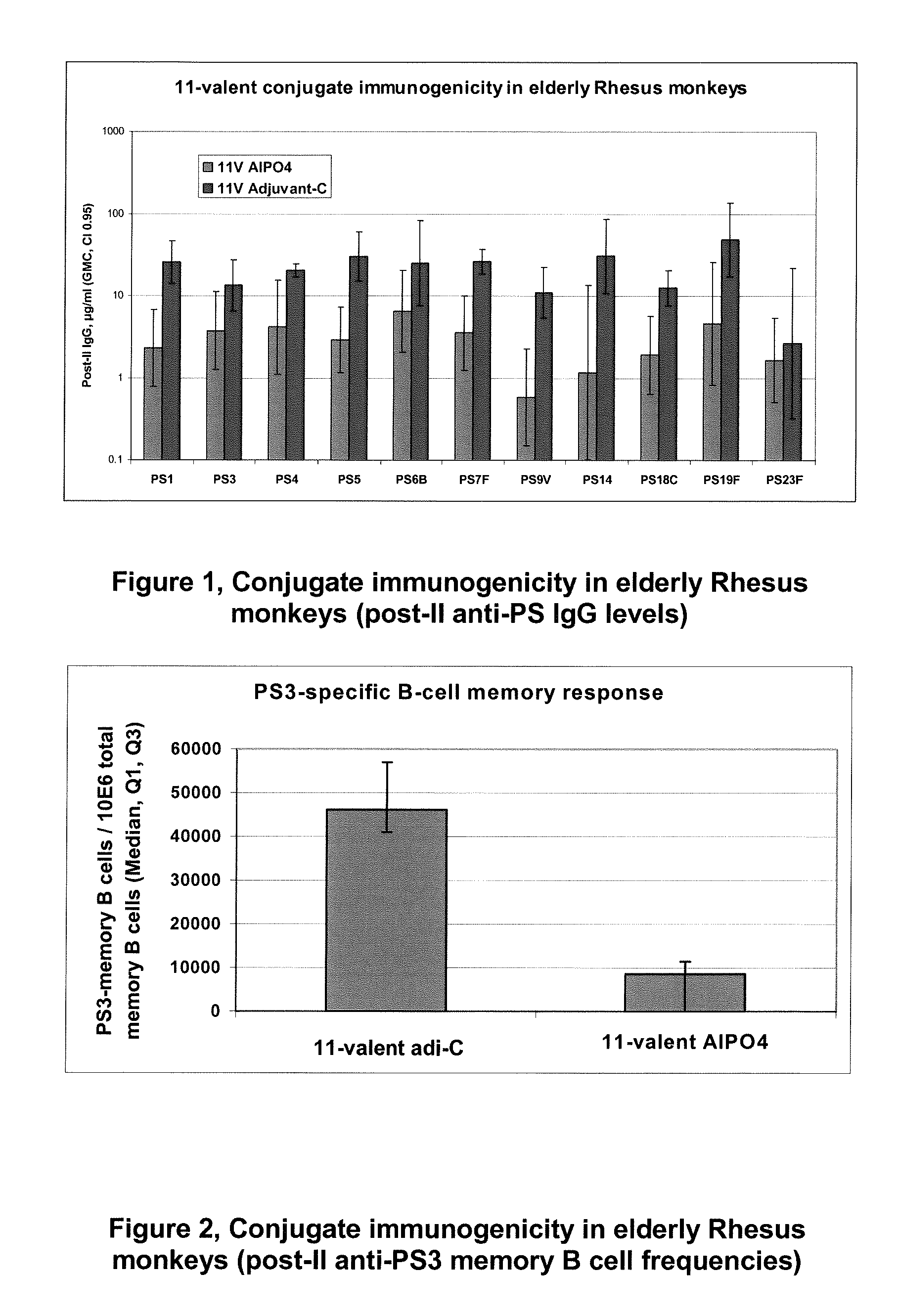
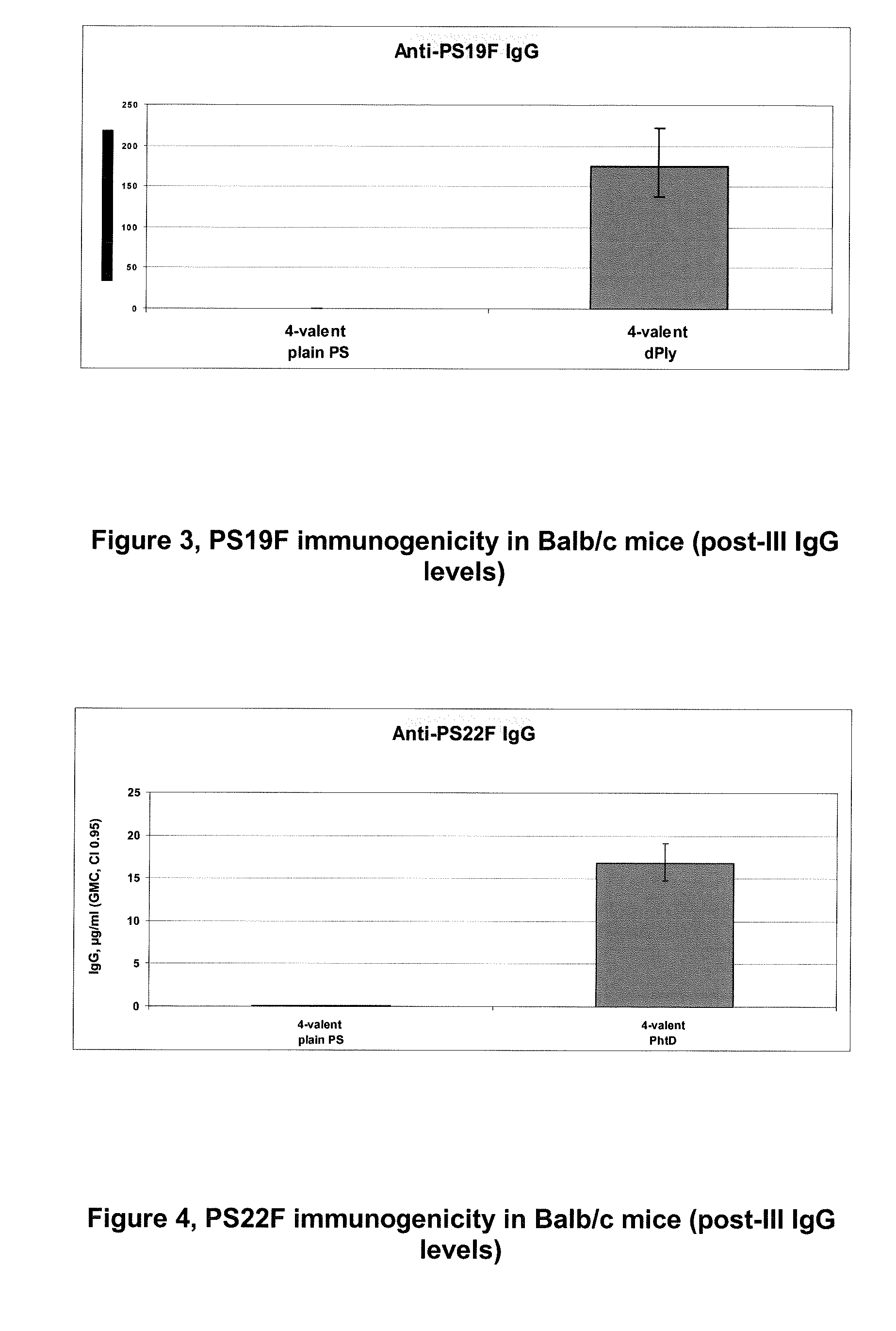
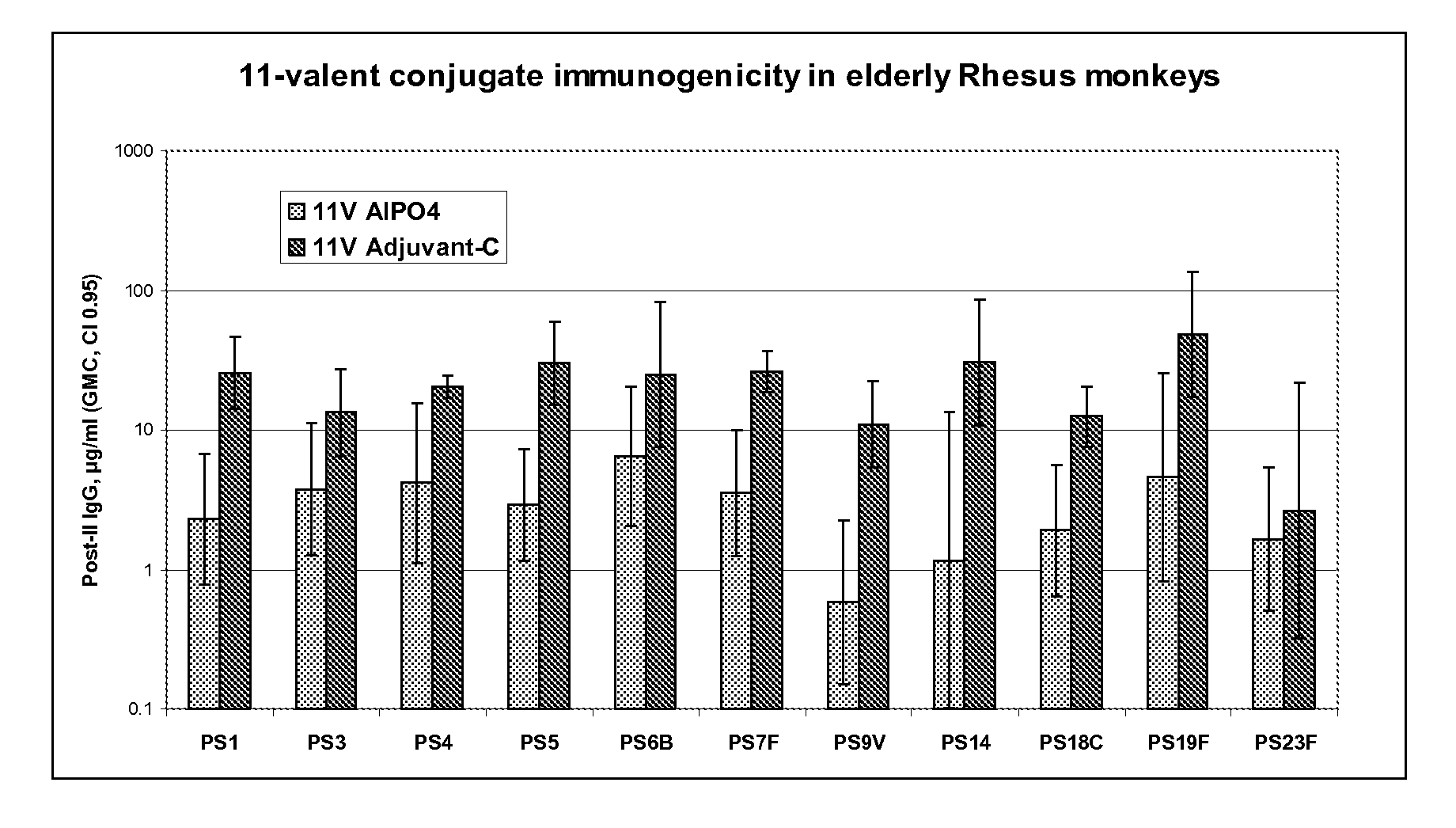
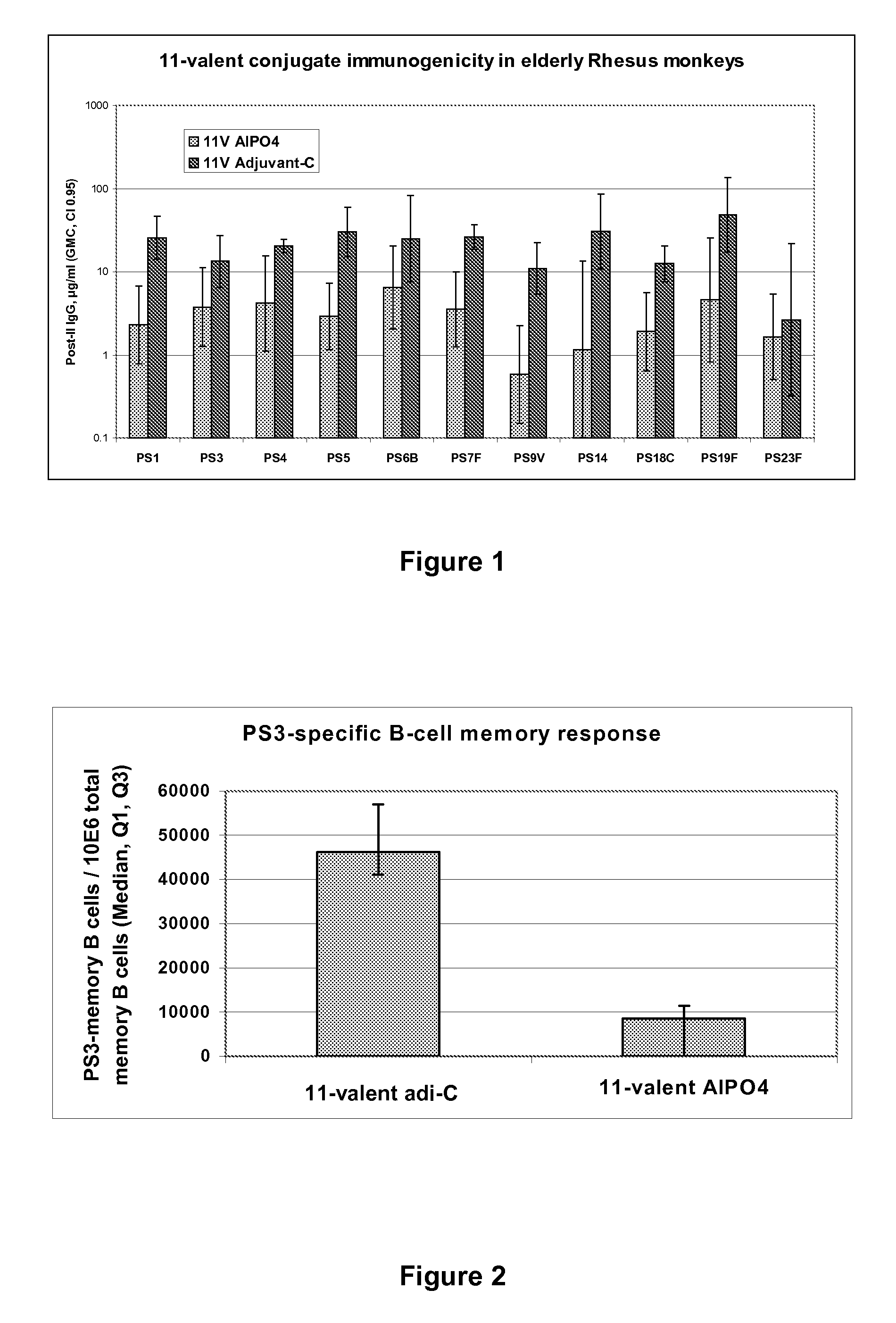
Patents
Literature
52 results about "Streptococcus pneumoniae serotype" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
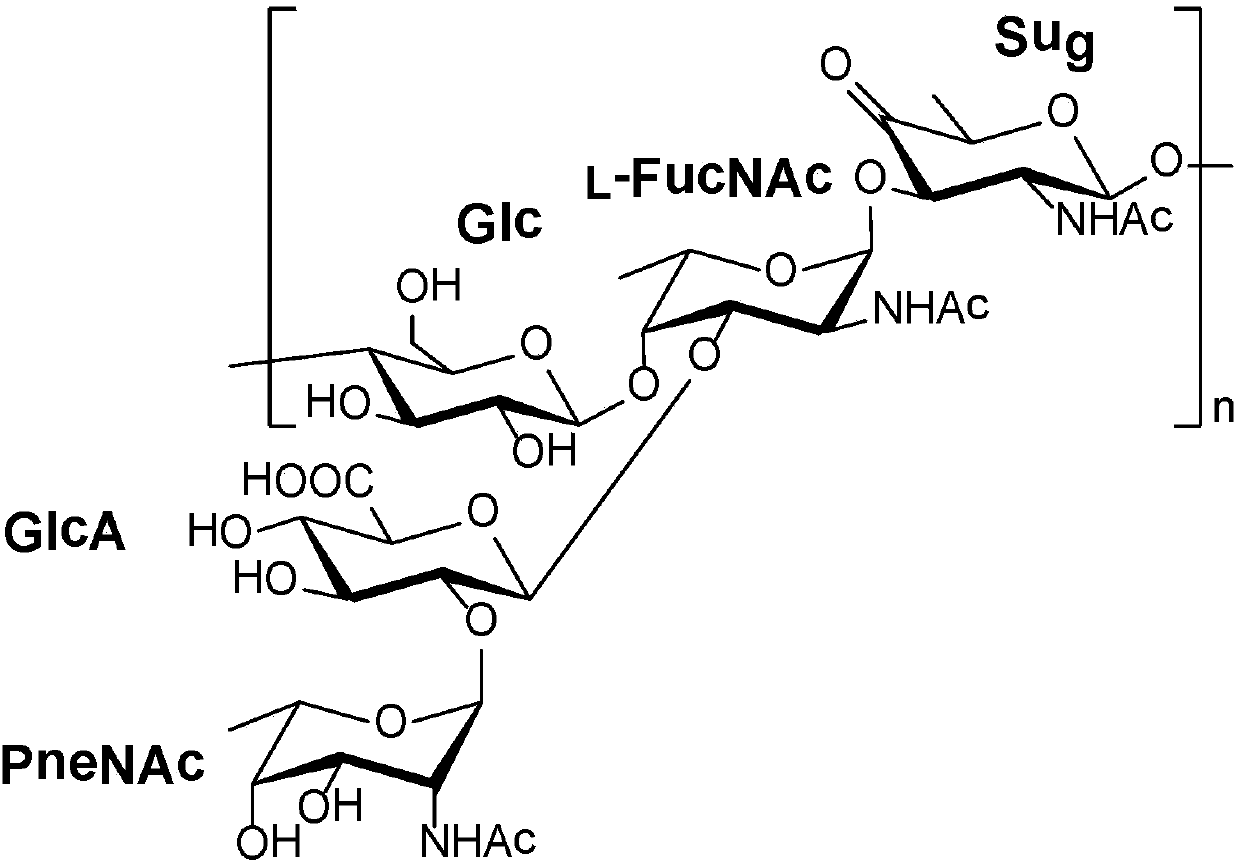
Streptococcus pneumoniae (S. pneumoniae) are lancet-shaped, gram-positive, facultative anaerobic bacteria with over 90 known serotypes. Most S. pneumoniae serotypes can cause disease, but only a minority of serotypes produce the majority of pneumococcal infections.
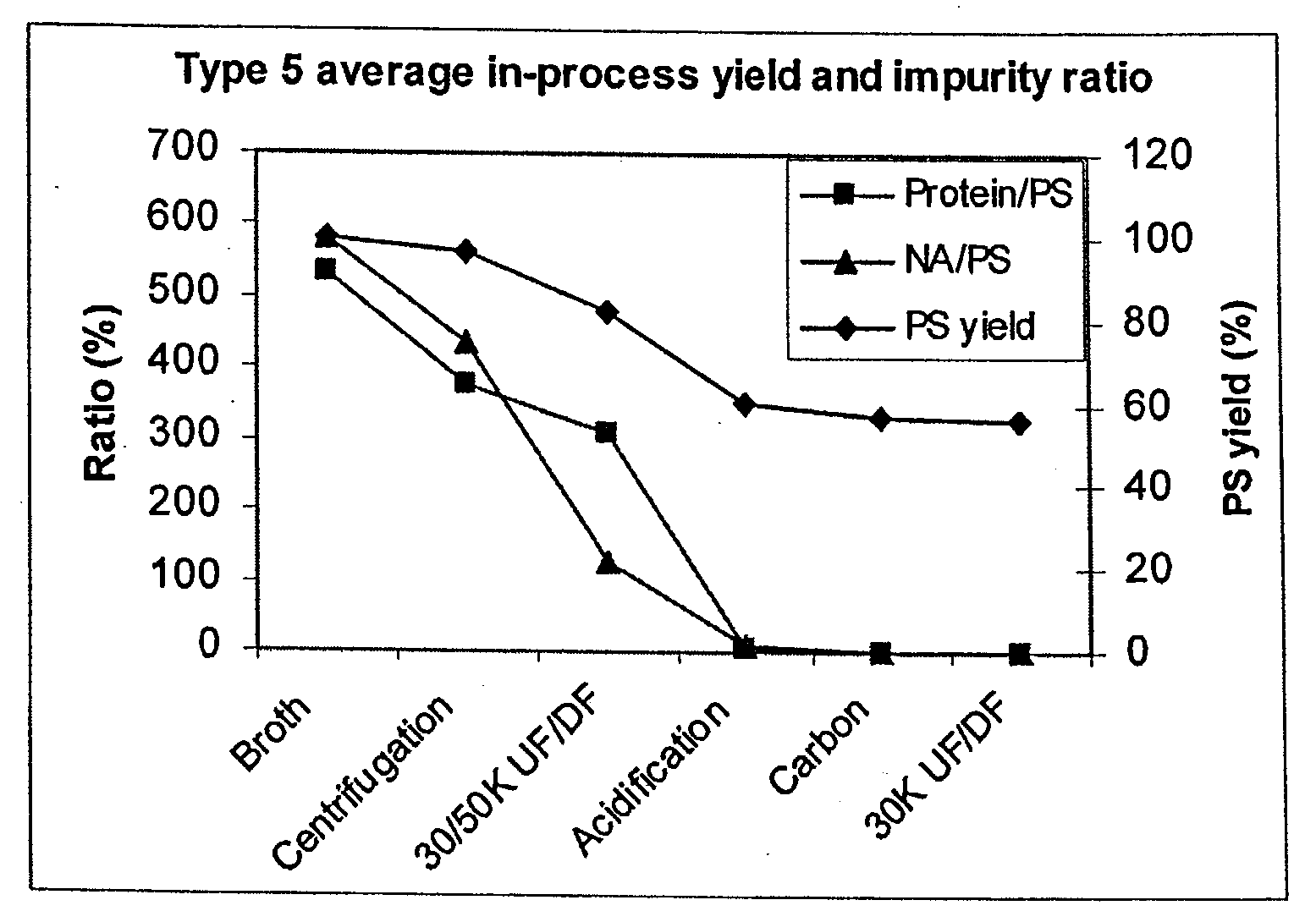
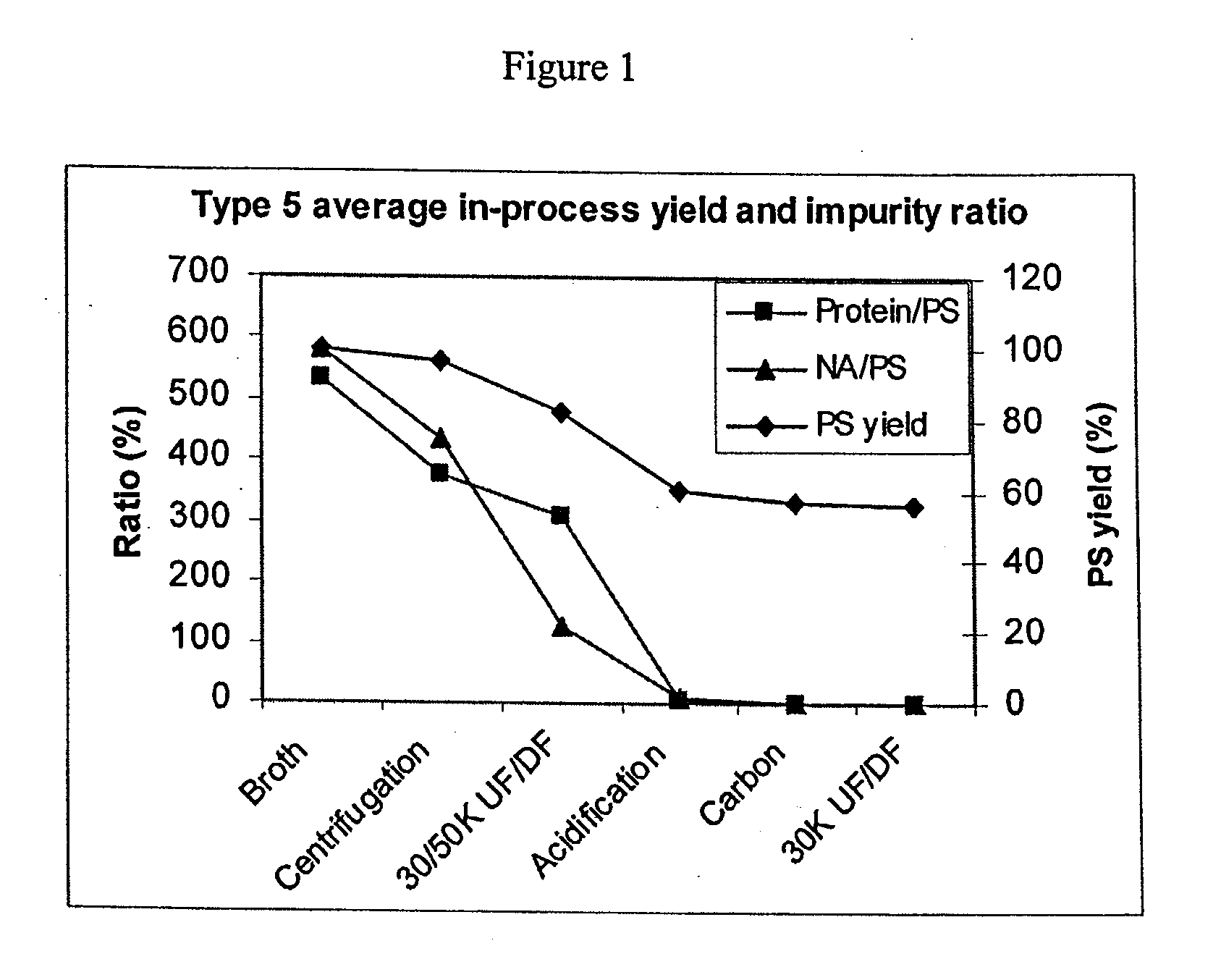
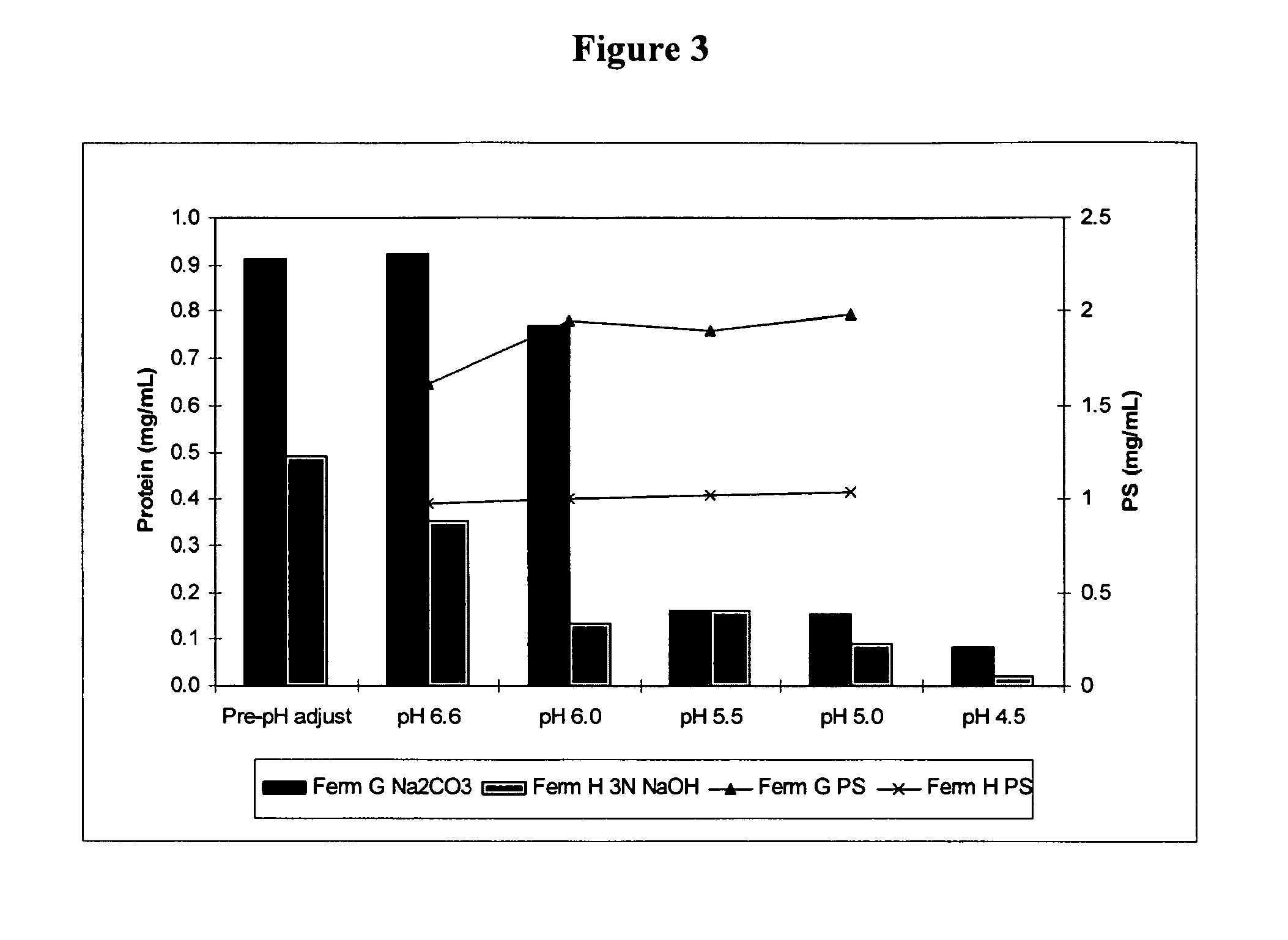
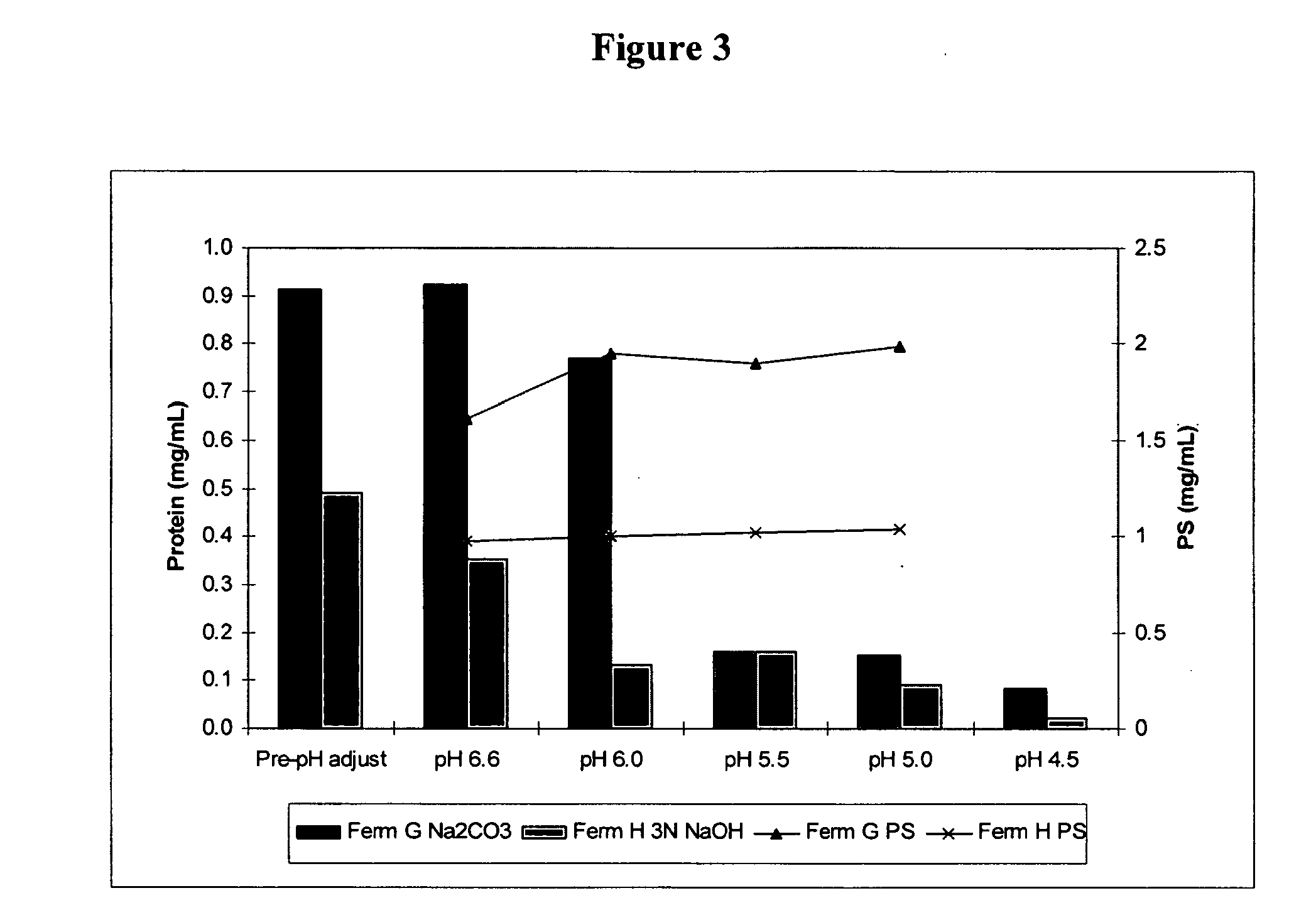
Shortened purification process for the production of capsular streptococcus pneumoniae polysaccharides
ActiveUS20080286838A1Increase polysaccharide concentrationReduce the presence of impuritiesAntibacterial agentsSugar derivativesPurification methodsActivated carbon filtration
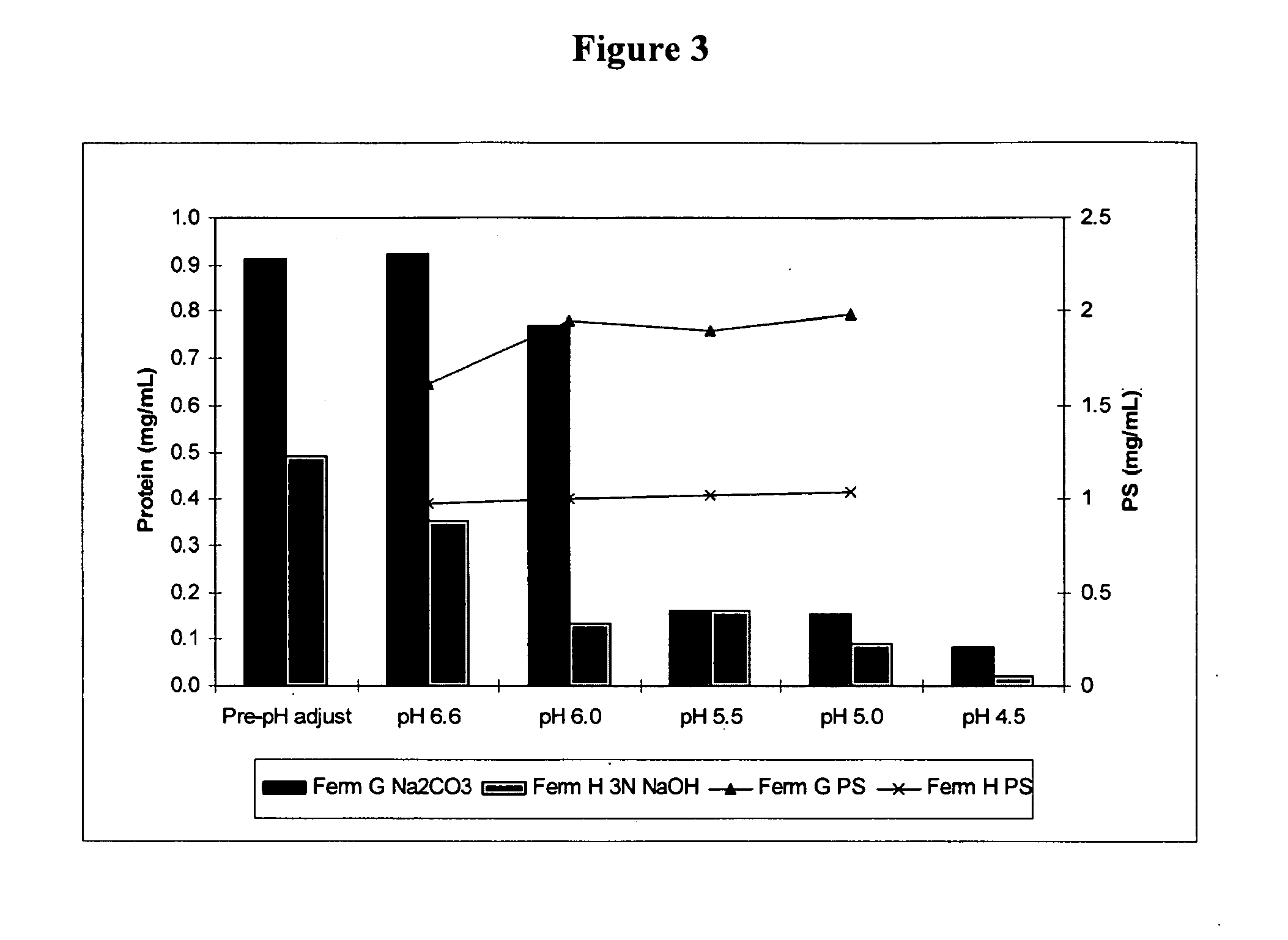
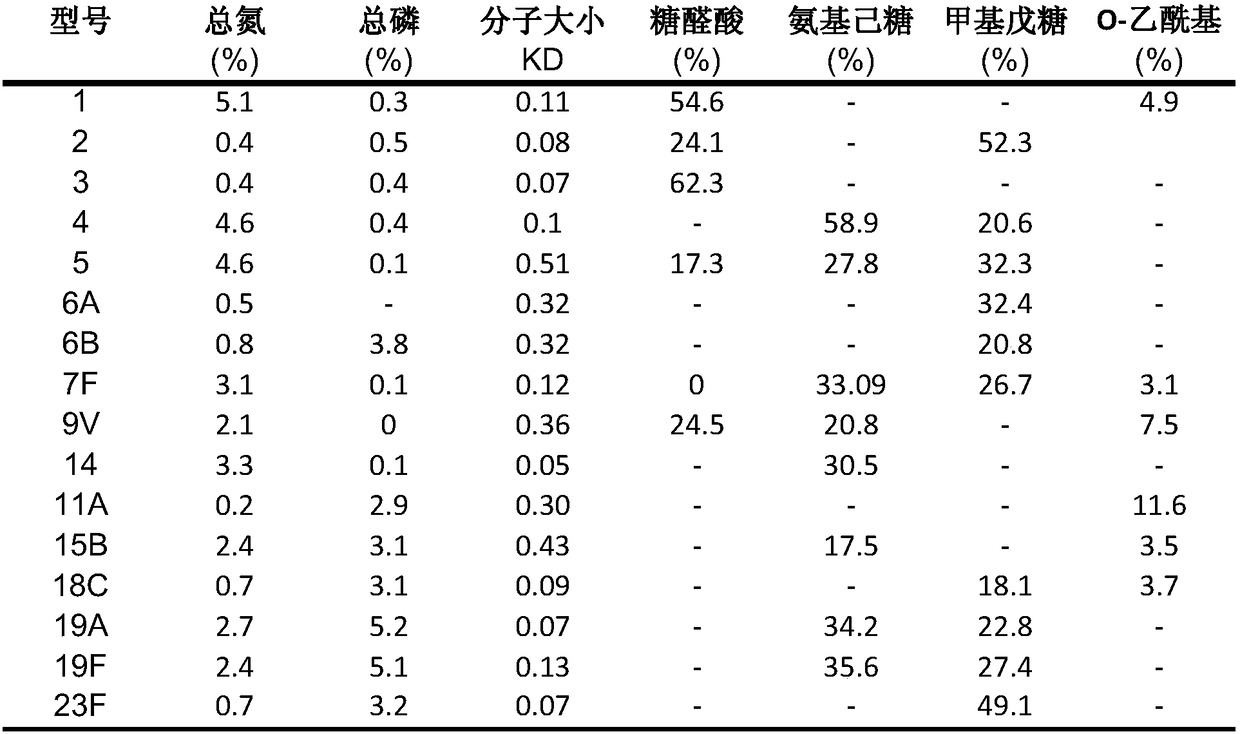
A shortened process for producing a solution containing substantially purified capsular polysaccharides from a cellular Streptococcus pneumoniae lysate broth is described. Ultrafiltering and diafiltering a clarified S. pneumoniae lysate followed by pH adjustment to less than 4.5, preferably about 3.5, precipitated at least 98% of the protein in the solution without seriously affecting polysaccharide yield. Furthermore, following ultrafiltration and diafiltration and acidification to a pH of less than 4.5, filtration using activated carbon precipitated at least 90% of remaining protein without seriously affecting polysaccharide yield. Exemplary, non-limiting S. pneumoniae serotypes that can be purified using the shortened process of the invention are 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. In one embodiment, the Streptococcus pneumoniae cells are lysed using deoxycholate sodium (DOC), while in another embodiment the lytic agent is a non-animal derived lytic agent such as N-lauryl sarcosine sodium (NLS).
Owner:WYETH LLC
Pneumococcal Polysaccharide Conjugate Vaccine
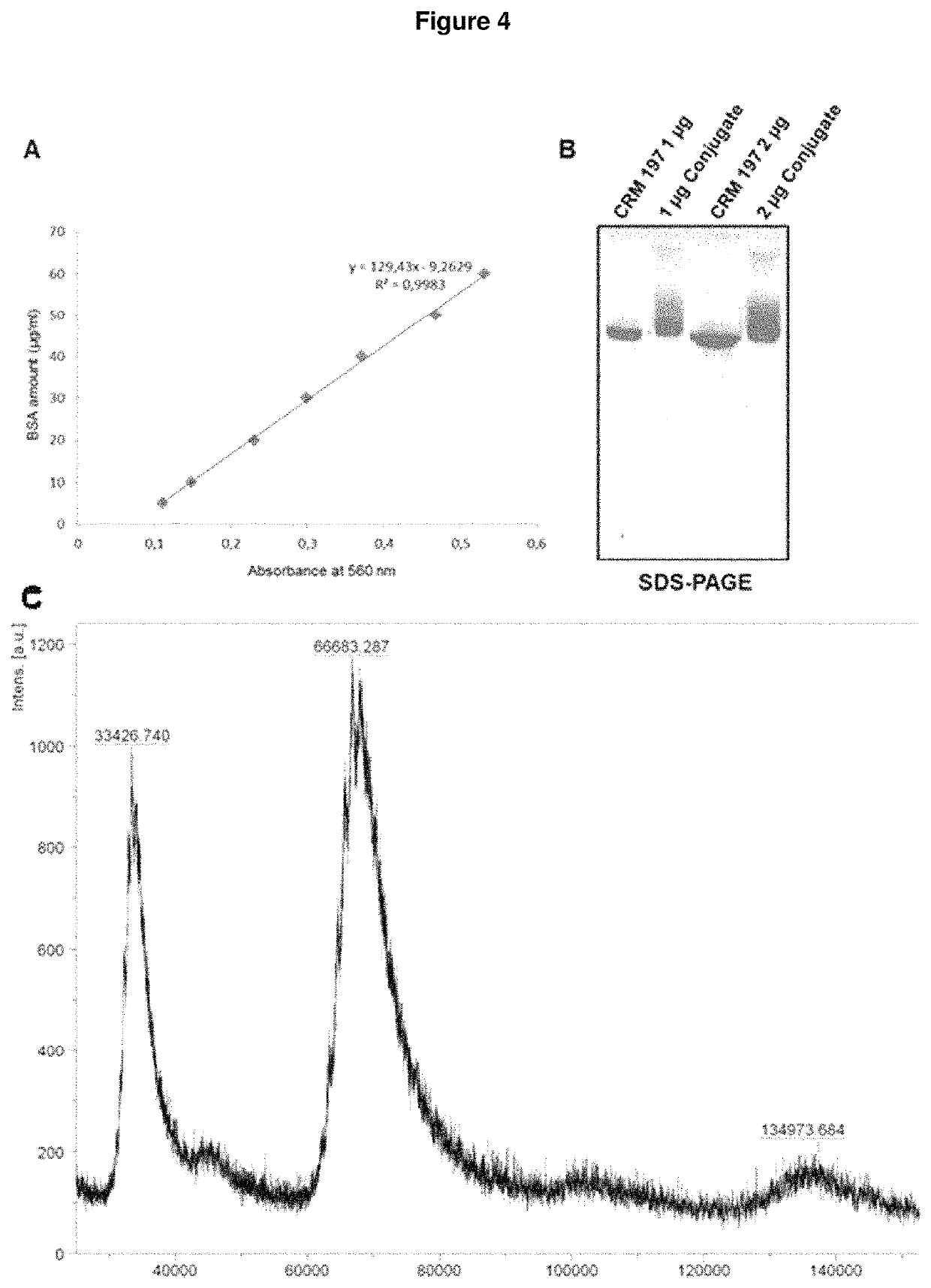
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid (DT) or CRM197, optionally wherein 19F is the only saccharide in the composition conjugated to diphtheria toxoid (DT) or CRM197.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vaccine comprising streptococcus pneumoniae capsular polysaccharide conjugates
InactiveUS20100209450A1Antibacterial agentsSenses disorderConjugate vaccineStreptococcus pneumoniae capsular polysaccharide
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various conjugated capsular saccharides from different S. pneumoniae serotypes conjugated to 2 or more different carrier proteins, where the composition comprises serotype 19F capsular saccharide conjugated to diphtheria toxoid. Methods of making and uses thereof are also described.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Shortened purification process for the production of capsular Streptococcus pneumoniae polysaccharides
ActiveUS8652480B2Reduce molecular weightImprove concentrationAntibacterial agentsBiocideActivated carbon filtrationSarcosine
A shortened process for producing a solution containing substantially purified capsular polysaccharides from a cellular Streptococcus pneumoniae lysate broth is described. Ultrafiltering and diafiltering a clarified S. pneumoniae lysate followed by pH adjustment to less than 4.5, preferably about 3.5, precipitated at least 98% of the protein in the solution without seriously affecting polysaccharide yield. Furthermore, following ultrafiltration and diafiltration and acidification to a pH of less than 4.5, filtration using activated carbon precipitated at least 90% of remaining protein without seriously affecting polysaccharide yield. Exemplary, non-limiting S. pneumoniae serotypes that can be purified using the shortened process of the invention are 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. In one embodiment, the Streptococcus pneumoniae cells are lysed using deoxycholate sodium (DOC), while in another embodiment the lytic agent is a non-animal derived lytic agent such as N-lauryl sarcosine sodium (NLS).
Owner:WYETH LLC
Methods and products for identifying strains of bacteria
InactiveUS20110053793A1Reduce and avoid needRemove and diminishes needPeptide librariesLibrary tagsSerum igeCapture antibody
Methods for identifying strains of bacteria, particularly methods for serotyping Streptococcus pneumoniae in a sample, methods for detecting and / or classifying S. pneumoniae infection by serotype, array devices and kits for use in such methods are disclosed. Array devices comprise a set of capture antibodies immobilised on a substrate at pre-determined array positions, wherein the set of capture antibodies comprises serotype-distinguishing antibodies which differ in their binding specificity for different S. pneumoniae serotypes. Serotyping methods may employ whole cell detection utilising one or more detectable labels, including in situ labelling of array-bound cells.
Owner:PROTEOMIKA +1
Vaccine
The present invention is in the field of pneumococcal capsular saccharide conjugate vaccines. Specifically, a multivalent Streptococcus pneumoniae immunogenic composition is provided with various (for instance 9 or more) conjugated capsular saccharides from different S. pneumoniae serotypes, wherein the composition comprises conjugated capsular saccharide 18C which is less than 80, 70, 60, 50, 40, 30, 20, 15 or 10% O-Acetylated.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
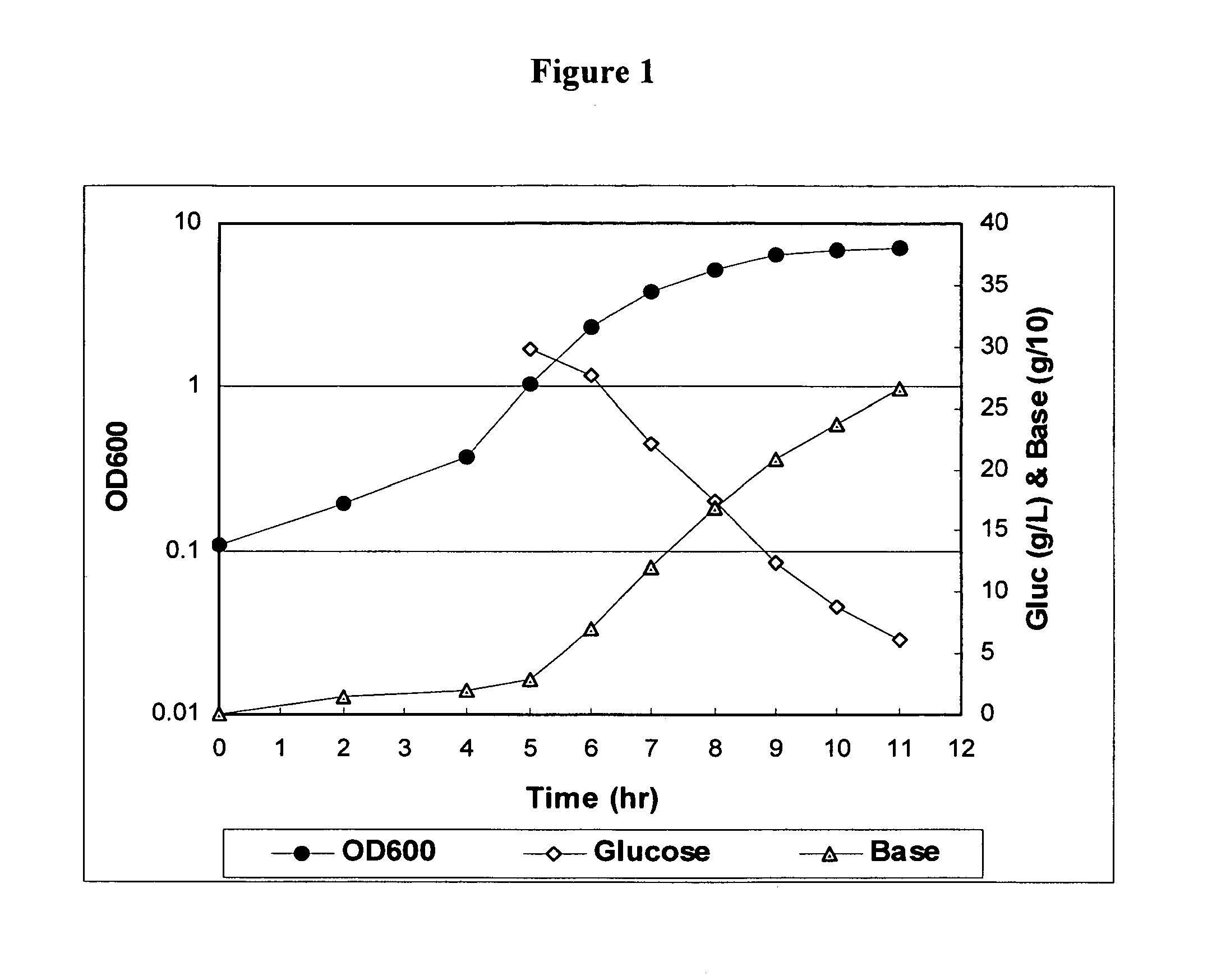
Method for controlling streptococcus pneumoniae serotype 19a polysaccharide molecular weight
The present invention provides improved methods for producing a solution containing high molecular weight isolated Streptococcus pneumoniae 19A capsular polysaccharides. In certain methods, a fermentation culture of Streptococcus pneumoniae bacterial cells that produce serotype 19A capsular polysaccharides is fermented for less than 6 hours before the bacterial cells are lysed the capsular polysaccharides are harvested. In other methods, CO2 is supplied to the fermentation culture. Supplying CO2 to the fermentation culture includes adding bicarbonate ions to the fermentation culture, adding carbonate ions to the fermentation culture, adding mixtures of bicarbonate and carbonate ions to the fermentation culture, and overlaying the fermentation culture with CO2.
Owner:WYETH LLC
Pneumococcal serotypes
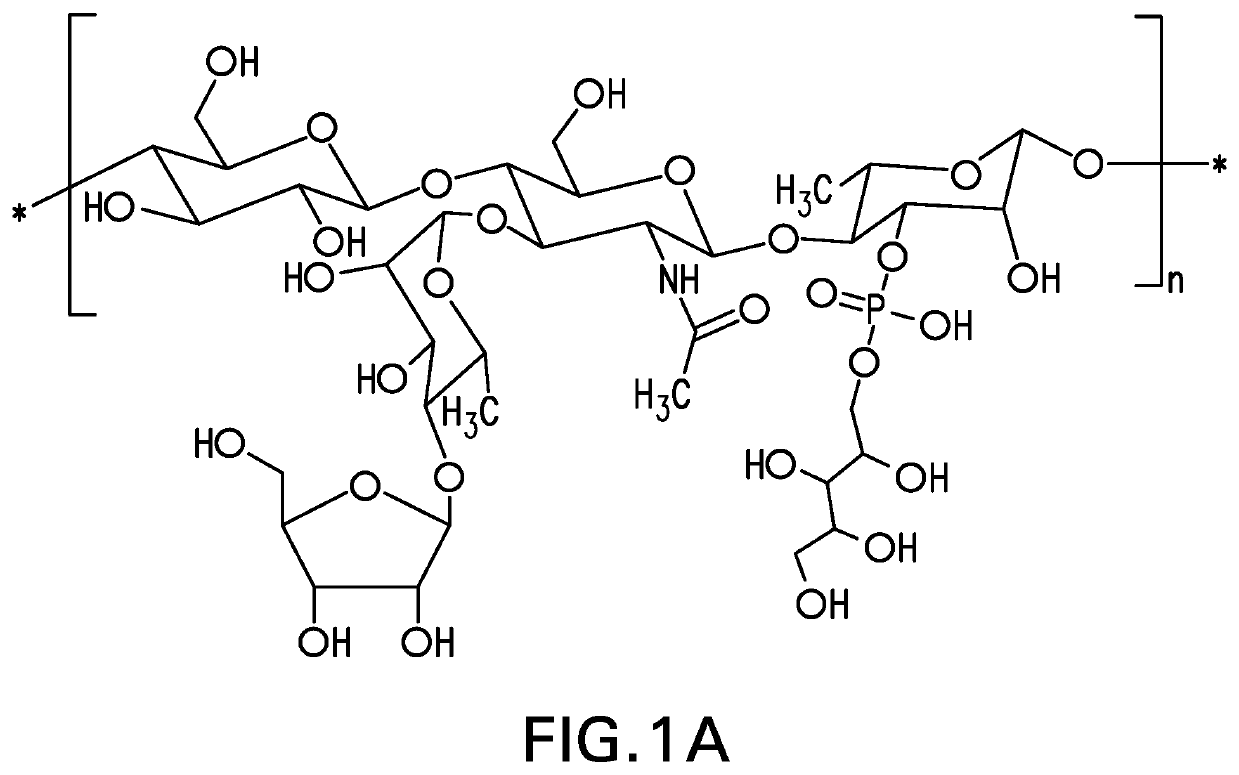
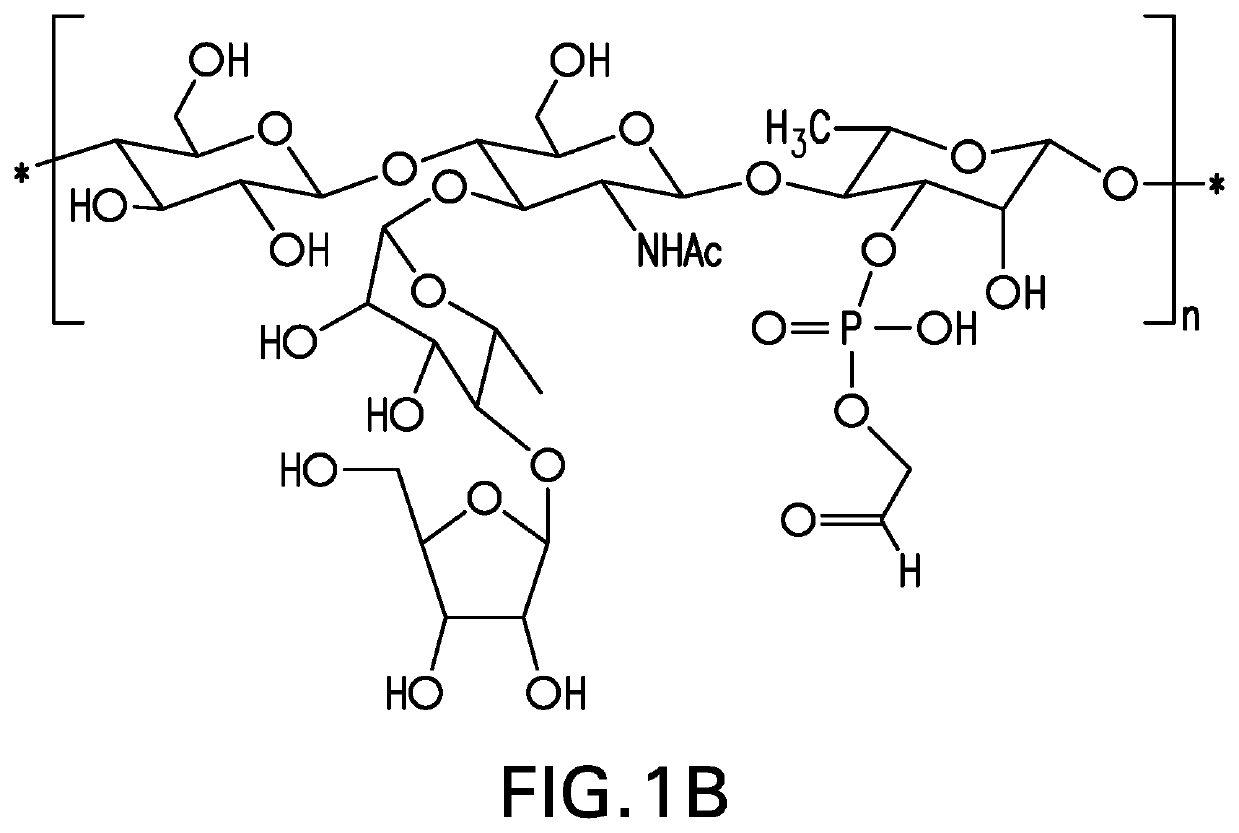
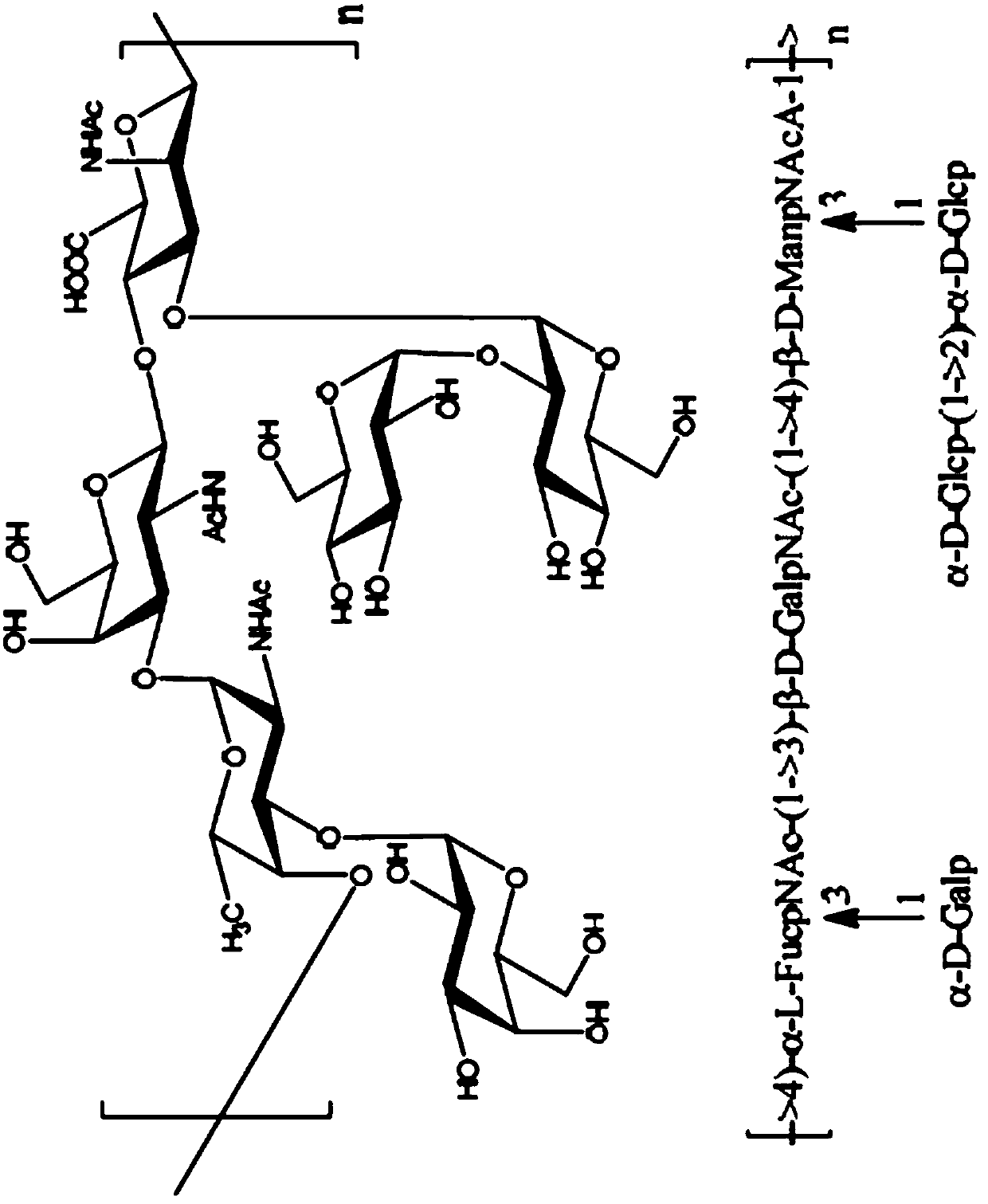
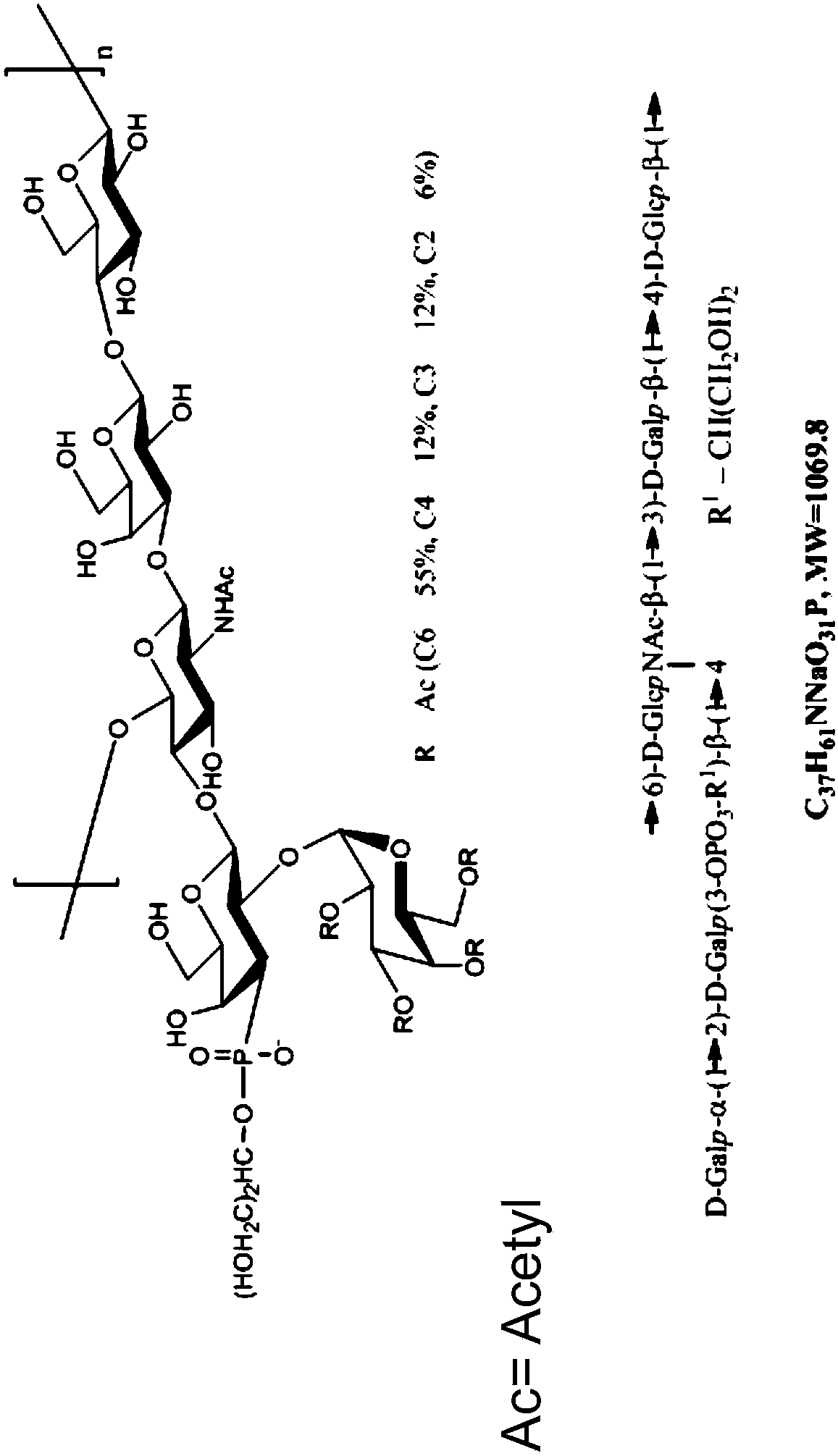
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {→2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:INST ADOLFO LUTZ +1
Compositions comprising streptococcus pneumoniae polysaccharide-protein conjugates and methods of use thereof
ActiveUS20190192648A1Improve stabilityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseCarrier protein
The invention is related to multivalent immunogenic compositions comprising more than one S. pneumoniae polysaccharide protein conjugates, wherein each of the conjugates comprises a polysaccharide from an S. pneumoniae serotype conjugated to a carrier protein, wherein the serotypes of S. pneumoniae are as defined herein. In some embodiments, at least one of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. In further embodiments, each of the polysaccharide protein conjugates is formed by a conjugation reaction comprising an aprotic solvent. Also provided are methods for inducing a protective immune response in a human patient comprising administering the multivalent immunogenic compositions of the invention to the patient. The multivalent immunogenic compositions are useful for providing protection against S. pneumoniae infection and diseases caused by S. pneumoniae. The compositions of the invention are also useful as part of treatment regimes that provide complementary protection for patients that have been vaccinated with a multivalent vaccine indicated for the prevention of pneumococcal disease.
Owner:MERCK SHARP & DOHME LLC
Identification of streptococcus penumoniae serotypes
InactiveUS20070020631A1Microbiological testing/measurementFermentationNucleotideNucleotide sequencing
The present invention relates to molecular methods of serotyping Streptococcus pneunoniae, as well as polynu-cleotides useful in such methods. These methods rely on analysing at least a portion of the nucleotide sequence between the 3′ end of the cpsA gene and the 5′ end of the cpsB gene, and / or analysing at least a portion of the szy and / or wzx gene(s).
Owner:TIANJIN BIOCHIP CORP +1
Pneumococcal serotypes
InactiveUS20130315958A1Organic active ingredientsBacterial antigen ingredientsPneumococcal serotypesCoccidia
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:UAB RES FOUND
Method for detecting serotyping of streptococcus pneumoniae
ActiveCN103290115AMicrobiological testing/measurementMicroorganism based processesMultiplex ligation-dependent probe amplificationFluorescence
The invention provides a method for carrying out serotyping on streptococcus pneumoniae by using MLPA (multiplex ligation dependent probe amplification). The method comprises the following steps of: firstly, extracting a sample genome DNA of streptococcus pneumoniae to be detected; carrying out pre-amplification on the extracted genome DNA by using a PCR pre-amplification primer; carrying out hybridization on pre-amplification products of the genome DNA by using an MLPA probe; adding a corresponding fluorescence detecting probe in a reaction system; connecting hybridized MLPA probes by using ligase, and carrying out PCR amplification on the hybridized and connected MLPA probes by using an MLPA universal amplification primer; and analyzing PCR products by using a multicolor fluorescence dissolution curve, so that the serotyping on streptococcus pneumoniae can be realized. The detecting method disclosed by the invention is good in specificity and high in sensitivity; according to the method, 10 kinds of serotypes can be subjected to genotyping simultaneously just in 2-4 hours, so that multiple uncapping operations in traditional MLPA detection are avoided, and the pollution possibility is reduced, therefore, the method can meet high-throughput sample detection, and is especially applicable to inspection departments.
Owner:江西南兴医疗科技有限公司 +1
Vaccines against Streptococcus pneumoniae serotype 8
ActiveUS10220083B2Improving immunogenicityImprove flow characteristicsAntibacterial agentsOrganic active ingredientsProtection sexAntibody
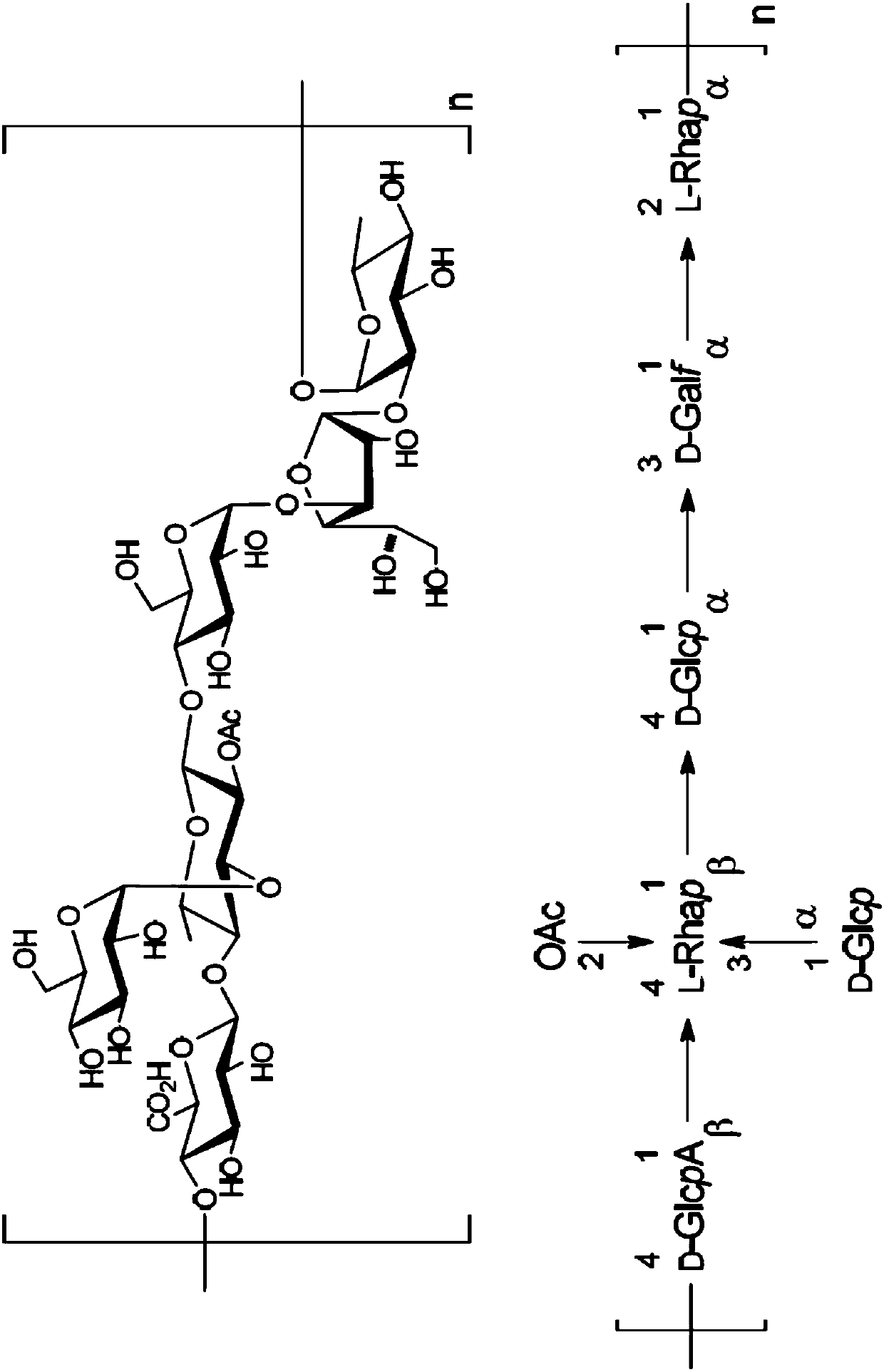
The present invention relates to synthetic saccharides of general formula (I) that are related to Streptococcus pneumoniae serotype 8 capsular polysaccharide, conjugates thereof and the use of said saccharides and conjugates for raising a protective immune response in a human and / or animal host. Furthermore, the synthetic saccharide structures of general formula (I) are useful as marker in immunological assays for detection of antibodies against Streptococcus pneumoniae bacteria.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
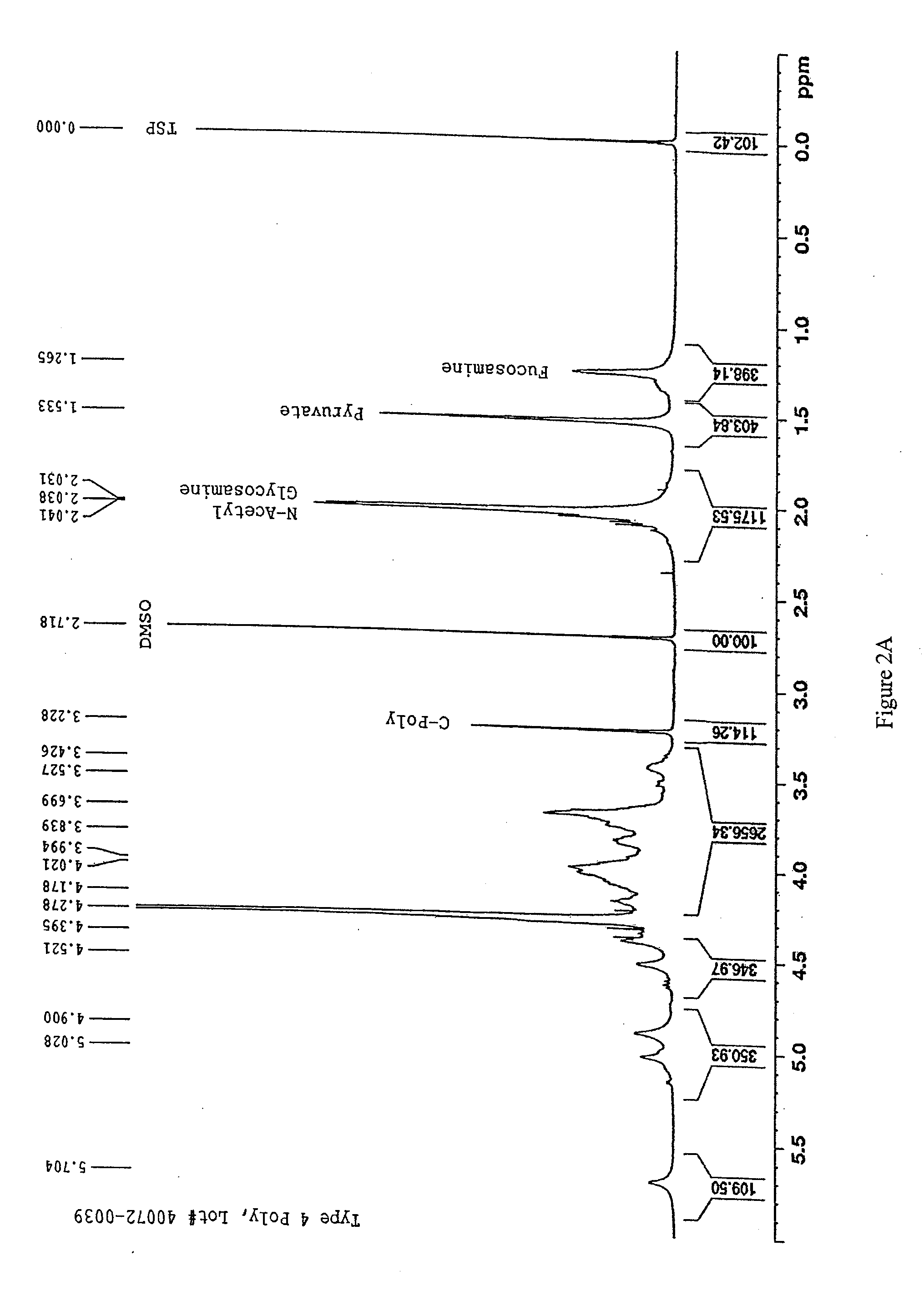
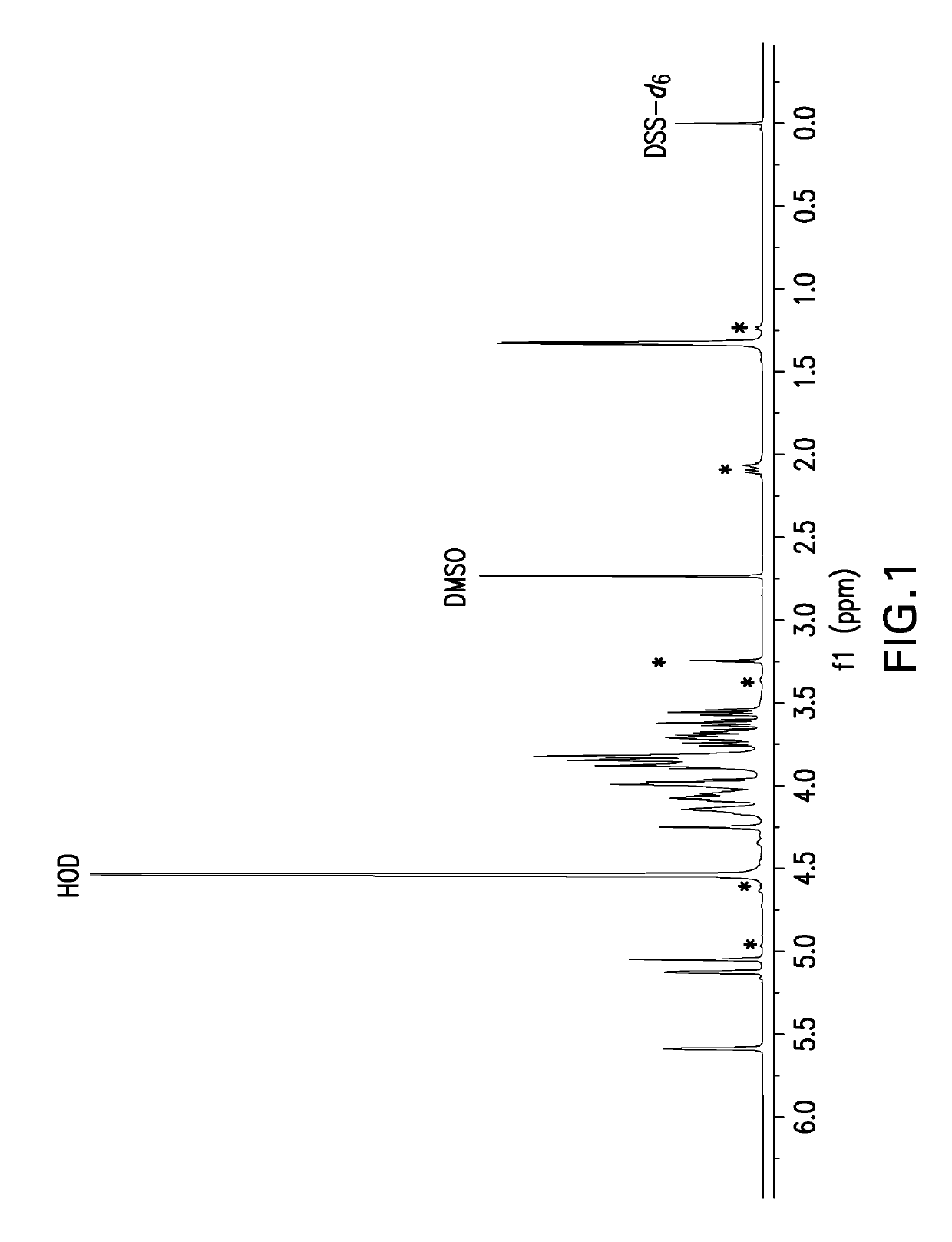
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
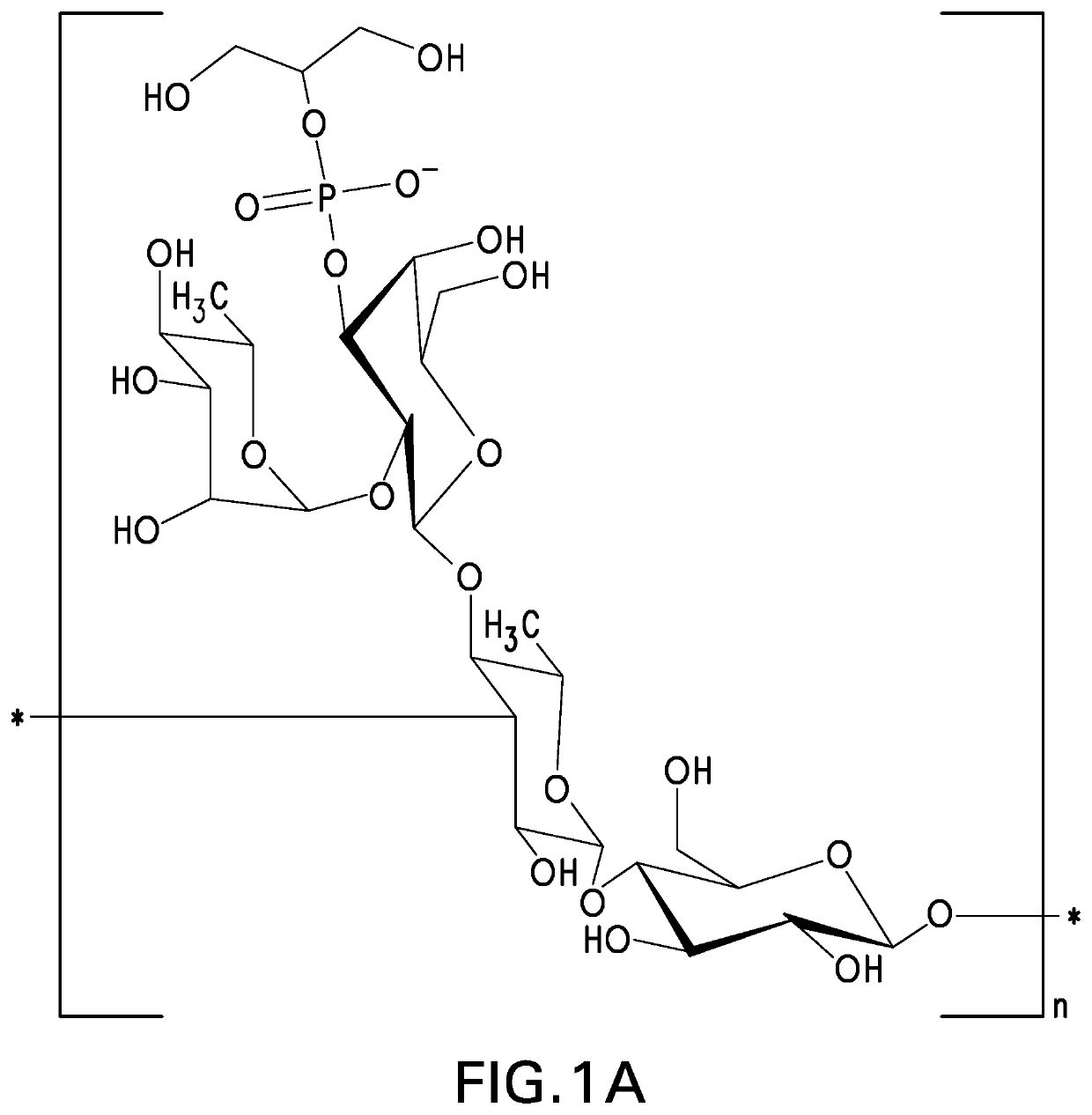
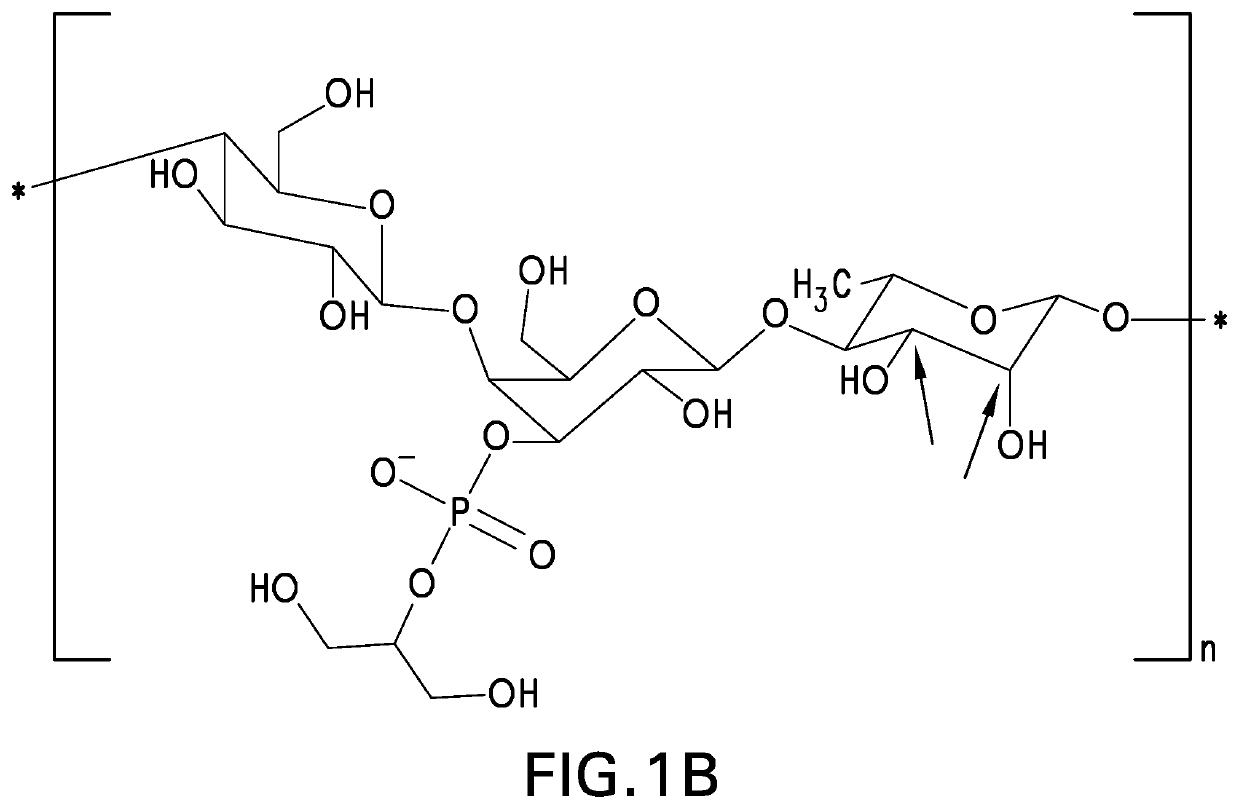
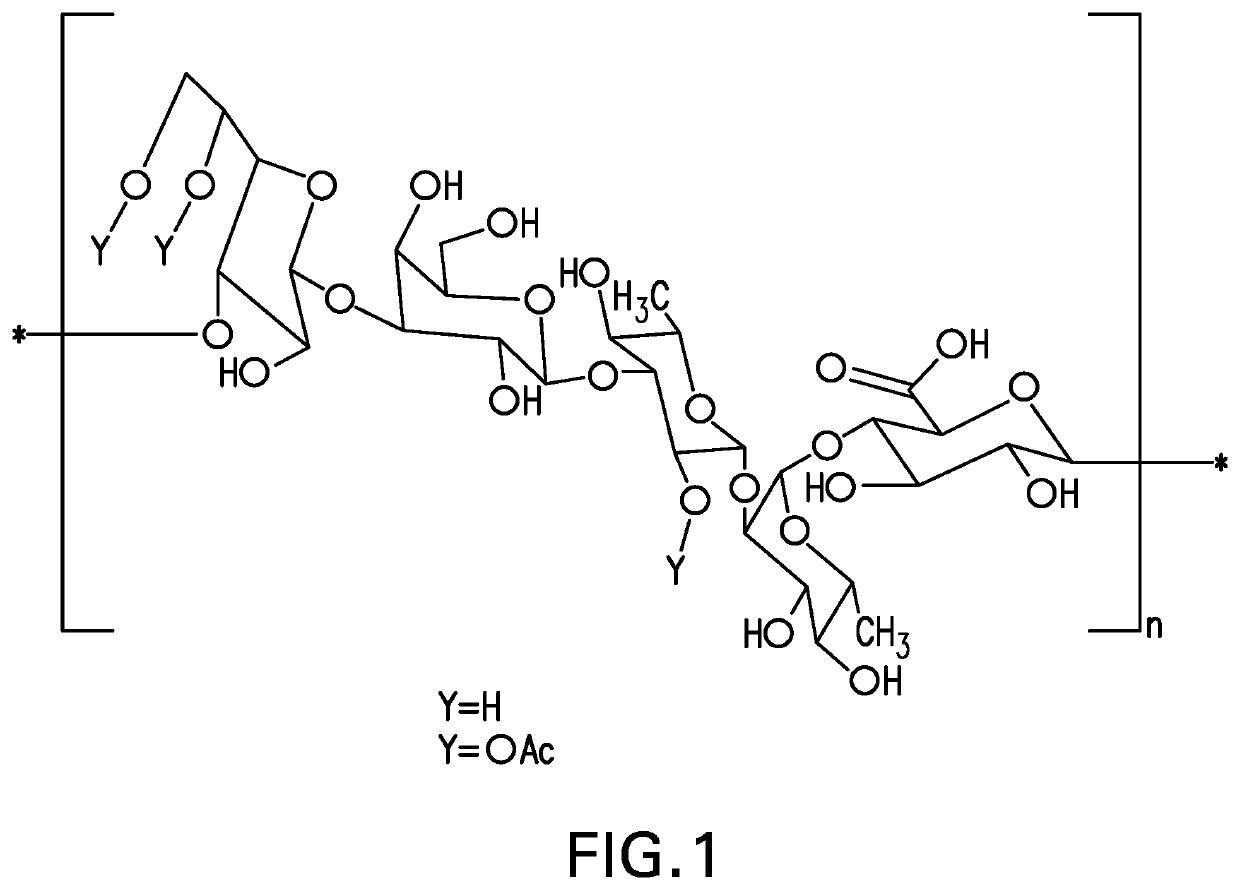
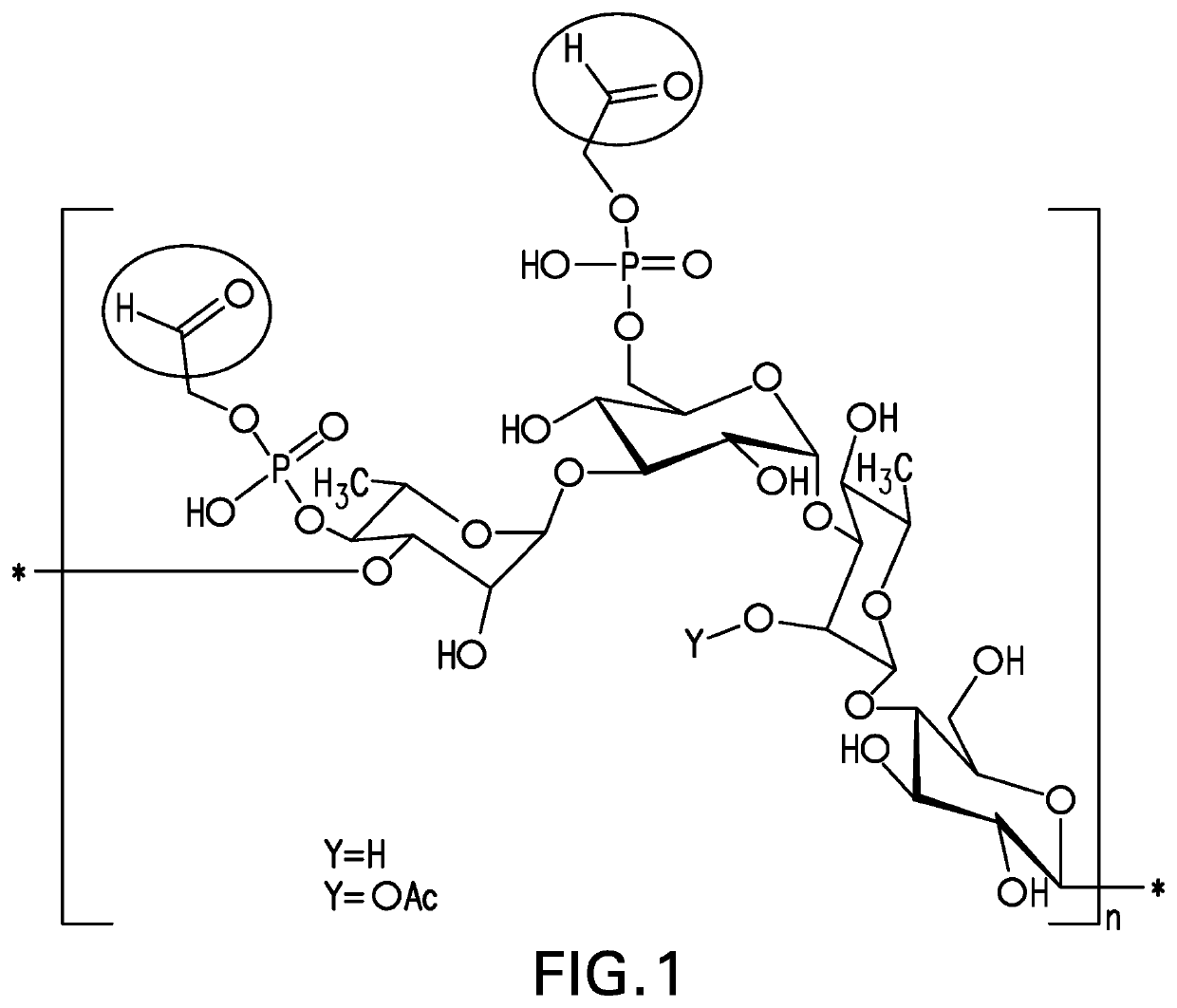
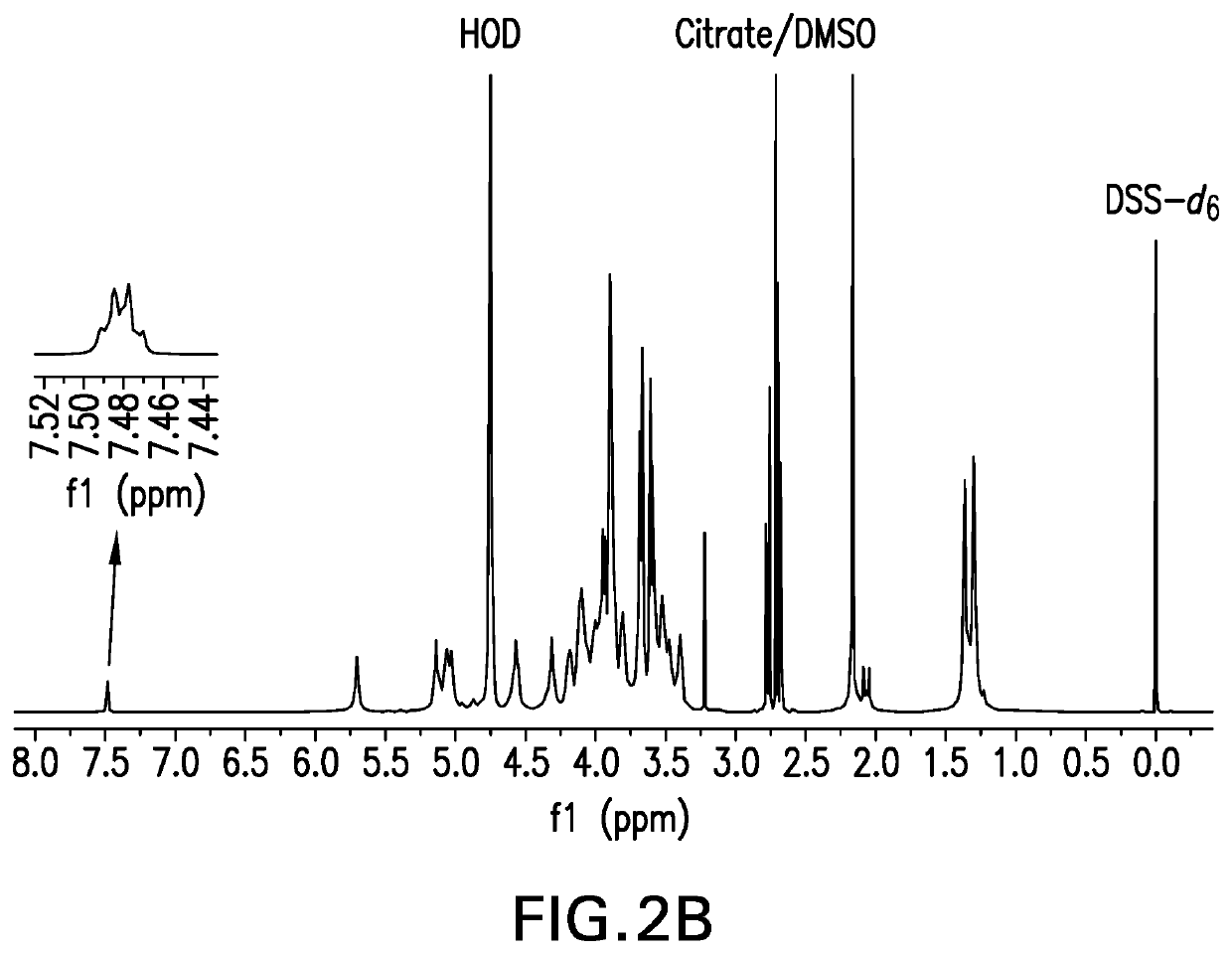
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Streptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
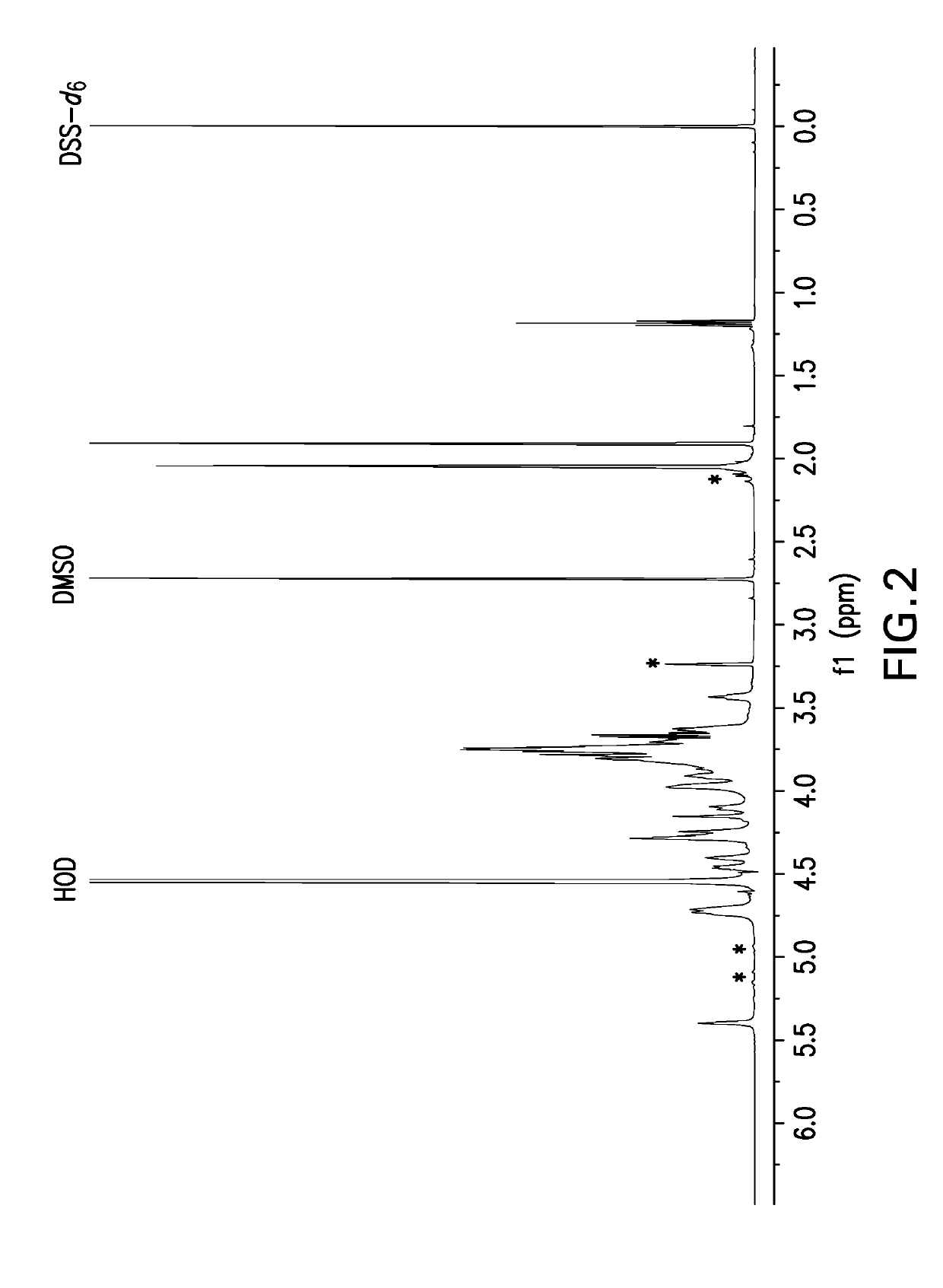
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Streptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
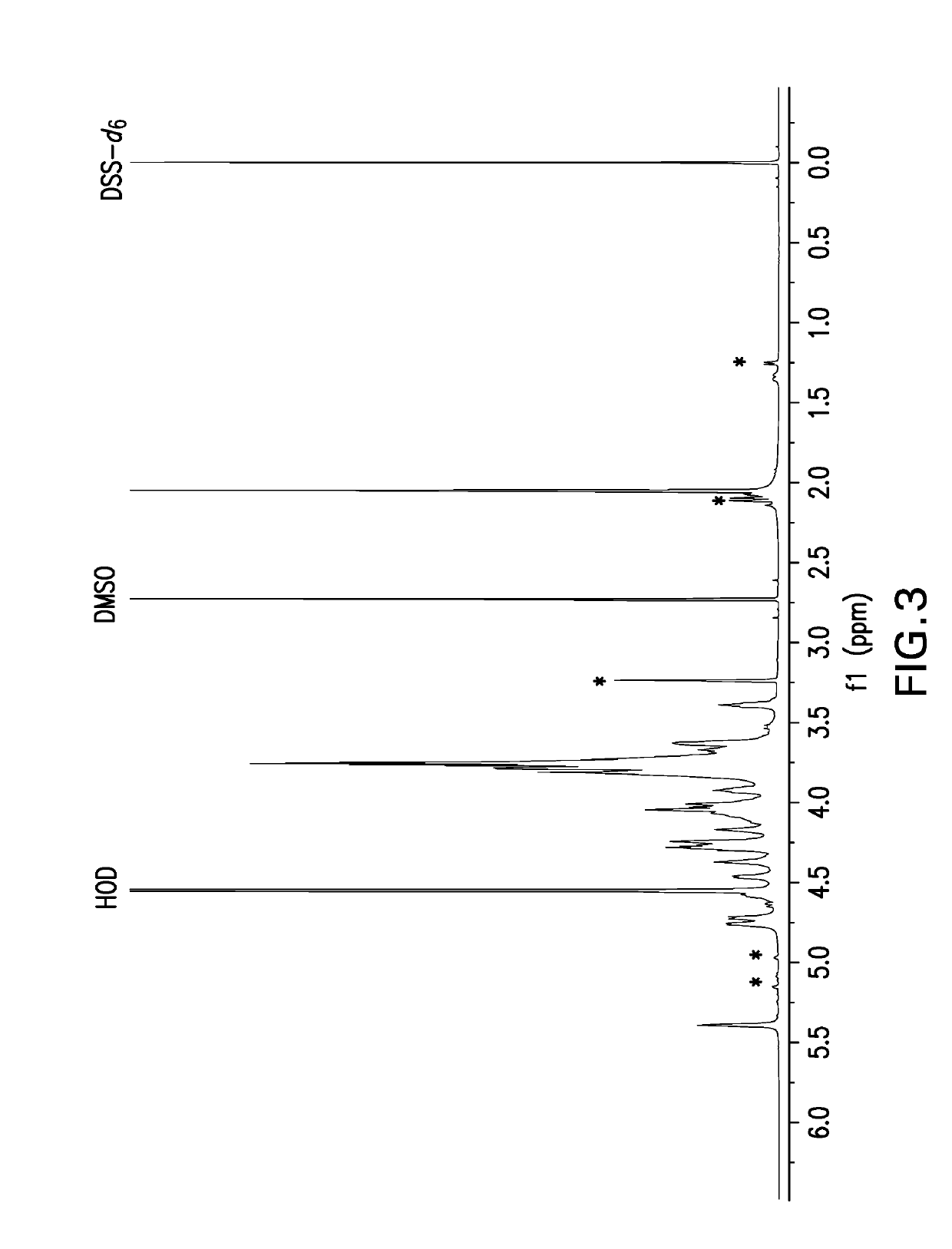
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these N serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Steptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
Pneumococcal serotypes
InactiveUS9778266B2Organic active ingredientsBacterial antigen ingredientsPneumococcal serotypesCoccidia
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6C, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit {2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→3) ribitol (5→phosphate}. This new serotype may be included in pneumococcal vaccines.
Owner:UAB RES FOUND
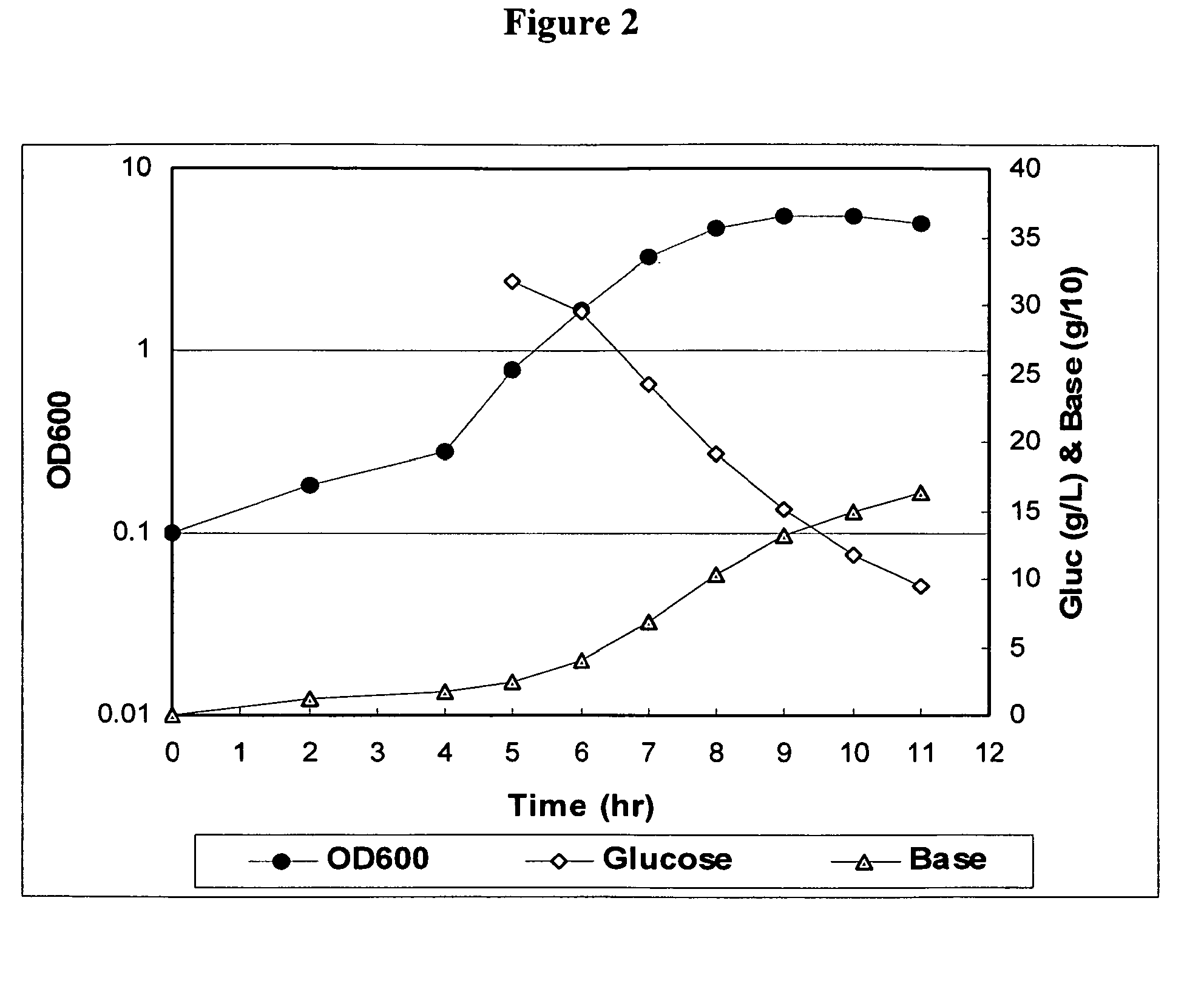
Method for controlling Streptococcus pneumoniae serotype 19A polysaccharide molecular weight
The present invention provides improved methods for producing a solution containing high molecular weight isolated Streptococcus pneumoniae 19A capsular polysaccharides. In certain methods, a fermentation culture of Streptococcus pneumoniae bacterial cells that produce serotype 19A capsular polysaccharides is fermented for less than 6 hours before the bacterial cells are lysed the capsular polysaccharides are harvested. In other methods, CO2 is supplied to the fermentation culture. Supplying CO2 to the fermentation culture includes adding bicarbonate ions to the fermentation culture, adding carbonate ions to the fermentation culture, adding mixtures of bicarbonate and carbonate ions to the fermentation culture, and overlaying the fermentation culture with CO2.
Owner:WYETH LLC
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
ActiveUS20210038723A1Antibacterial agentsPharmaceutical delivery mechanismCarrier proteinStreptococcus halichoeri
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Steptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
Biochip for streptococcus pneumoniae serotype detection, and its detecting method and kit
InactiveCN1710108AImprove accuracyGood repeatabilityMicrobiological testing/measurementBiochipStreptococcus constellatus
The invention relates to one kind of gene chip used in the pneumonia streptococcus blood serum examination and its examination method and the reagent box used thereof. The gene chip includes the solid phase carrier and the oligonucleotide probe fixed on this carrier. The probe includes the DNA fragment selected from the streptococcus pneumoniae 16s rDNA and the DNA fragment selected from the polyase gene of streptococcus pneumoniae. Use designed primer to enlarge and mark the gene group of the sample to be detected, then to hybrid the gene group using the biological chip, the different type of serum of the streptococcus pneumoniae can be detected.
Owner:TIANJIN BIOCHIP TECH CO LTD
Pneumococcal serotype 6D
Disclosed is a new and emerging serotype of Streptococcus pneumoniae designated serotype 6D, and assays and monoclonal antibodies useful in identifying same. Also disclosed is a novel pneumococcal polysaccharide with the repeating unit →2) glucose 1 (1→3) glucose 2 (1→3) rhamnose (1→4) ribitol (5→phosphate. This new serotype may be included in pneumococcal vaccines.
Owner:UAB RES FOUND
Pneumococcal serotypes
ActiveUS20180136224A1Organic active ingredientsBacterial antigen ingredientsPneumococcal serotypesCoccidia
Owner:UAB RES FOUND +1
Immunogenic compositions comprising conjugated capsular saccharide antigens, kits comprising the same and uses thereof
PendingCN108367063AAntibacterial agentsBacterial antigen ingredientsVaccinationStreptococcus pneumoniae conjugated
The present invention relates to new immunogenic compositions comprising conjugated Streptococcus pneumoniae capsular saccharide antigens (glycoconjugates), kits comprising said immunogenic compositions and uses thereof. Immunogenic compositions of the present invention will typically comprise at least one glycoconjugate from a S. pneumoniae serotype not found in PREVNAR , SYNFLORIX and / or PREVNAR 13 . The invention also relates to vaccination of human subjects, in particular infants and elderly, against pneumoccocal infections using said novel immunogenic compositions.
Owner:PFIZER INC
16-valent streptococcus pneumoniae conjugate vaccine composition
PendingCN109091668ABroad-spectrum high-efficiency protectionAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniae capsular polysaccharideCarrier protein
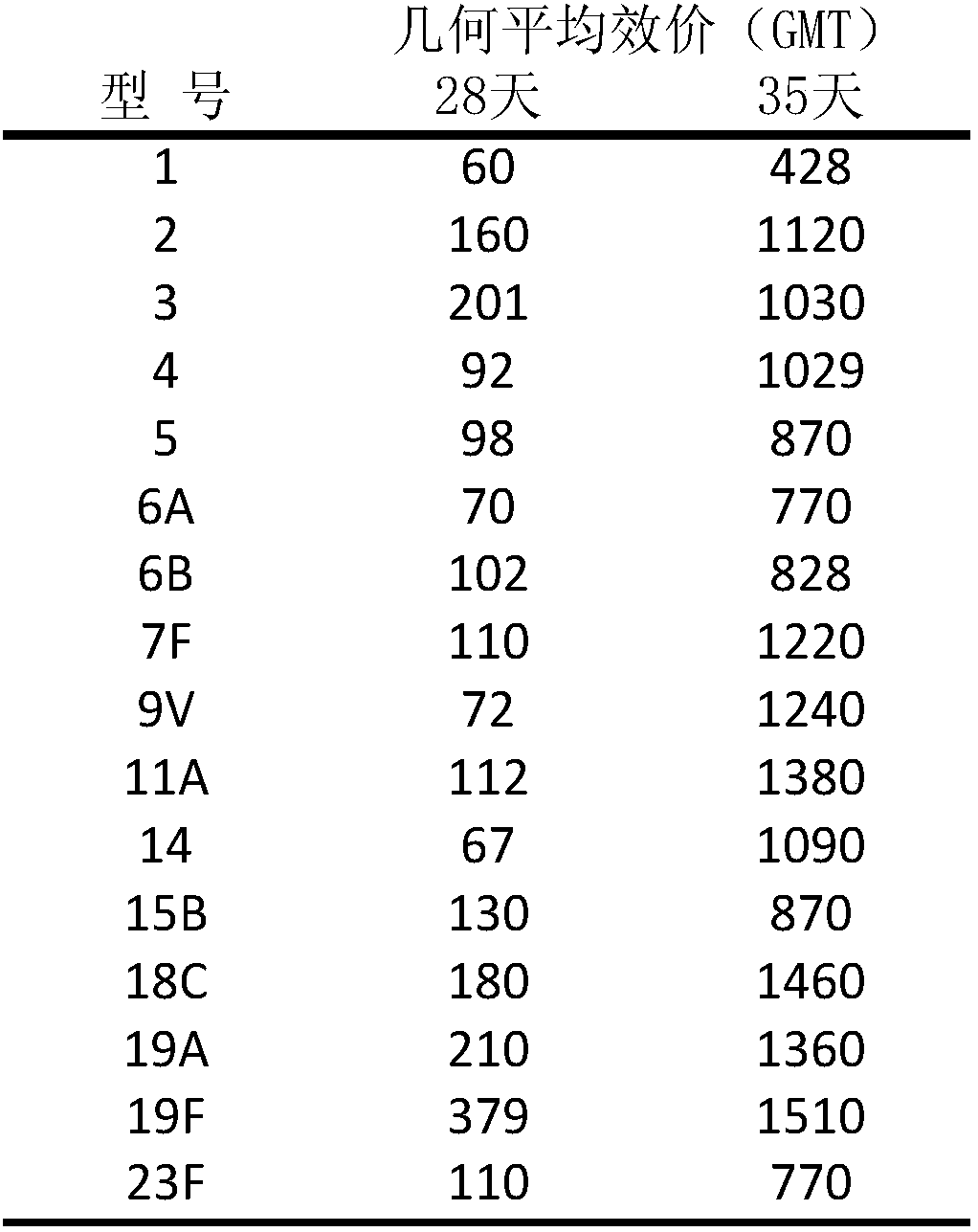
The invention discloses a 16-valent streptococcus pneumoniae conjugate vaccine composition. The vaccine composition is obtained in a manner that streptococcus pneumoniae capsular polysaccharide afterseparation and purification and carrier protein are subjected to coupling combination to be mixed, wherein the capsular polysaccharide includes 16 streptococcus pneumoniae serotypes of 1, 2, 3, 4, 5,6A, 6B, 7F, 9V, 11A, 14, 15B, 18C, 19A, 19F and 23F. The 16-valent streptococcus pneumoniae conjugate vaccine composition can cover most of common pathogenic bacterial types or drug-resistance bacterial types of our country, and has the broad-spectrum and efficient protection effect on high-risk susceptible populations such as new-born infants, old people and children under two years old.
Owner:SHANGHAI RUIZHOU BIOTECH CO LTD
Vaccines against streptococcus pneumoniae serotype 5
The present invention relates to well-defined synthetic saccharides of general formula (I) that are related to the repeating unit of Streptococcus pneumoniae serotype 5 capsular polysaccharide and conjugates thereof. The conjugates and pharmaceutical compositions containing said conjugates are useful for prevention and / or treatment of diseases associated with Streptococcus pneumoniae, and more specifically against diseases associated with Streptococcus pneumoniae serotype 5. Furthermore, the synthetic saccharides of general formula (I) (as represented in the specification) are useful as markerin immunological assays for detection of antibodies against Streptococcus pneumoniae bacteria.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
S. pneumonia and capsular polysaccharide synthesis method, SPD-0064 gene coding product and purpose of coding product
InactiveCN105950511AIncrease productionInterfere with or inhibit bindingBacteriaMicrobiological testing/measurementSequence pointGene cluster
The invention discloses S. pneumonia in the technical field of bio-pharmacy. The S. pneumonia comprises an S. pneumonia serum type strain, wherein the S. pneumonia serum type strain includes one or a plurality of kinds of capsular polysaccharide generating inhabiting genes with integral lack, partial lack, sequence point mutation or sequence point lack. When the capsular polysaccharide generating inhabiting genes have one or a plurality of kinds of changes of partial lack, sequence point mutation or lack, the function and the activity of the SPD-0064 genes or coded products of the SPD-0064 genes can be influenced; the goal of interfering or inhibiting the combination of SPD-0064 gene coding products and capsular polysaccharide gene clusters is achieved, so that the transcription function of the capsular polysaccharide gene clusters is interfered or damaged; the capsular polysaccharide yield is finally increased.
Owner:遵义医学院第三附属医院
Method for controlling streptococcus pneumoniae polysaccharide molecular weight using carbon dioxide
InactiveUS20100160622A1Esterified saccharide compoundsBacteriaBacteroidesStreptococcus pneumoniae capsular polysaccharide
The present invention provides improved methods for producing a solution containing high molecular weight isolated Streptococcus pneumoniae capsular polysaccharides having phosphodiester linkages between saccharide repeat units. In certain methods, CO2 is supplied to a fermentation culture of Streptococcus pneumoniae bacterial cells that produce capsular polysaccharide serotypes containing phosphodiester linkages between saccharide repeat units. Exemplary Streptococcus pneumoniae serotypes containing a phosphodiester linkage between saccharide repeat units include serotypes 6A, 6B, 19A, and 19F. Supplying CO2 to the fermentation culture includes adding bicarbonate ions to the fermentation culture, adding carbonate ions to the fermentation culture, adding mixtures of bicarbonate and carbonate ions to the fermentation culture, and overlaying the fermentation culture with CO2.
Owner:WYETH LLC
Vaccines against Streptococcus pneumoniae serotype 5
ActiveUS10596272B2Improving immunogenicityEasy accessAntibacterial agentsOrganic active ingredientsDiseaseStreptococcus pneumoniae conjugated
The present invention relates to well-defined synthetic saccharides of general formula (I) that are related to the repeating unit of Streptococcus pneumoniae serotype 5 capsular polysaccharide and conjugates thereof. The conjugates and pharmaceutical compositions containing said conjugates are useful for prevention and / or treatment of diseases associated with Streptococcus pneumoniae, and more specifically against diseases associated with Streptococcus pneumoniae serotype 5. Furthermore, the synthetic saccharides of general formula (I) are useful as marker in immunological assays for detection of antibodies against Streptococcus pneumoniae bacteria.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Synthetic vaccines against Streptococcus pneumoniae serotype 2
ActiveUS10864261B2Improving immunogenicityAntibacterial agentsAntipyreticStreptococcus pneumoniae conjugatedAssay
The present invention relates to a synthetic saccharide of general formula (I) that is related to Streptococcus pneumoniae serotype 2 capsular polysaccharide, a conjugate thereof and the use of said saccharide and conjugate for raising a protective immune response in a human and / or animal host. Furthermore, the synthetic saccharide of general formula (I) is useful as marker in immunological assays for detection of antibodies against Streptococcus pneumoniae type 2 bacteria.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
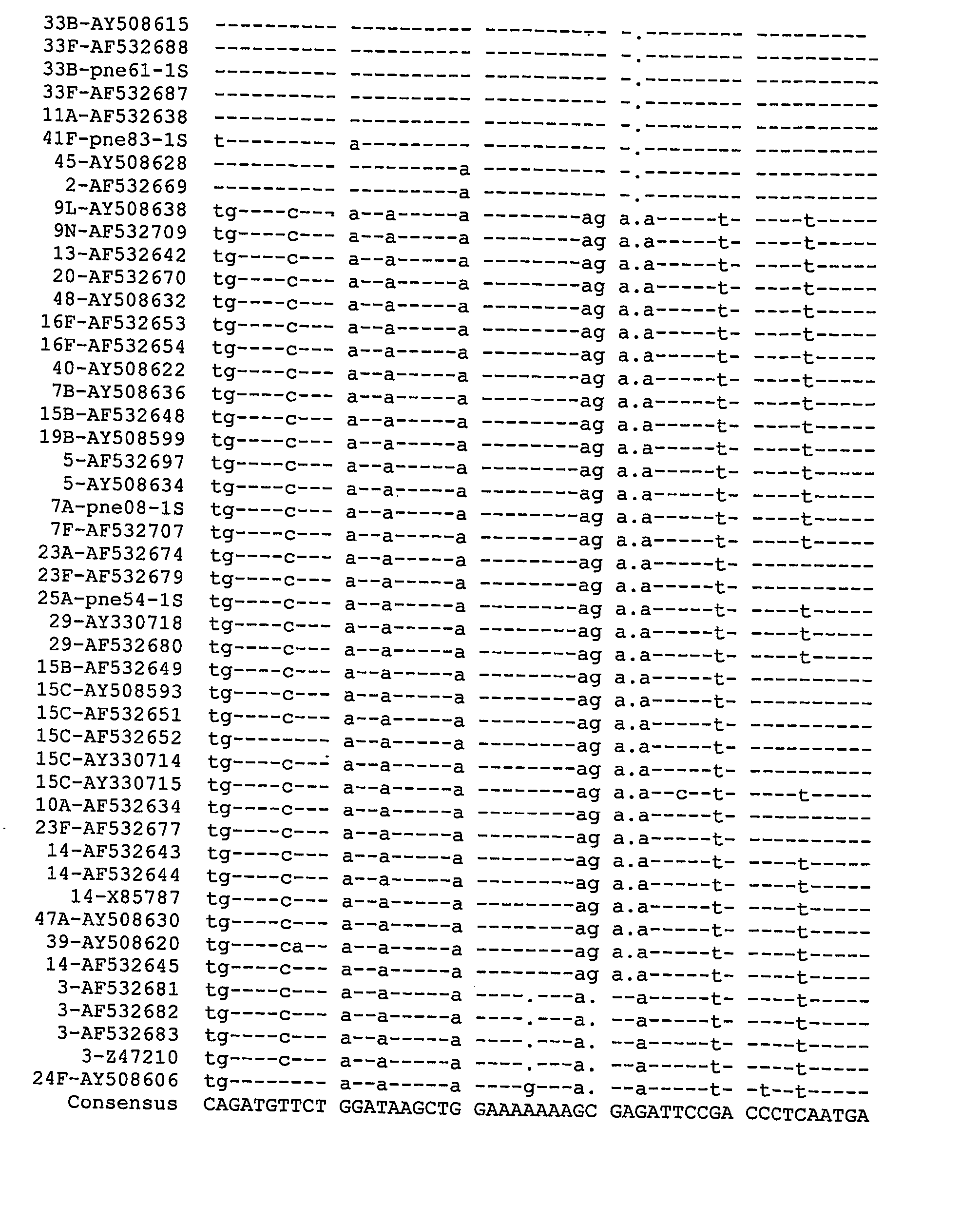
Multivalent pneumococcal polysaccharide-protein conjugate compositions and methods of use thereof
Provided is a multivalent pneumococcal conjugate composition comprising 22 to 27 different pneumococcal capsular polysaccharide-protein conjugates wherein each pneumococcal capsular polysaccharide-protein conjugate comprises a protein carrier conjugated to a capsular polysaccharide from a different serotype of Streptococcus pneumoniae, wherein the Streptococcus pneumoniae serotype is selected from the group consisting of 1, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15A, 15B, 15C, 18C, 19A, 19F, 22F, 23A, 23B, 23F, 24F, 33F, and 35B. Also provided are methods of producing the multivalent pneumococcal conjugate compositions and methods of using the multivalent pneumococcal conjugate compositions to prevent a Streptococcus pneumoniae infection or disease in a subject. Also provided are immunogenic compositions comprising at least one polysaccharide-protein conjugate, wherein the polysaccharide is a capsular polysaccharide from Streptococcus pneumoniae serotypes 15A, 15C, 23A, 23B, 24F and / or 35B; and methods of making the immunogenic compositions.
Owner:圣诺菲·帕斯图尔公司 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com