Multivalent pneumococcal polysaccharide-protein conjugate compositions and methods of use thereof
A technology for pneumococcus and pneumococcus, which can be used in drug combinations, multivalent vaccines, non-active components of polymer compounds, etc., and can solve problems such as the increase of virulent pneumococcal strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0361] Example 1. Preparation of Streptococcus pneumoniae capsular polysaccharide
[0362] Cultivation of S. pneumoniae and purification of capsular polysaccharides are performed as known to those skilled in the art. S. pneumoniae serotypes were obtained from the American Type Culture Collection (ATCC) (Serotype 1: ATCC No. 6301; Serotype 3: ATCC No. 6303; Serotype 4: ATCC No. 6304; Serotype 5: ATCC No. 6305; Serotype 6A: ATCC No. 6306; Serotype 6B: ATCC No. 6326; Serotype 7F: ATCC No. 10351; Serotype 9N: ATCC No. 6309; Serotype 9V: ATCC No. 10368; Serotype 14: ATCC No. 6314; Serotype 18C: ATCC No. 10356; Serotype 19A: ATCC No. 10357; Serotype 19F: ATCC No. 6319; Serotype 23B: ATCC No. 10364; Serotype 23F: ATCC No. 6323). For serotypes 8, 10A, 11A, 12F, 15A, 15B, 15C, 22F, 23A, 23B, 24F, 33F, and 35B, internal strains or strains obtained from other sources were used, but any publicly available strain could be used. Streptococcus pneumoniae is characterized by capsular and mo...
Embodiment 2
[0369] Example 2. Conjugates of Streptococcus pneumoniae capsular polysaccharide and carrier protein (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, Preparation of 18C, 19A, 19F, 22F, 23F and 33F)
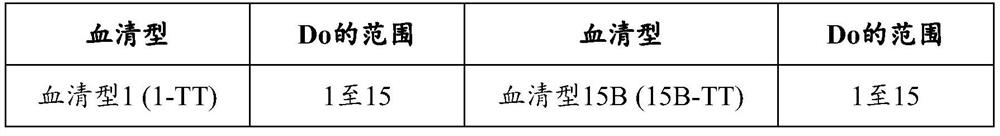
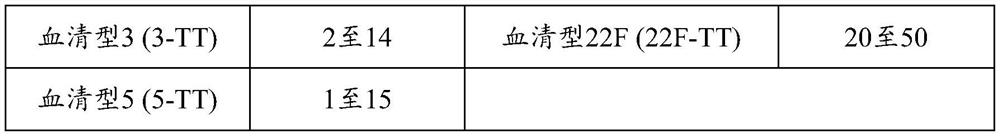
[0370] The polysaccharides of different serotypes are activated according to different pathways, and then combined with the carrier protein CRM 197 or TT conjugated. Specifically, serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15A, 15B, 15C, 18C, Polyvalent pneumococcal polysaccharide-protein conjugates of capsular polysaccharides of 19A, 19F, 22F, 23A, 23B, 23F, 24F, 33F and 35B: serotypes 3, 4, 6A, 6B, 7F, 8, 9N, Each capsular polysaccharide and CRM of 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F and 33F 197 Each capsular polysaccharide of serotypes 1 and 5 was conjugated and conjugated to TT. Serotypes 15A, 15C, 23A, 23B, 24F and 35B and CRMs are described in Examples 3-8 197 conjugation. Compounds from serotypes 1, 3, 4, 5, 6A...
Embodiment 3
[0416] Example 3. Serotype 15A and CRM 197 Preparation of Single Conjugates
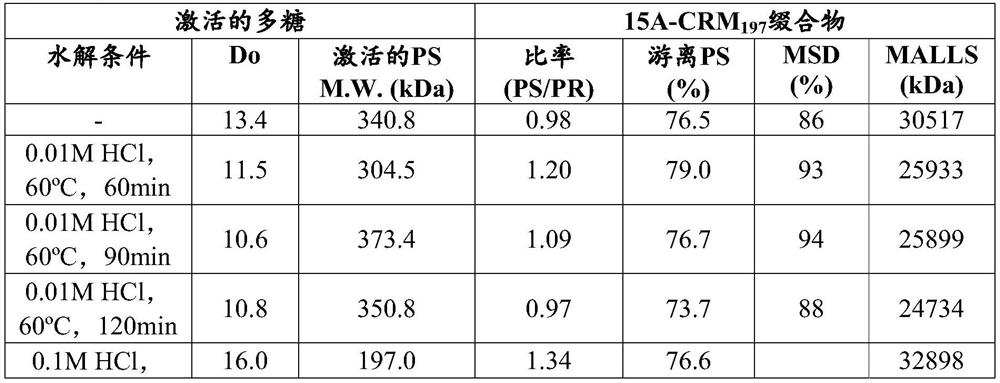
[0417] The serotype 15A polysaccharide can be purified as discussed above or with reference to the methods described in WO2013 / 191459 for the purification of polysaccharides of other serotypes. Acid hydrolysis was performed by applying acid and high temperature to purified serotype 15A polysaccharides as shown in Table 1, followed by an activation process. The conditions of hydrolysis were observed to affect the degree of oxidation (Do) and molecular weight of activated polysaccharides as well as conjugation results. The activation process and the conjugation process were performed under the same conditions. Sodium periodate was added and the oxidation reaction was allowed to proceed at 21 to 25°C for 16 to 20 hours. will activate polysaccharides and CRMs 197Proteins were lyophilized and suspended in DMSO. The activated polysaccharide and protein were mixed in a 1:1 ratio, and the reaction conce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com