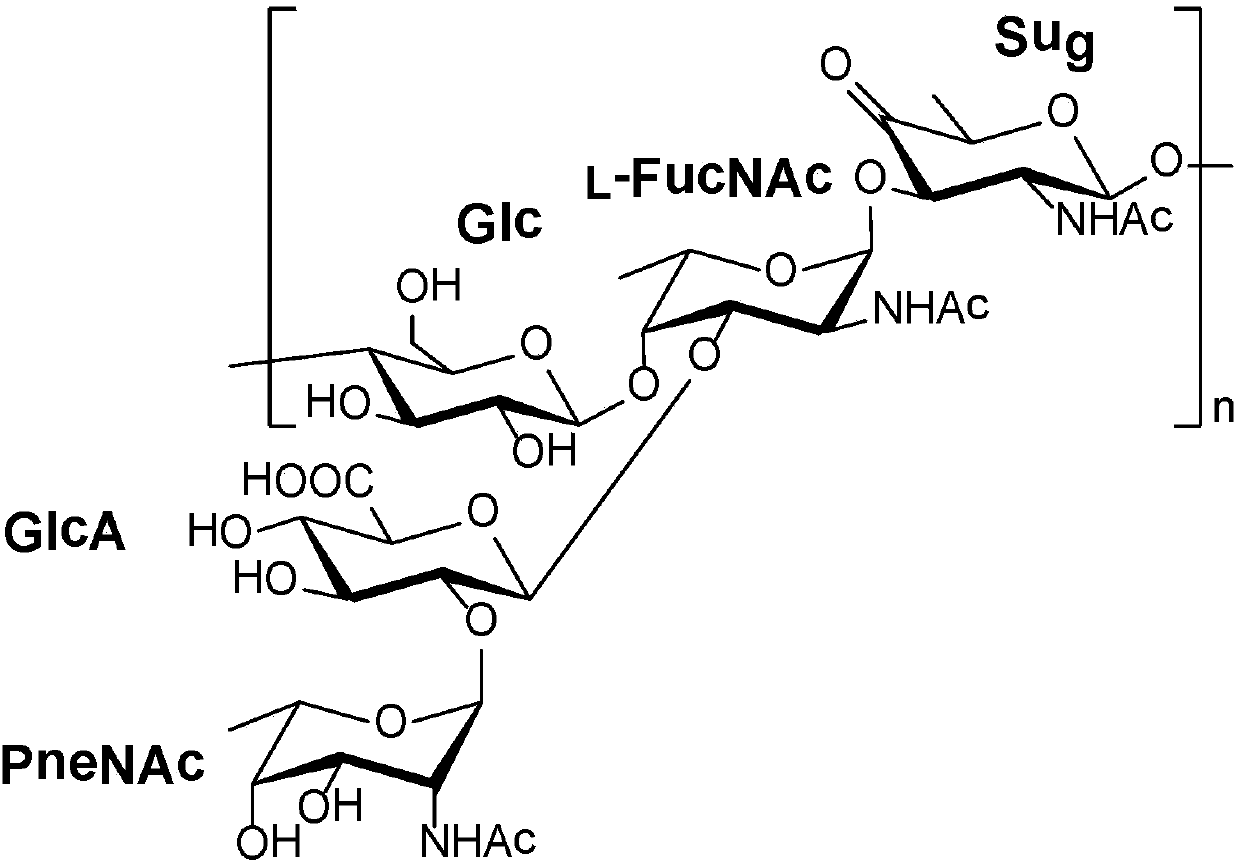
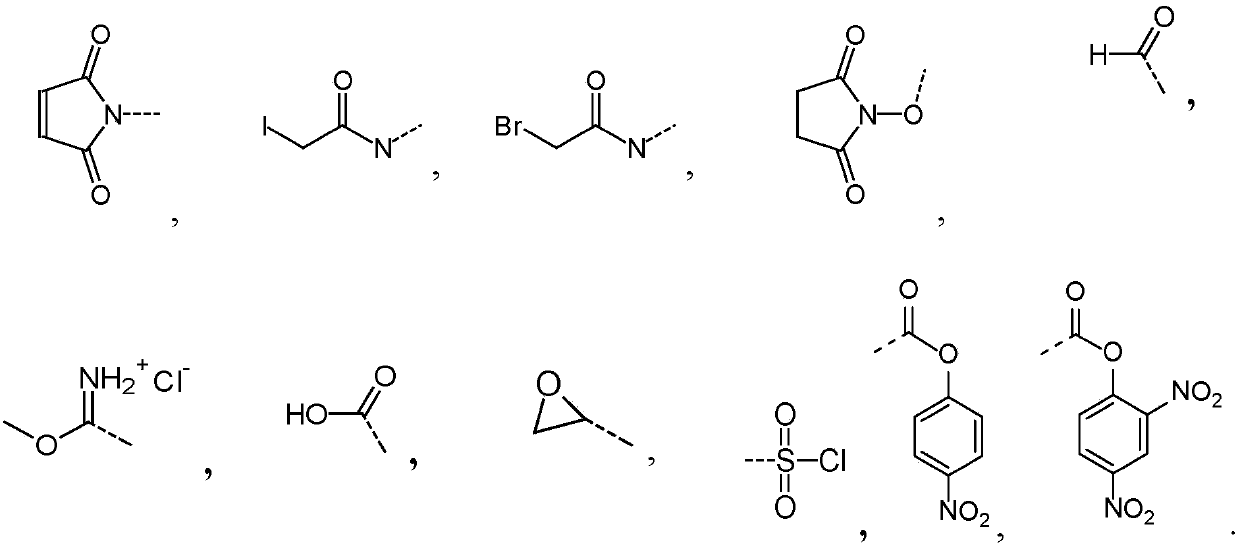
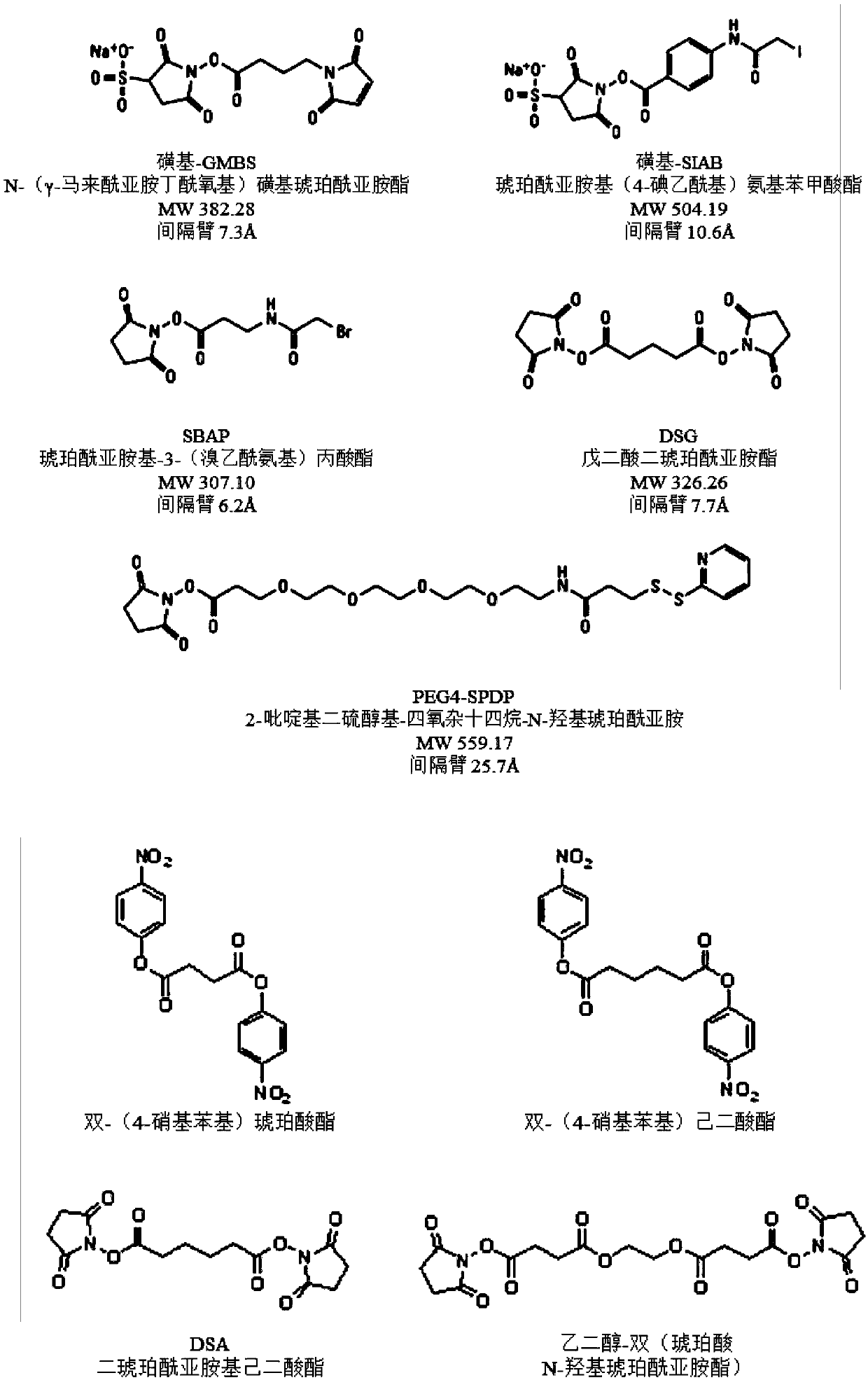
Vaccines against streptococcus pneumoniae serotype 5
一种肺炎、选自的技术,应用在合成糖及其缀合物领域,能够解决疫苗有效制造不利等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0342] Example 1: Synthesis of phenyl 2-azido-2-deoxy-1-seleno-α-D-fucopyranoside (1*)
[0343]
[0344] At -30°C, fucotenose (Bedini, E.et.al.Synlett 2006, 6, 825-830) (4.6g, 21.6mmol) and Ph 2 Se 2 (6.75g, 21.6mmol) To a solution in DCM (70mL) was added PhI(OAc) 2 (6.96 g, 21.6 mmol). TMSN was then added dropwise to the resulting solution 3 (5.74 mL, 43.2 mmol). The solution was warmed to -10°C. The reaction was complete after 4 h at -10 °C. The reaction mixture was warmed to room temperature and washed with NaHCO 3 Wash with saturated solution and then with brine. The solvent was evaporated and the impure was dissolved in MeOH (190 mL). NaOMe 0.5M (4.75 mmol, 9.5 mL) solution was added dropwise to the mixture at room temperature. The reaction was stirred at room temperature for 1 h. Add Amberlite and stir until pH drops to 5. The reaction mixture was filtered and the solvent was evaporated. The residue was purified by silica gel chromatography (DCM 100% to ...
Embodiment 2
[0345] Example 2 : Synthesis of phenyl 2-azido-2-deoxy-3-O-p-methoxybenzyl-1-selenium-α-D-fucopyranoside (2*)
[0346]
[0347] Diol 2* (2.5 g, 7.62 mmol) was coevaporated twice with anhydrous toluene and allowed to dry under high vacuum for 30 min. Anhydrous toluene (80 mL) was then added followed by Bu 2 SnO (2.84 g, 11.43 mmol) and 4AMS. The reaction was stirred at reflux for 1 h. The reaction was cooled to 40 °C; PMBCl (3.11 mL, 22.85 mmol) and TBAB (3.68 g, 11.43 mmol) were added and stirred at room temperature overnight. In the morning the reaction was complete. The reaction was filtered and the solvent was evaporated. The residue was purified by silica gel chromatography (10%-20% EtOAC in hexane). Alcohol 2* was obtained as a yellow oil (3.09 g, 6.89 mmol, 90%). [α] D 20 =+177.4° (c=0.77, CHCl 3 ); IRν max (Film) 3493, 2935, 2111, 1613, 1514, 1249, 1091, 740cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ7.79–7.47(m,2H),7.41–7.12(m,5H),6.92(d,J=8.7Hz,2H),5.88(d,J=5....
Embodiment 3
[0348] Example 3 :Phenyl 2-azido-2-deoxy-3-O-p-methoxybenzyl-4-O-(2-naphthylmethyl)-1-selenium-α-D-fucopyranoside -Synthesis of 2-(bromomethyl)-naphthalene(3*)
[0349]
[0350] 2-(Bromomethyl)-naphthalene (2.8 g, 12.67 mmol) was added to a solution of alcohol 2* (2.84 g, 6.33 mmol) in DMF:THF (1:1, 64 mL). The mixture was then cooled to 0 °C and NaH 60% (304 mg, 7.60 mmol) was added. The reaction was allowed to warm to room temperature and stirred for 1 h. The reaction is complete. It was then cooled to 0°C again and 100 mg of NaH was added. After 1 h the reaction was complete. The reaction mixture was cooled to 0 °C, MeOH was added and the reaction was allowed to warm to room temperature. The reaction mixture was diluted with ether, washed with HCl, NaHCO 3 and washed with brine. The solvent was evaporated and the residue was purified by silica gel chromatography (5%-10% EtOAC in hexane). The product 3* was obtained as a colorless oil (2.56 g, 4.35 mmol, 69%). ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com