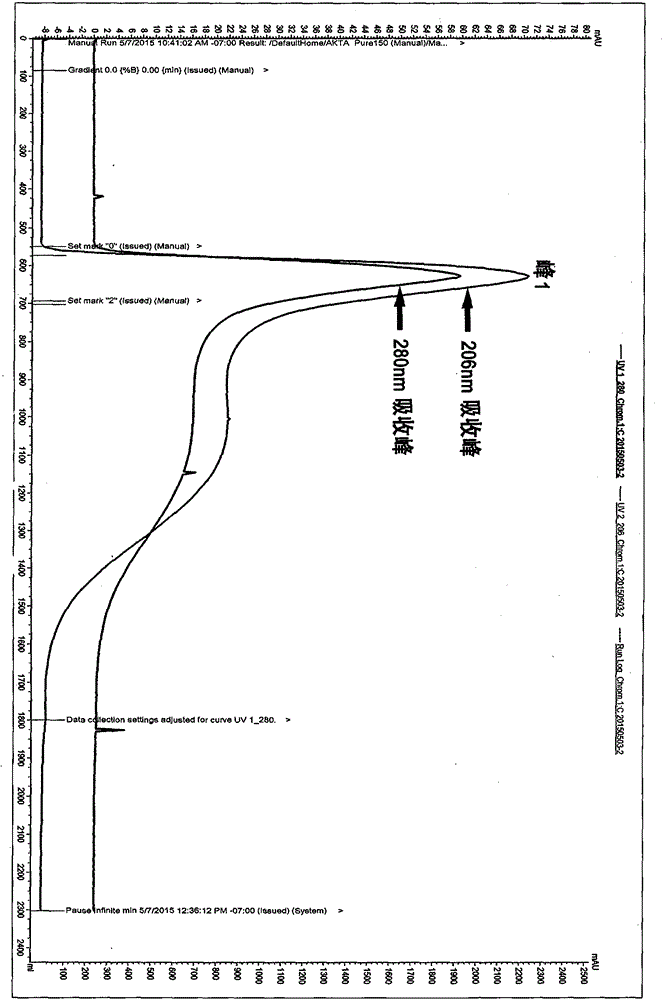
Image
Examples
Embodiment 1
[0009] Describe the present invention below in conjunction with reaction example
[0010] Group C capsular polysaccharides are dissolved to 5-10 mg / ml, add ADH 1-3 times the mass of polysaccharides to the polysaccharide solution, stir and mix well, adjust the pH of the solution to pH 5.5-6.3 with dilute hydrochloric acid; EDAC at a ratio of 0.2 to 0.6 mg / mg, stirred at room temperature and reacted; during the reaction, dilute hydrochloric acid was used to maintain the pH of the solution at 5.5 to 6.3.
[0011] After reacting for 1 to 2 hours, the polysaccharide derivative solution is ultrafiltered with a 30KD ultrafiltration membrane to remove small molecular substances. Store at 2-8°C after filtration with a 0.45 μm filter membrane.
[0012] Weigh the CRM197 protein and dissolve it to 10-20 mg / ml, mix the polysaccharide derivative solution with the CRM197 protein solution so that the polysaccharide-to-protein mass ratio is 1-2 mg / mg, and put it in an ice bath; adjust the pH ...
Embodiment 2
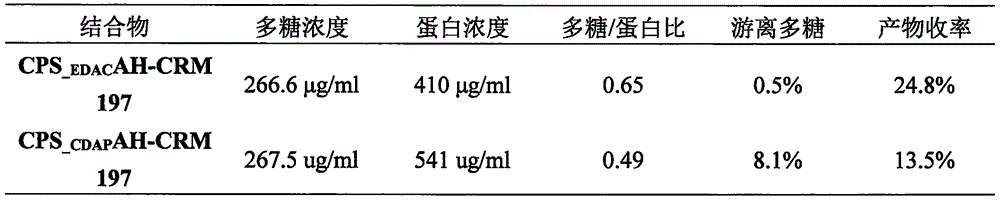
[0015] Compared with the traditional activation method using CNBr or CDAP, the polysaccharide derivatization method adopted in this study has milder conditions and relatively simple operation. However, whether the polysaccharide conjugates of group C prepared by this method can reach or even exceed those prepared by traditional methods in terms of glycoprotein ratio, yield, and free polysaccharide level is also worthy of attention. The detection of the main indicators was carried out, and the detection results were compared.
[0016] Comparison of biochemical properties of conjugates prepared by different methods
[0017] In order to compare with the traditional method, the CDAP-activated polysaccharide method was used as the control method to prepare the conjugate in this study.
[0018] The main operation steps are as follows:
[0019] Preparation of group C polysaccharide-protein conjugates by indirect binding of CDAP-activated polysaccharides to proteins
[0020] Group ...
Embodiment 3
[0031] Study on Stability of Polysaccharide Conjugates of Group C Meningococcal Meningitis
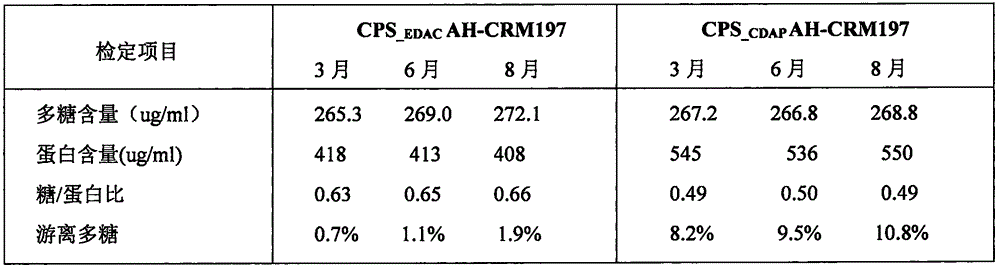
[0032]In view of the possible exposure temperature of 2-8°C and short-term room temperature under the future production conditions, we investigated the storage of the two batches of the original solution at 2-8°C for 3, 6, 8 months and 20-25°C in the above example. The quality stability after 4 weeks and 6 weeks, and the stability of 3 days, 7 days, and 14 days at 37°C in an extreme environment, and compare the two.
[0033] Two batches of conjugated vaccine stock solution samples were stored at 2-8°C for 3 months, 6 months, and 8 months, and after 3 days, 7 days, and 14 days at 37°C, the indicators were tested respectively. The detection method is the same as above, and the test results are shown in the table. 2 and Table 3.
[0034] As shown in Table 2, the polysaccharide content, protein content, and glycoprotein ratio of the two batches of group C conjugate stock solutions did not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com