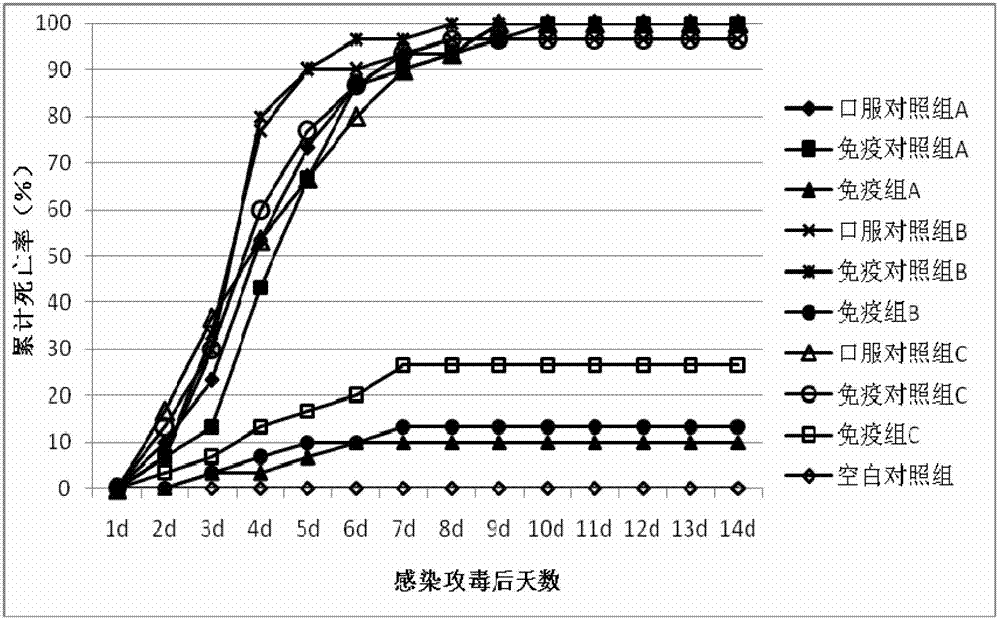
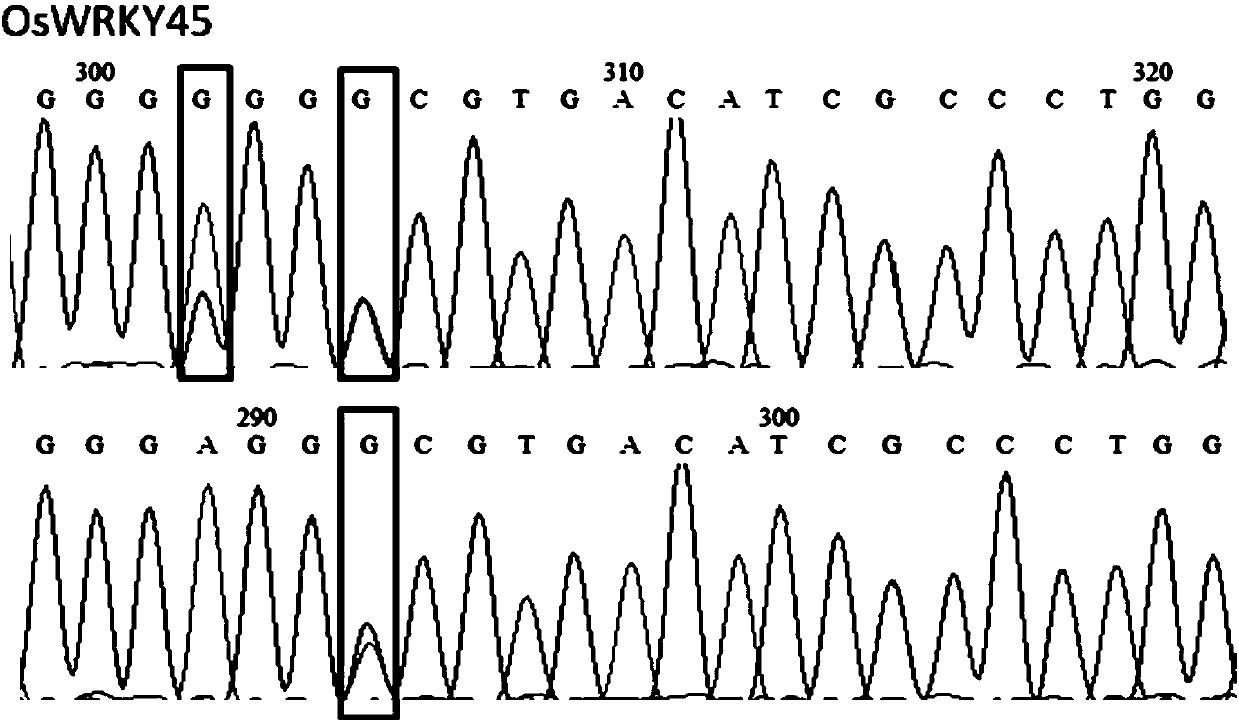
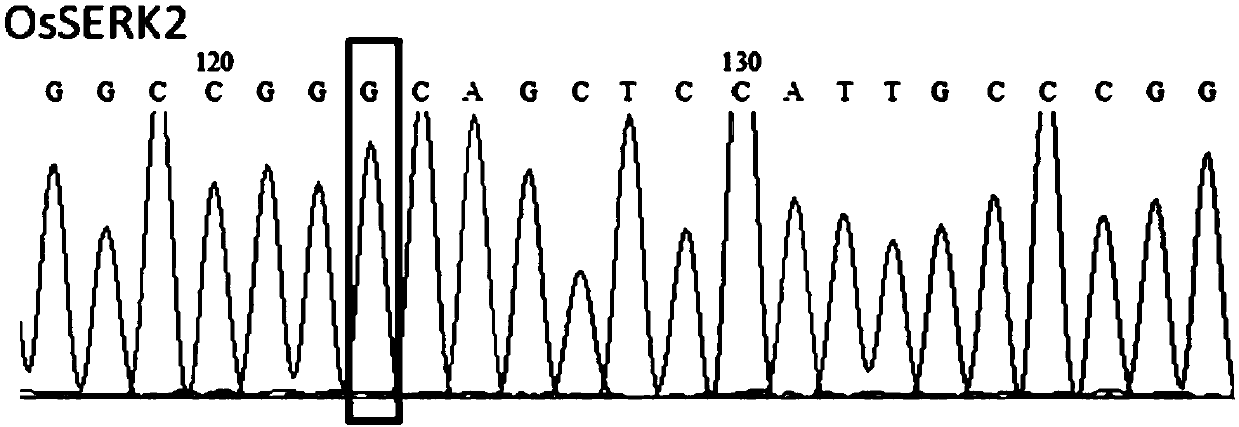
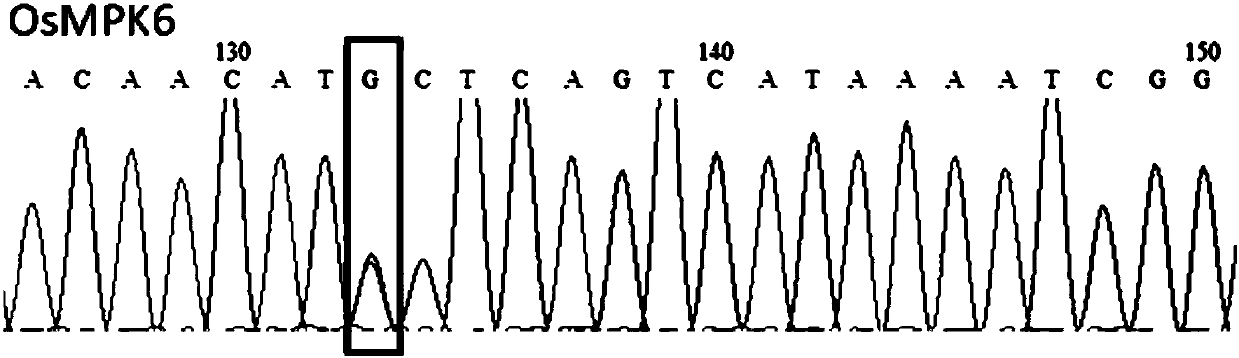
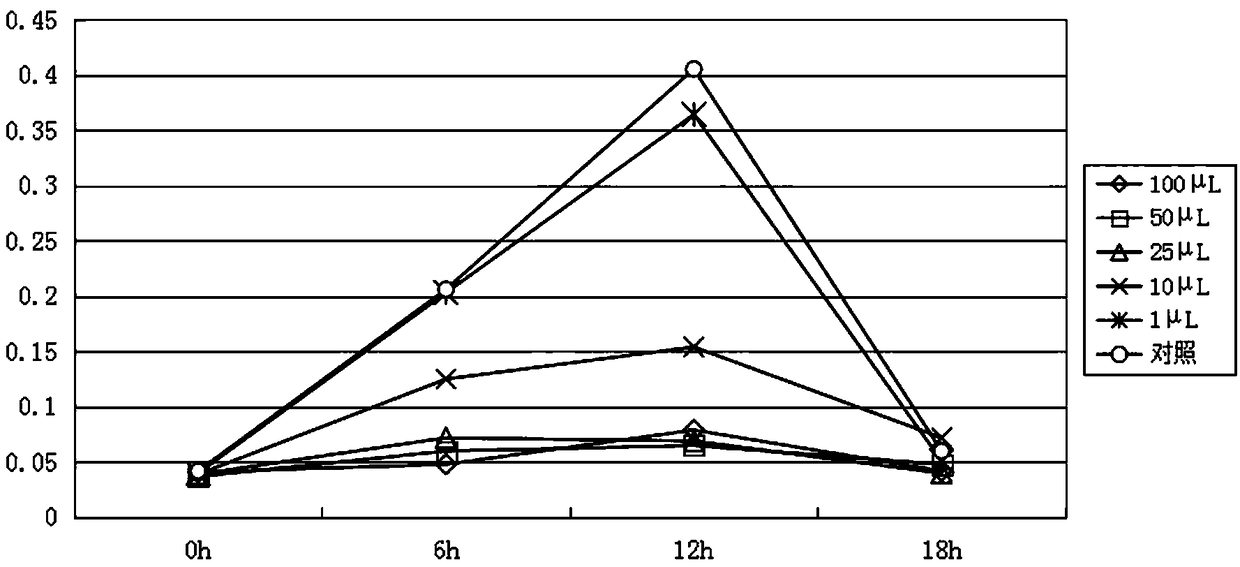
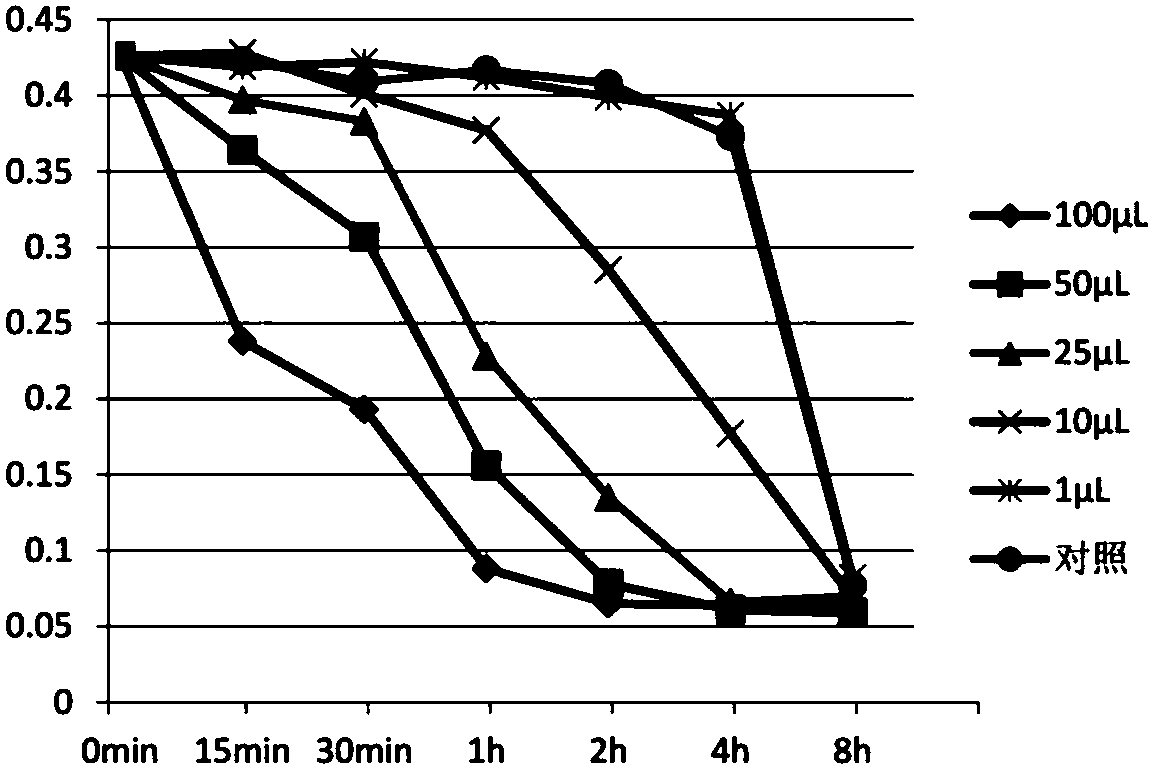
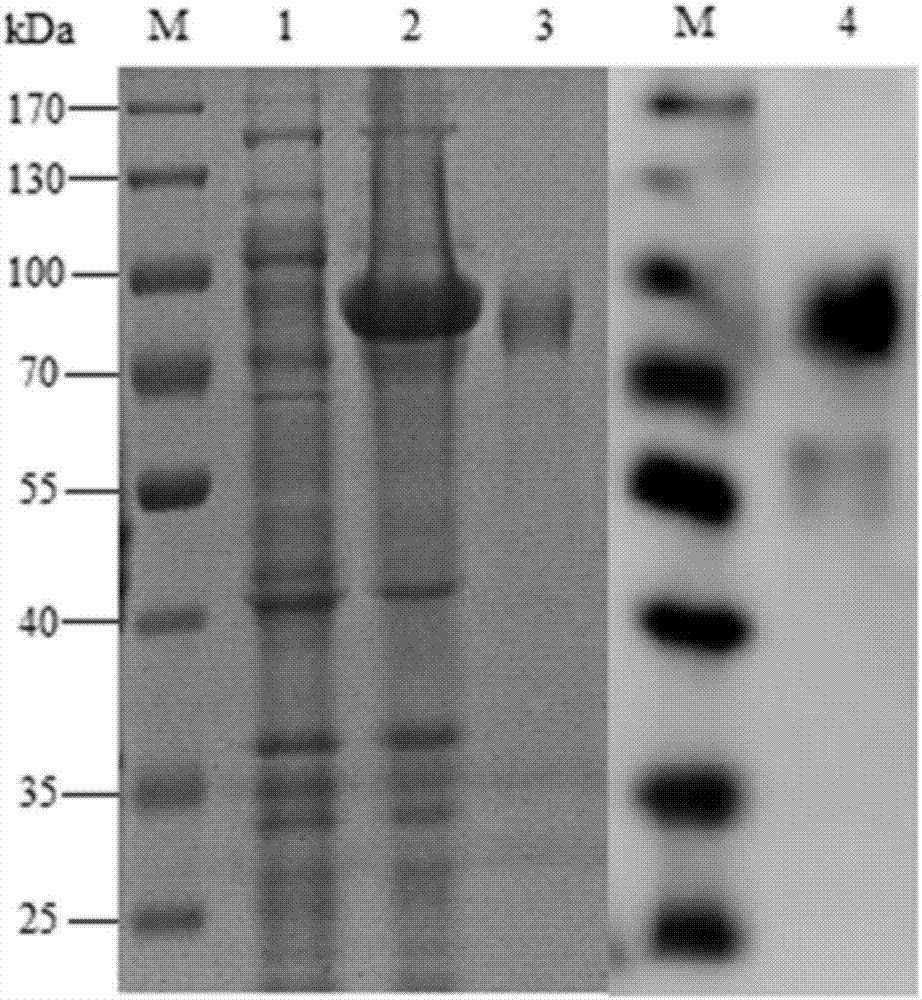
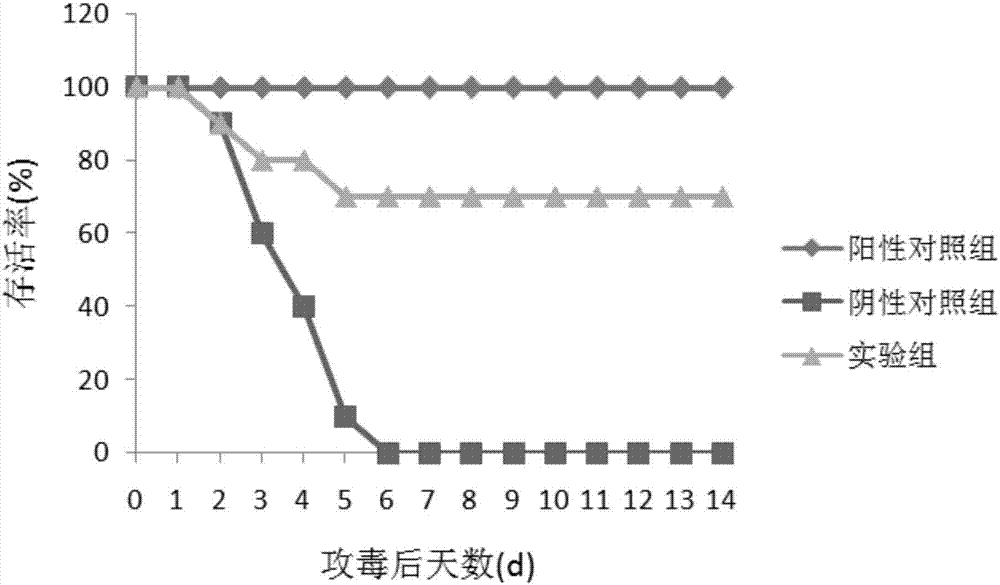
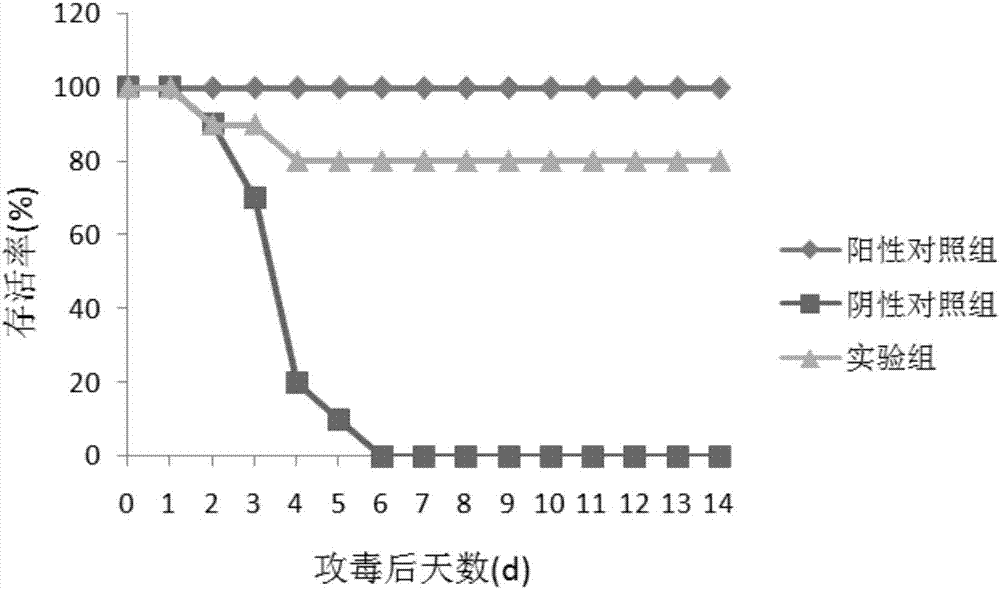
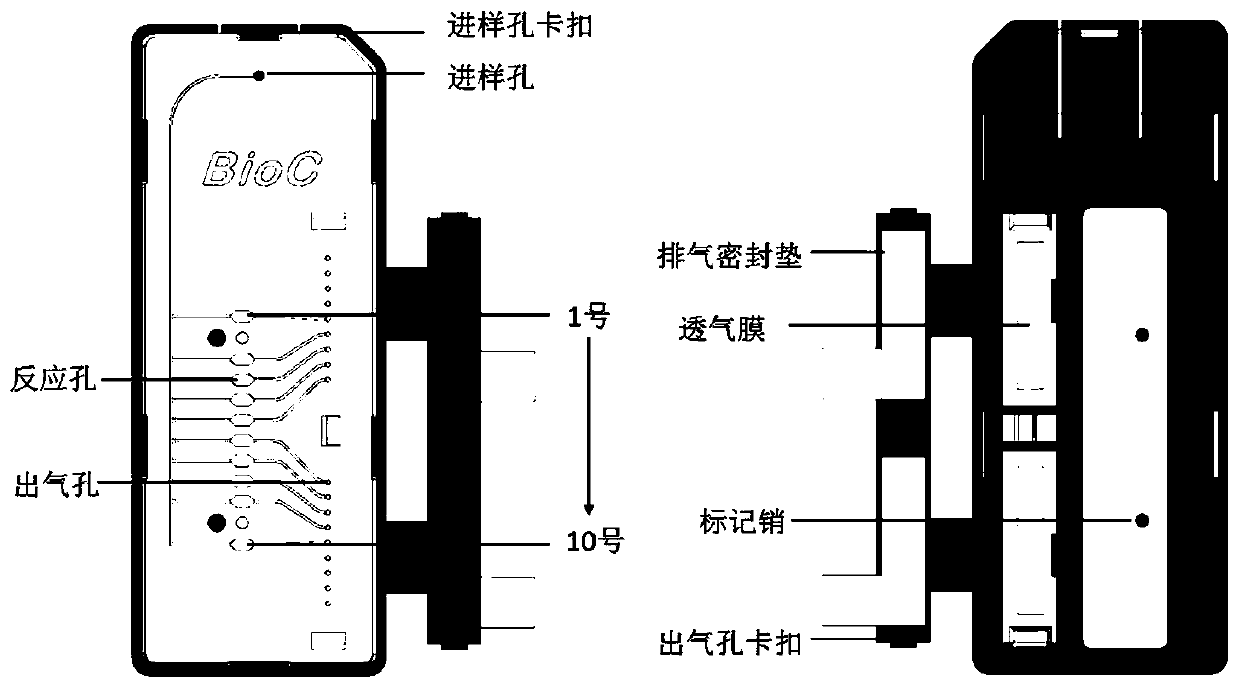
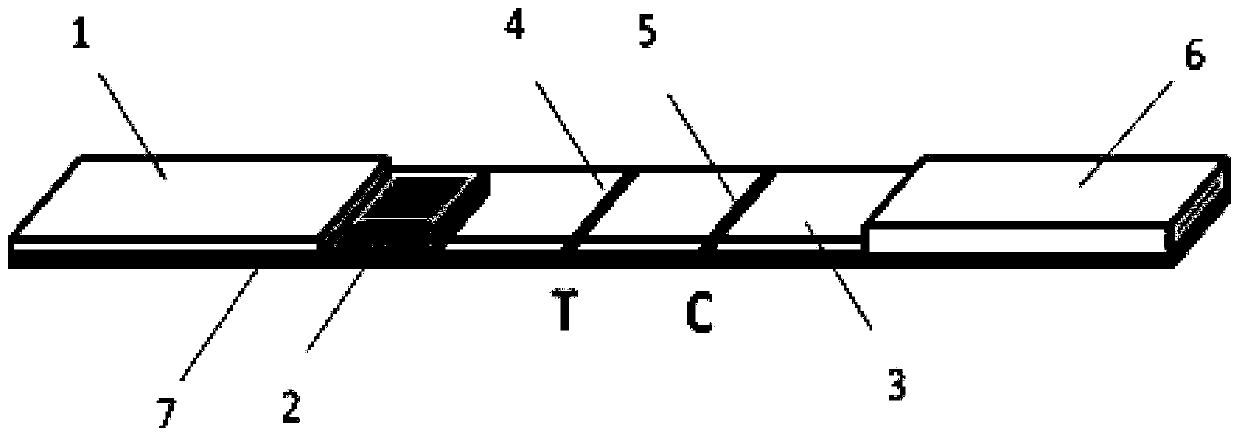
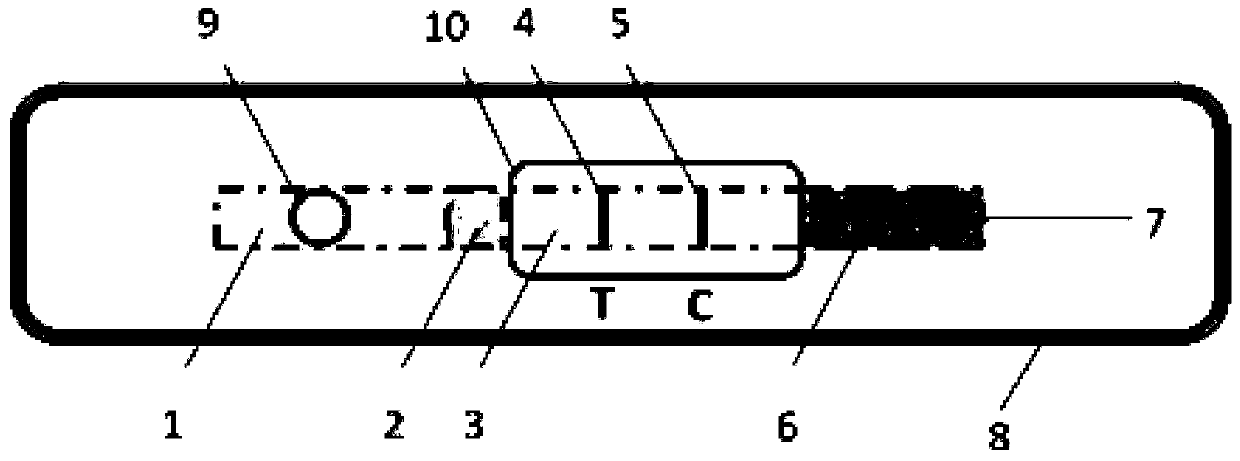
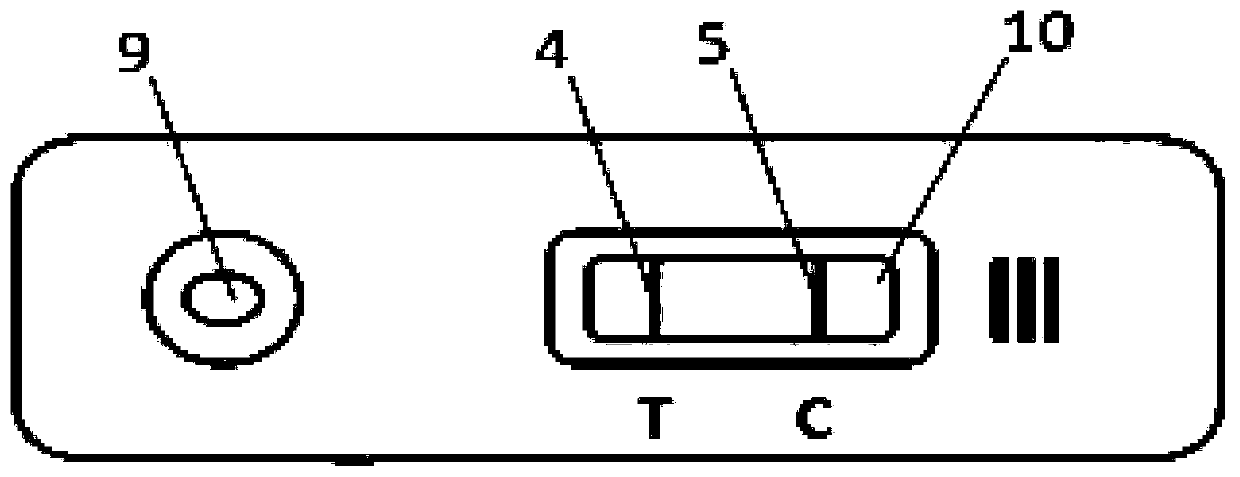
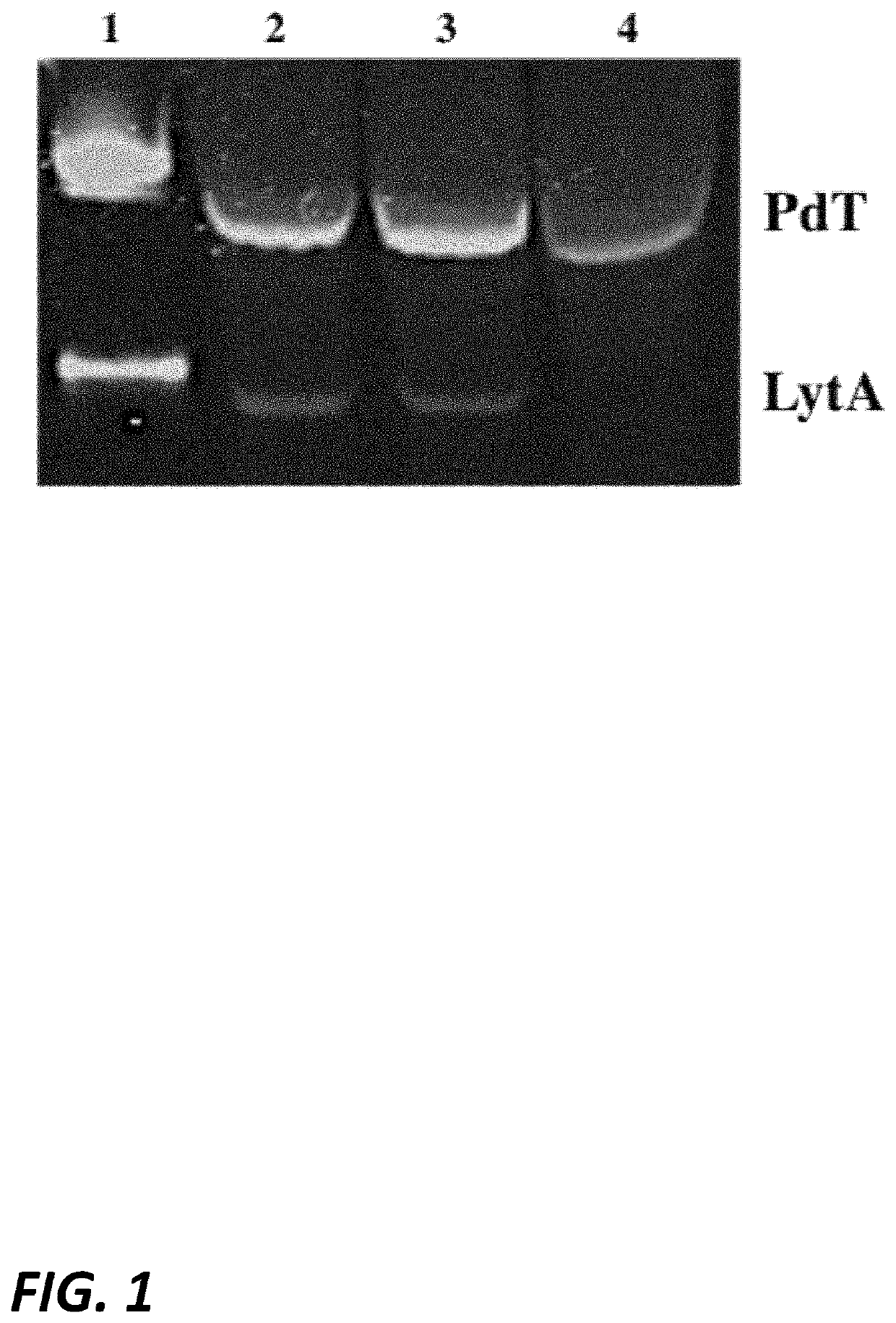
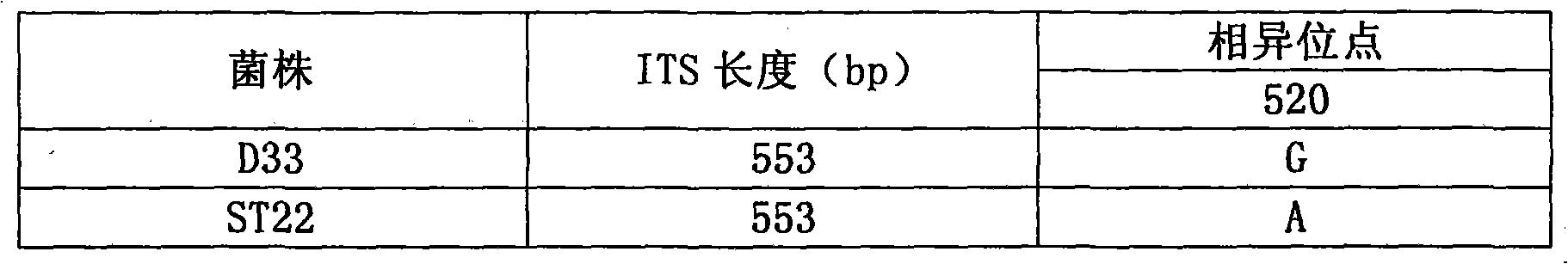
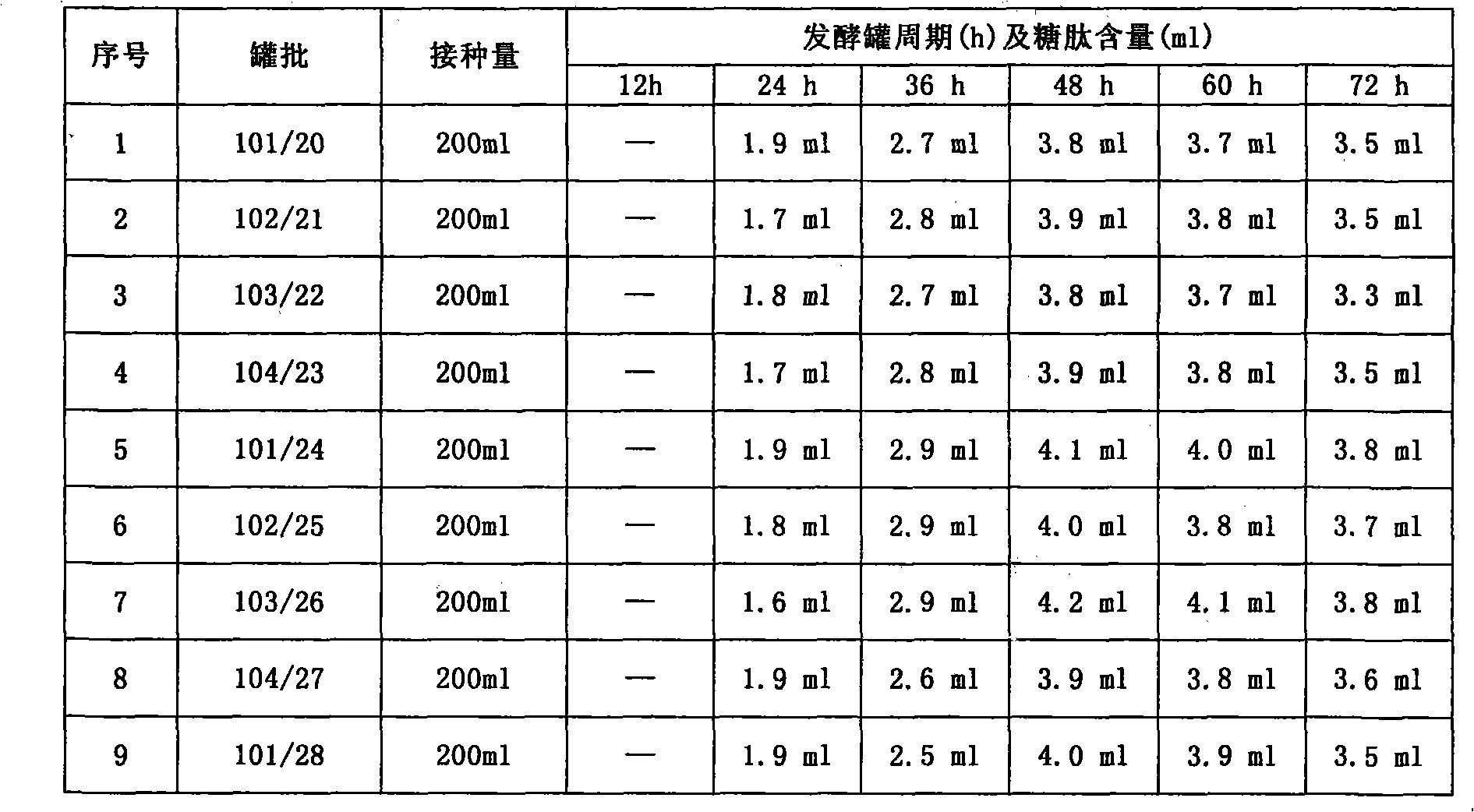
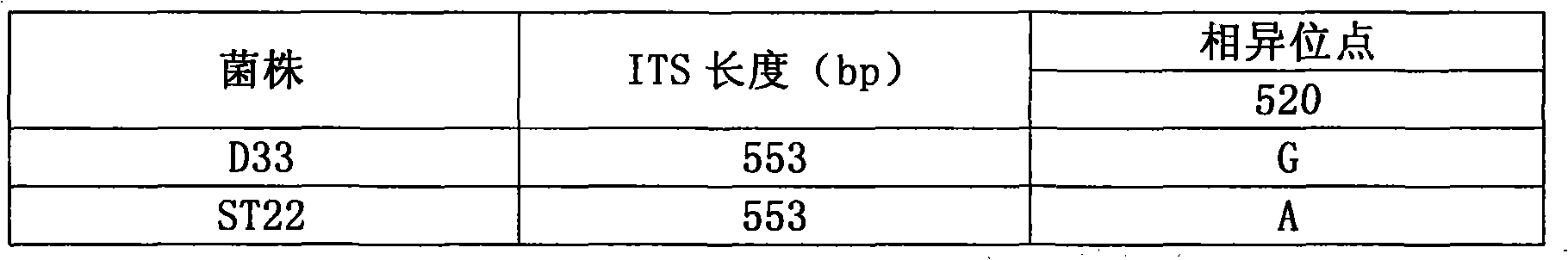
Patents
Literature
101 results about "Streptococcus halichoeri" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
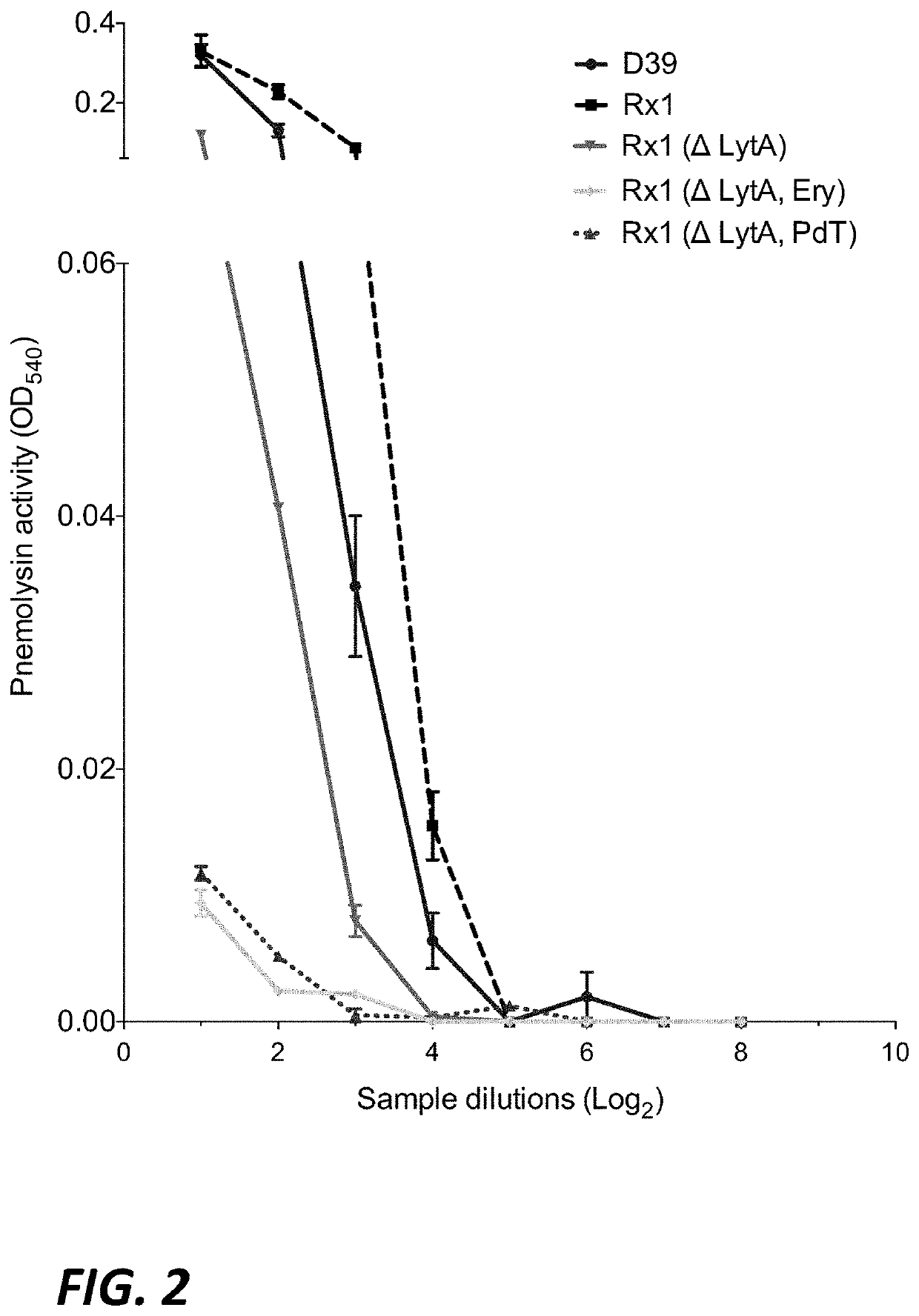
Phenotypic, genotypic, and antimicrobial characteristics of six phenotypically distinct human clinical isolates that most closely resembled the type strain of Streptococcus halichoeri isolated from a seal are presented.
Methods of use of one step immunochromatographic device for Streptococcus A antigen
InactiveUS6979576B1Reduce the numberLess manipulationBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenReagent
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:SEKISUI DIAGNOSTICS
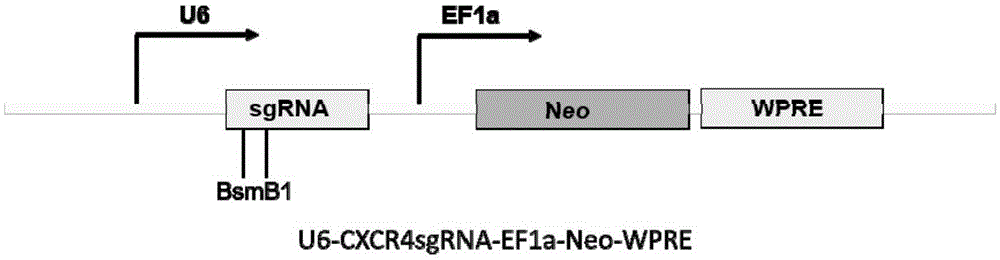
Streptococcus thermophilus derived human CXCR3 gene target sequence recognizable by CRISPR (clustered regularly interspaced short palindromic repeat)-Cas9 (CRISPR associated 9) system and sgRNA (single guide ribonucleic acid) and application thereof
InactiveCN105316324AAchieve preventionAchieve therapeutic effectOrganic active ingredientsHydrolasesProtein recruitmentA-DNA
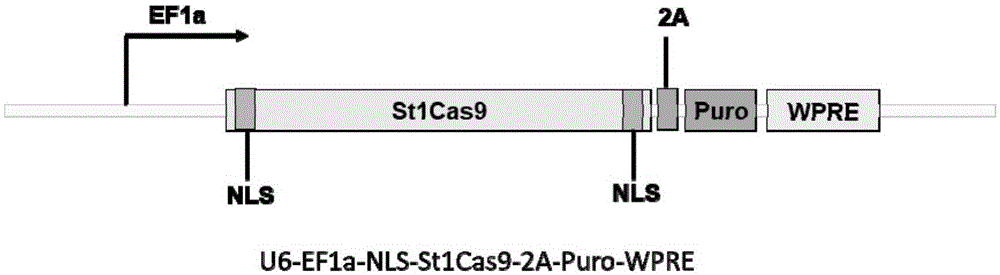
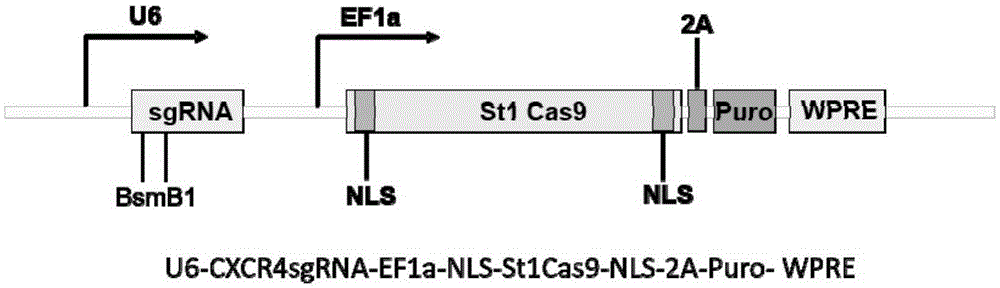
The invention belongs to the field of genetic engineering, discloses a streptococcus thermophilus derived target sequence recognizable by a CRISPR (clustered regularly interspaced short palindromic repeat)-Cas9 (CRISPR associated 9) system and further discloses a sgRNA (single guide ribonucleic acid) and a coding DNA (deoxyribonucleic acid) molecule thereof. The target sequence is shown as n-20th of any one of SEQ ID NO:1-63, wherein n=1-5. A sequence of the sgRNA is 5'-recognition sequence- Cas9 protein recruitment sequence-3', and a DNA sequence corresponding to the recognition sequence is identical to the target sequence. The invention also discloses the CRISPR-Cas9 system, and the CRISPR-Cas9 system comprises Cas9 proteins and sgRNA and / or comprises carriers carrying a Cas9 protein coding sequence and a sgRNA coding sequence. The invention further discloses application of the CRISPR-Cas9 system to CXCR4 gene editing and preparation of medicines for treating HIV (human immunodeficiency virus) infection. CXCR4 gene editing can be realized to protect cells from HIV infection.
Owner:张竞方
Immunogenic compositions for streptococcus pyogenes
ActiveUS7709009B2Antibacterial agentsAntibody mimetics/scaffoldsStreptococcus halichoeriBiochemistry
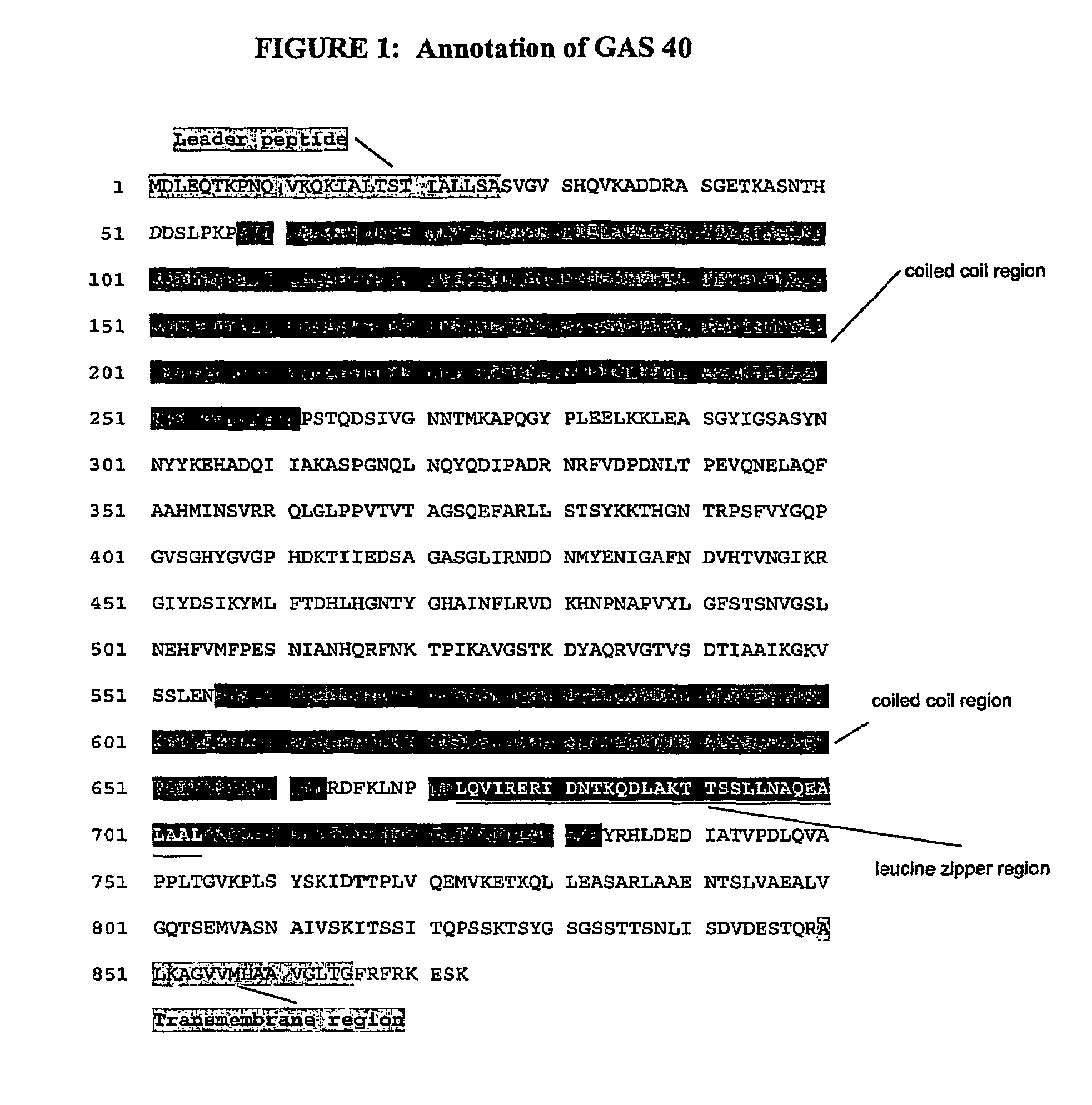
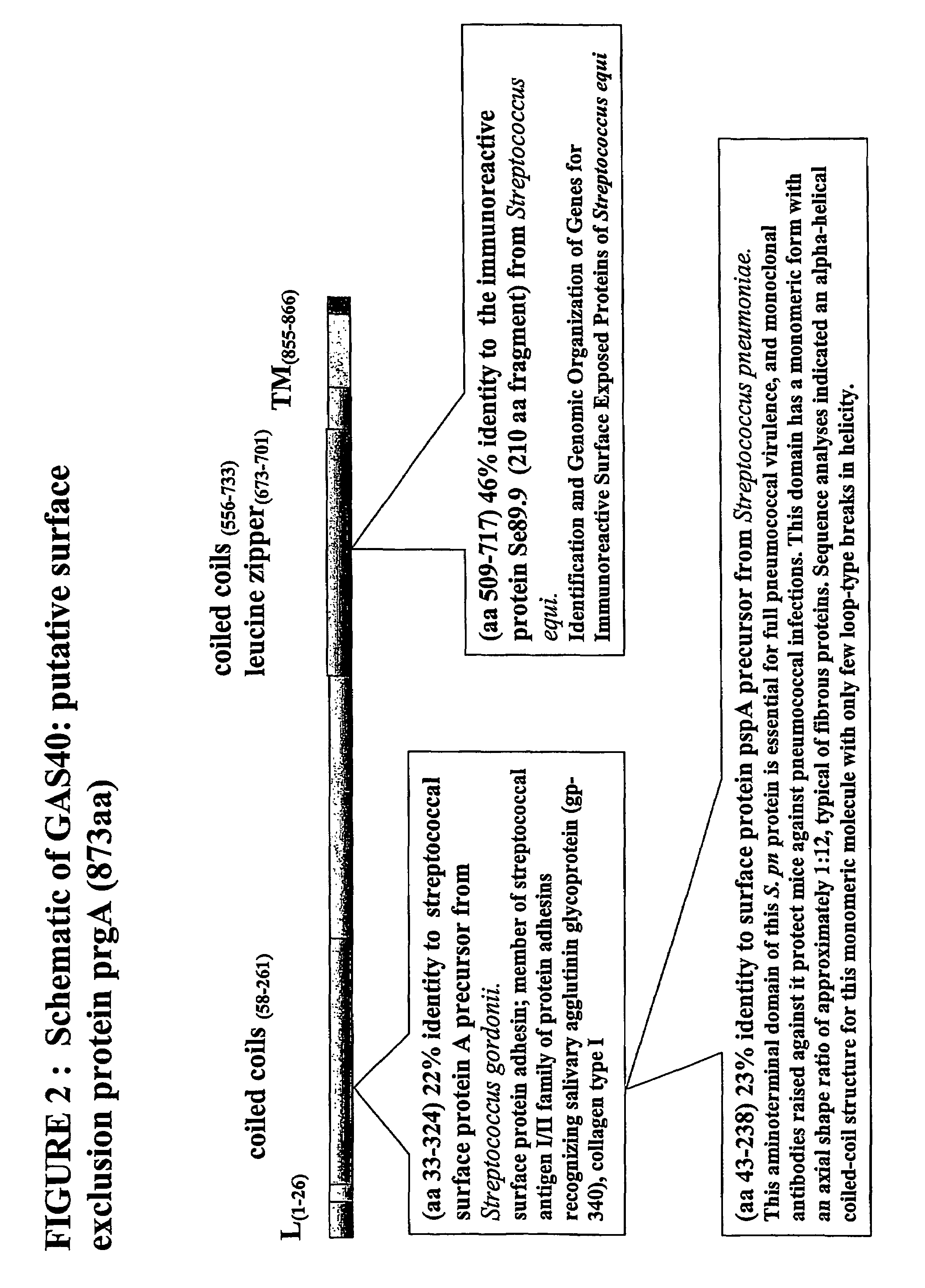
The invention includes a GAS antigen, GAS 40, which is particularly suitable for use either alone or in combinations with additional GAS antigens, such as GAS 117, GAS 130, GAS 277, GAS 236, GAS 40, GAS 389, GAS 504, GAS 509, GAS 366, GAS 159, GAS 217, GAS 309, GAS 372, GAS 039, GAS 042, GAS 058, GAS 290, GAS 511, GAS 533, GAS 527, GAS 294, GAS 253, GAS 529, GAS 045, GAS 095, GAS 193, GAS 137, GAS 084, GAS 384, GAS 202, and GAS 057.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
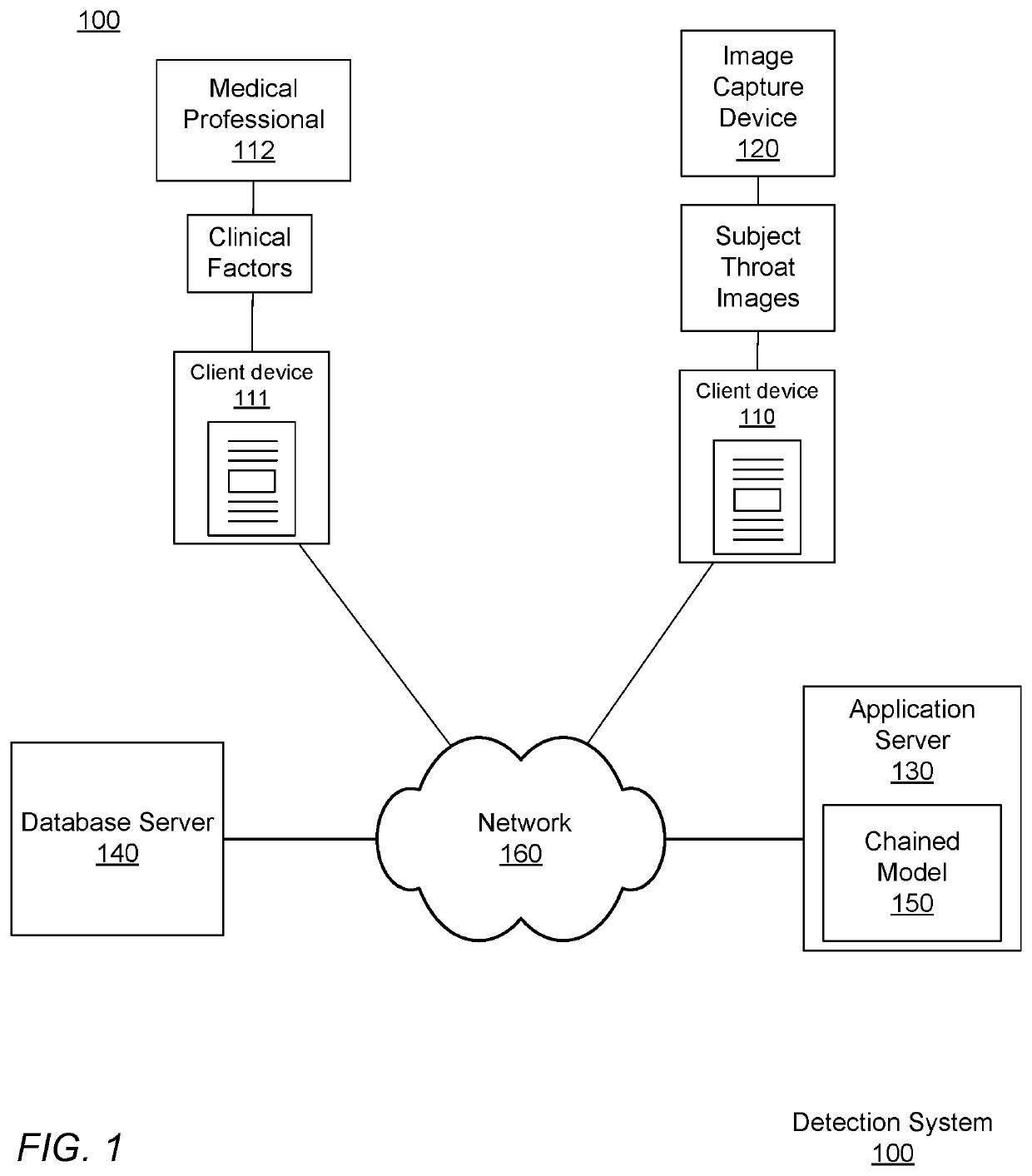
Image Processing of Streptococcal Infection in Pharyngitis Subjects
A method for determining a disease state prediction, relating to a potential disease or medical condition of a subject, includes accessing a set of subject images, the subject images capturing a part of a subject's body, and accessing a set of clinical factors from the subject. The clinical factors are collected by a device or a medical practitioner substantially contemporaneously with the capture of the subject images. The subject images are inputted into an image model to generate disease metrics for disease prediction for the subject. The disease metrics generated by the image model and the clinical factors are inputted into a classifier to determine the disease state prediction, and the disease state prediction is returned.
Owner:LIGHT AI INC
Efficient process for purification of high molecular weight hyaluronic acid
Owner:RELIANCE LIFE SCI PVT
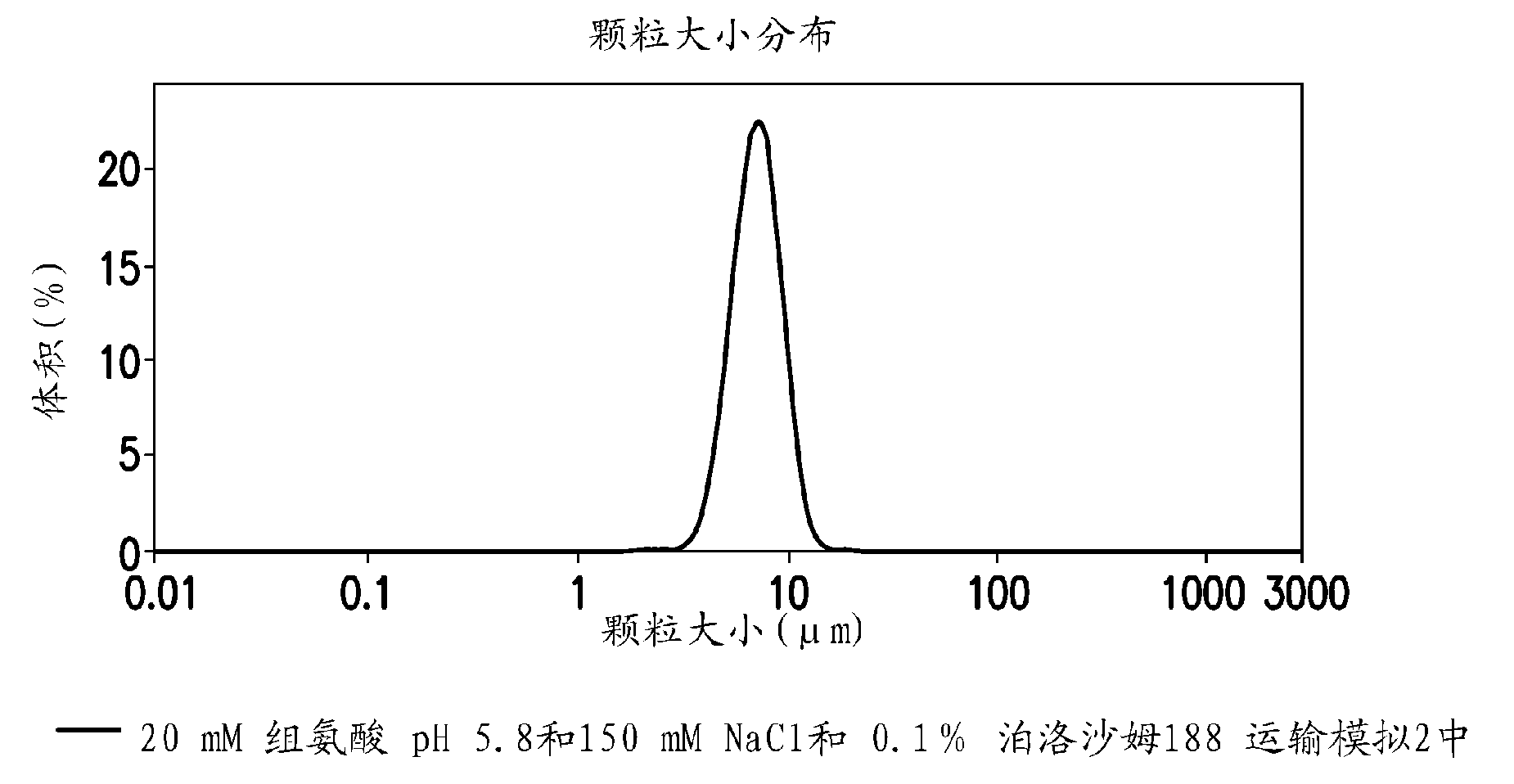
Novel formulations which mitigate agitation-induced aggregation of immunogenic compositions
The present invention provides novel formulations which mitigate agitation-induced aggregation of immunogenic compositions particularly those having polysaccharide-protein conjugates. Specifically, the novel formulations comprise a poloxamer within a molecular weight range of 1100 to 17,400 which provides significant advantages over previously used surfactants including polysorbate 80. In one embodiment, the present invention provides a multivalent immunogenic composition having 15 distinct polysaccharide-protein conjugates and a poloxamer. Each conjugate consists of a capsular polysaccharide prepared from a different serotype of Streptococcus pneumoniae (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F or 33F) conjugated to a carrier protein, preferably CRM197.
Owner:MERCK & CO INC
Production of highly purified sodium hyaluronate (HANa) with controlled molecular weight
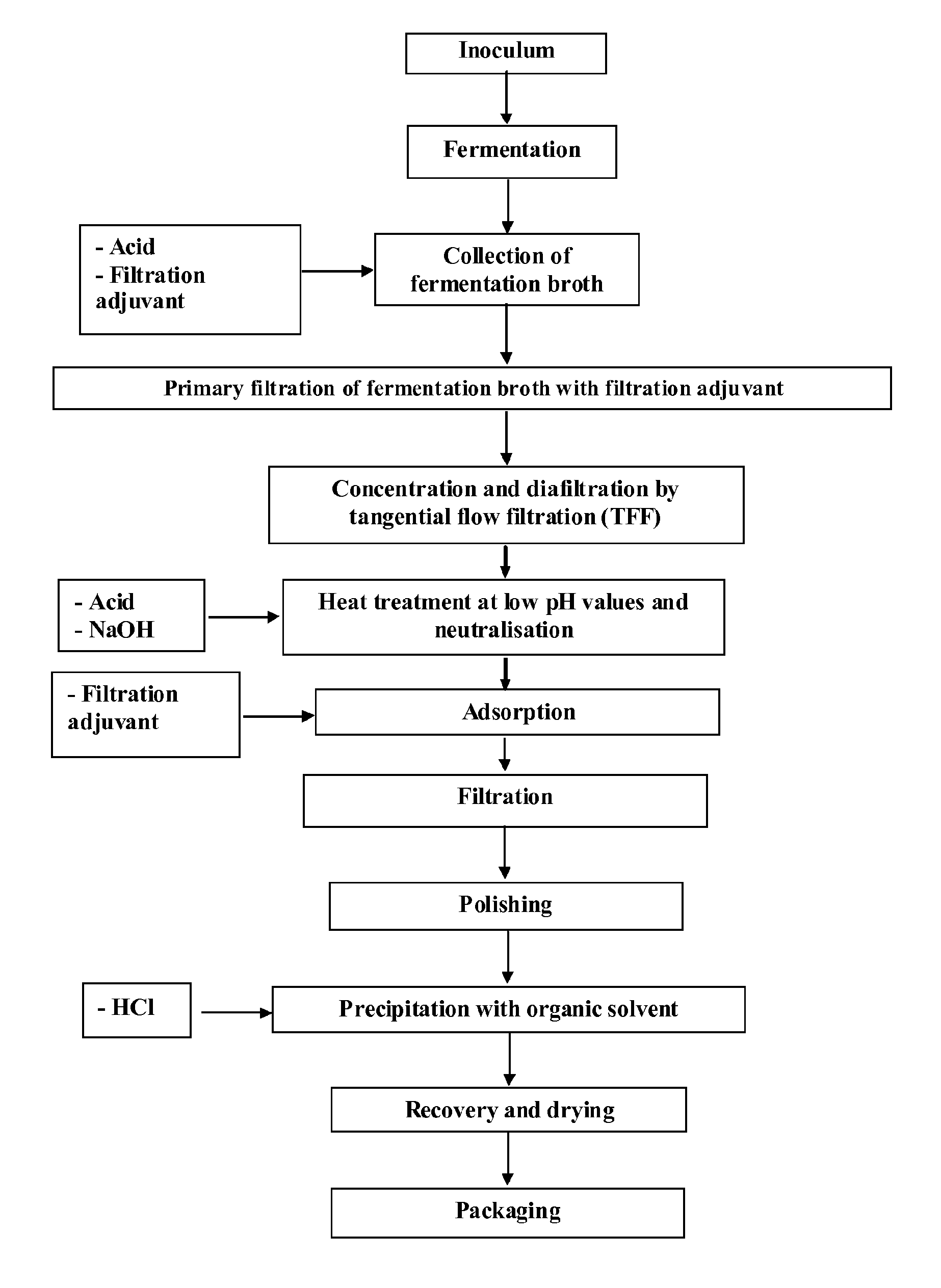
Disclosed is a process for the production of sodium hyaluronate with a molecular weight of between 60 and 2400 kDa and low polydispersity (1.4 Mw / Mn), which comprises: a) a step of fermentation of Streptococcus equi subsp. Zooepidemicus CNCM 1-4645 in a suitable culture medium; b) a step of ultrafiltration of the cell-free filtered solution; by concentrating and diafiltering the solution under differential pressure conditions (ΔP) of 1.0-5.0 bar(g) and transmembrane pressure (TMP) of 0.5-4 bar(g).
Owner:ALTERGON ITAL
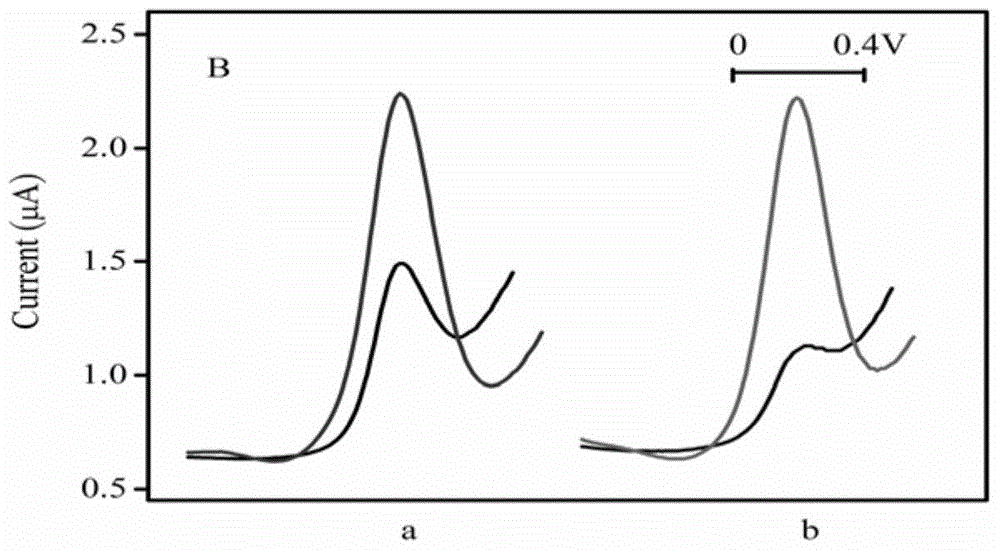
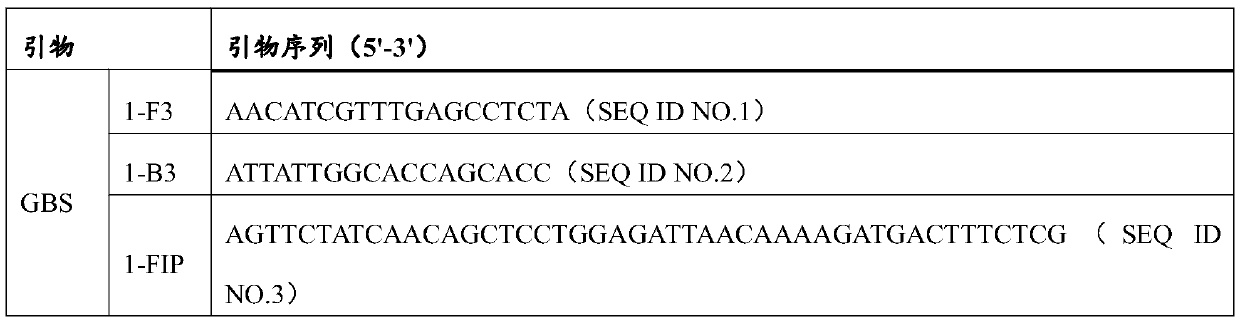
A kind of electrochemical sensor for detecting b group streptococcus and its preparation and application
InactiveCN104730128BSensitive detectionQuick checkMaterial electrochemical variablesNucleic acid detectionStreptococcus halichoeri
The invention relates to the field of nucleic acid detection, and particularly relates to an electrochemical sensor for detecting group B streptococcus (GBS) and preparation and an application thereof. The sensor comprises a working electrode, a reference electrode and a counter electrode, wherein the working electrode is obtained by fixing a capturing probe on the surface of a substrate electrode, namely a gold electrode, and the sensor further comprises a template probe, an extension probe and a detection probe which are matched with the capturing probe. The sensor can be used for detecting GBS sensitively, quickly and specifically.
Owner:CHONGQING MEDICAL UNIVERSITY
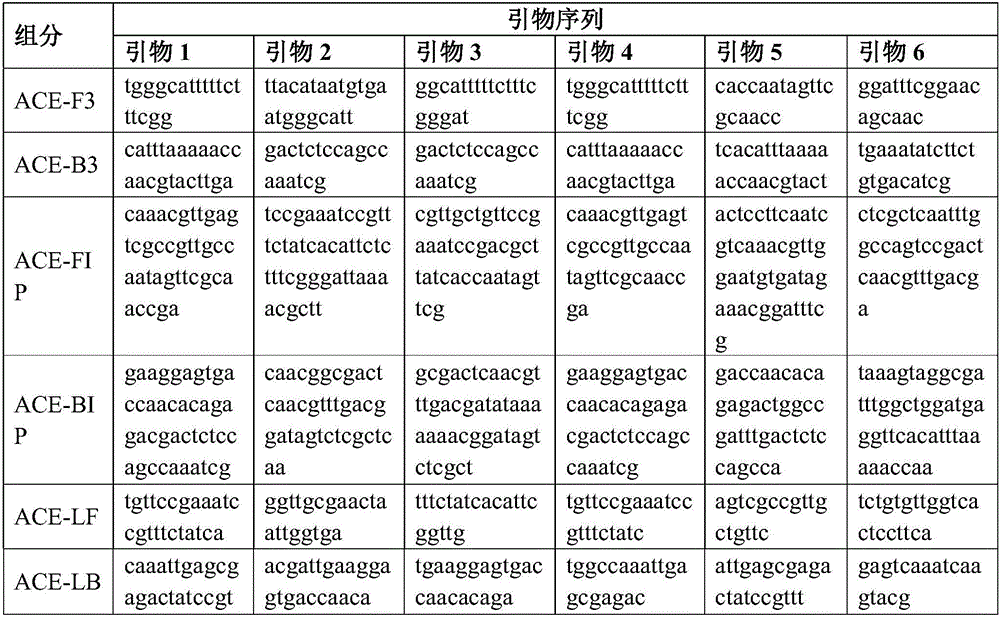
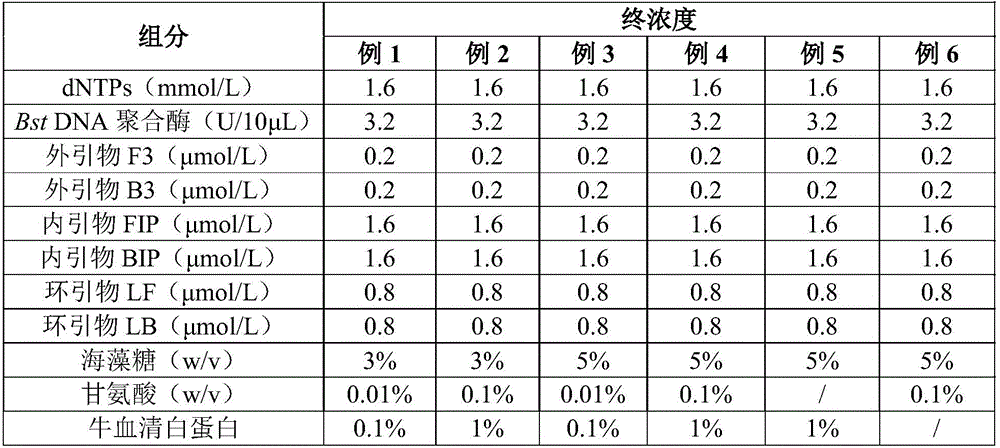
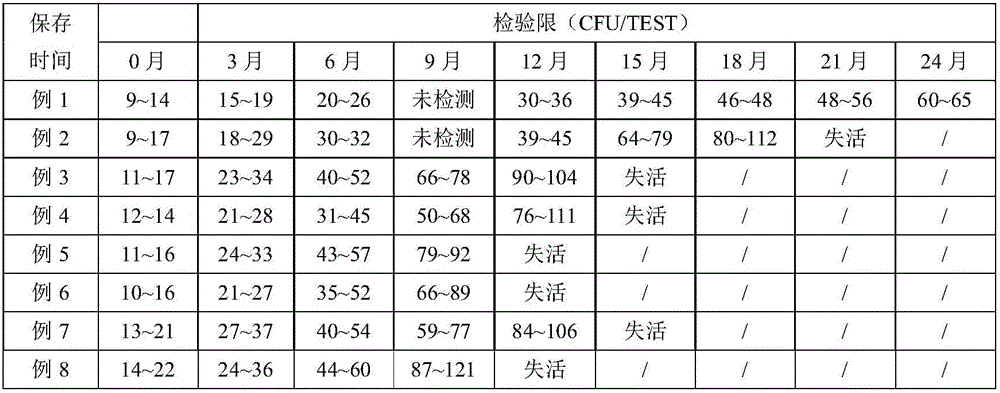
Streptococcus faecalis powdering LAMP rapid detection kit, and use method thereof
ActiveCN106544444AImprove stabilityLong shelf lifeMicrobiological testing/measurementMicroorganism based processesFreeze-dryingBiology
The invention discloses a Streptococcus faecalis powdering LAMP rapid detection kit, and a rapid detection method based on the kit. The kit comprises a freeze-dried isothermal amplification agent, a re-dissolving solution, a positive contrast dry powder, a negative contrast substance, a colorimetric solution, a lysate and a sealing solution, and the freeze-dried isothermal amplification agent contains dNTPs, Bst DNA polymerase, an amplimer and a freeze-drying protection agent. The primer in the kit corresponds to eight segments of a Streptococcus faecalis specific gene sequence, the colorimetric solution is added after a reaction, and a negative and positive colorimetric difference is obvious. The kit has the advantages of strong specificity, high sensitivity, easiness in operation and simplicity in result judgment, and is suitable for the quality control and the safe and rapid detection of foods, beverages, water and other raw and auxiliary materials. The kit is convenient for normal temperature transportation, is simple and fast to use without special devices, reduces the cost, widens the application range of products, is suitable for onsite rapid detection and base remote region related detection, and has wide application prospect.
Owner:GUANGDONG HUANKAI BIOLOGICAL SCI & TECH CO LTD +1
High-fidelity cas9 variants and applications thereof
ActiveUS20200149020A1On-off ratio can be increasedReduced off-target activityFusion with DNA-binding domainHydrolasesVariomeMutagenesis
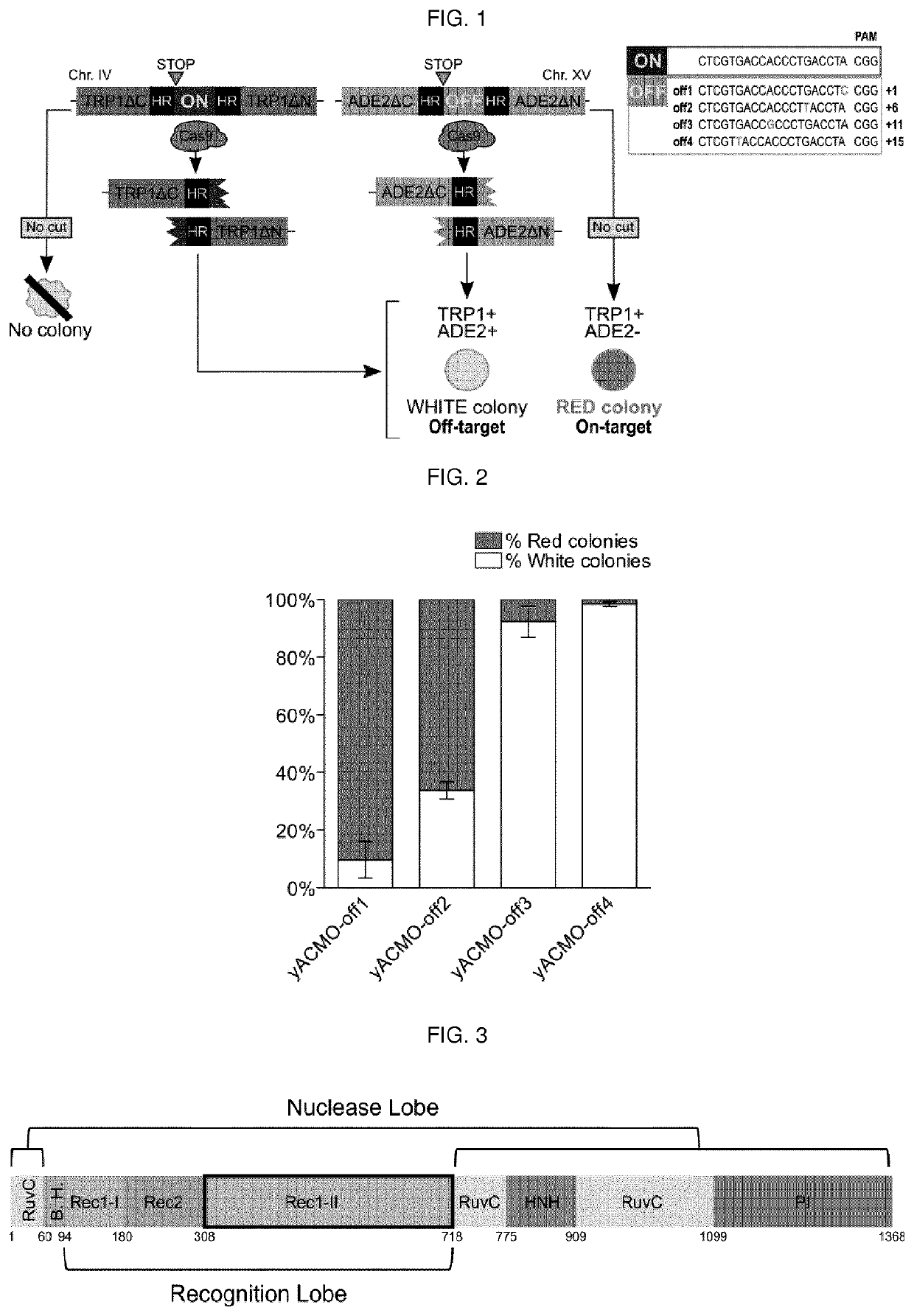
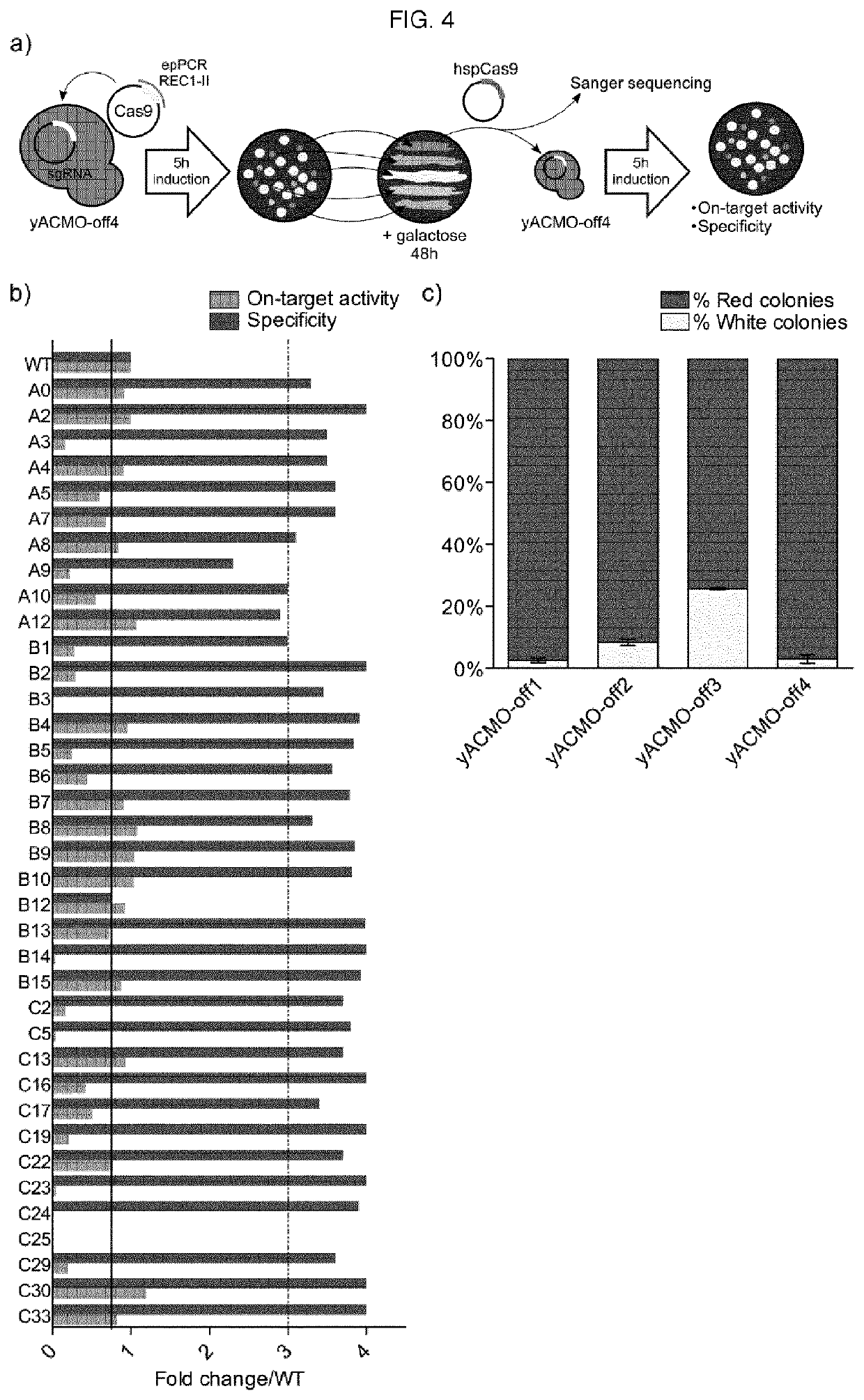
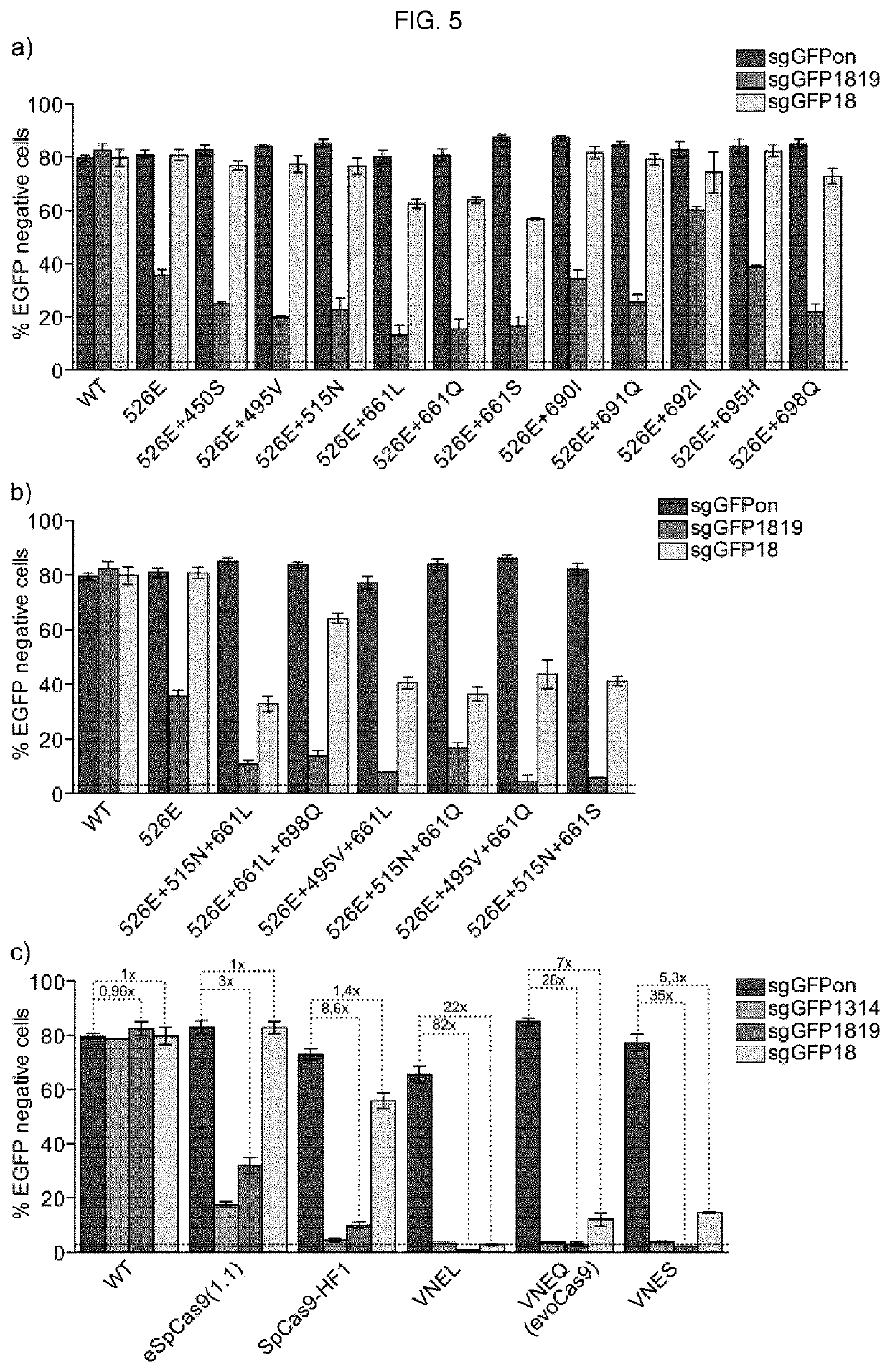
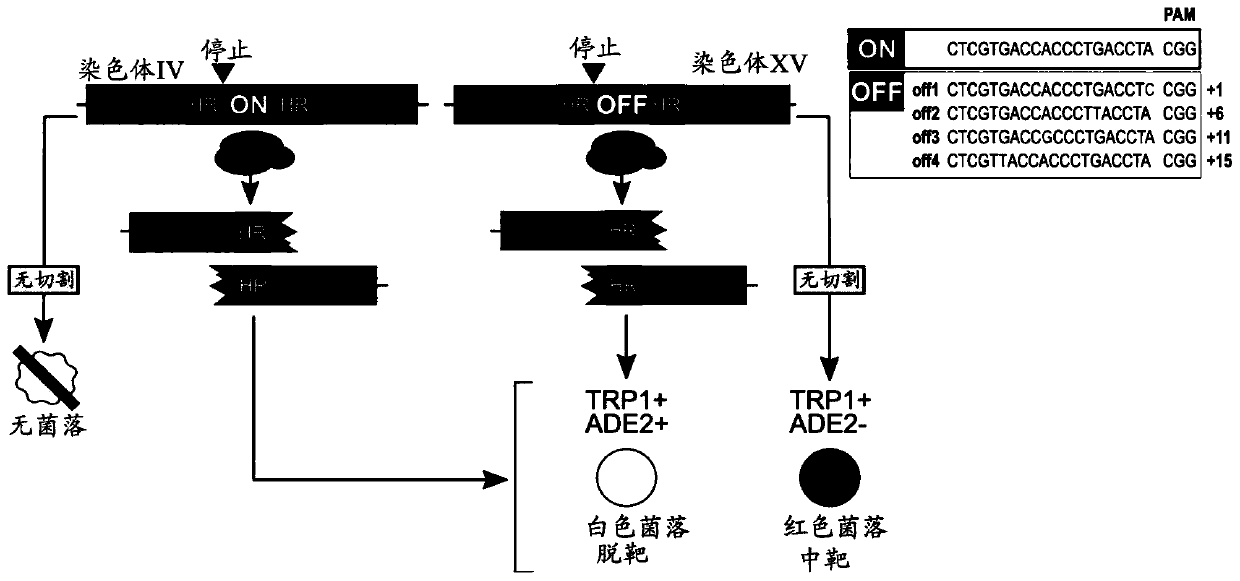
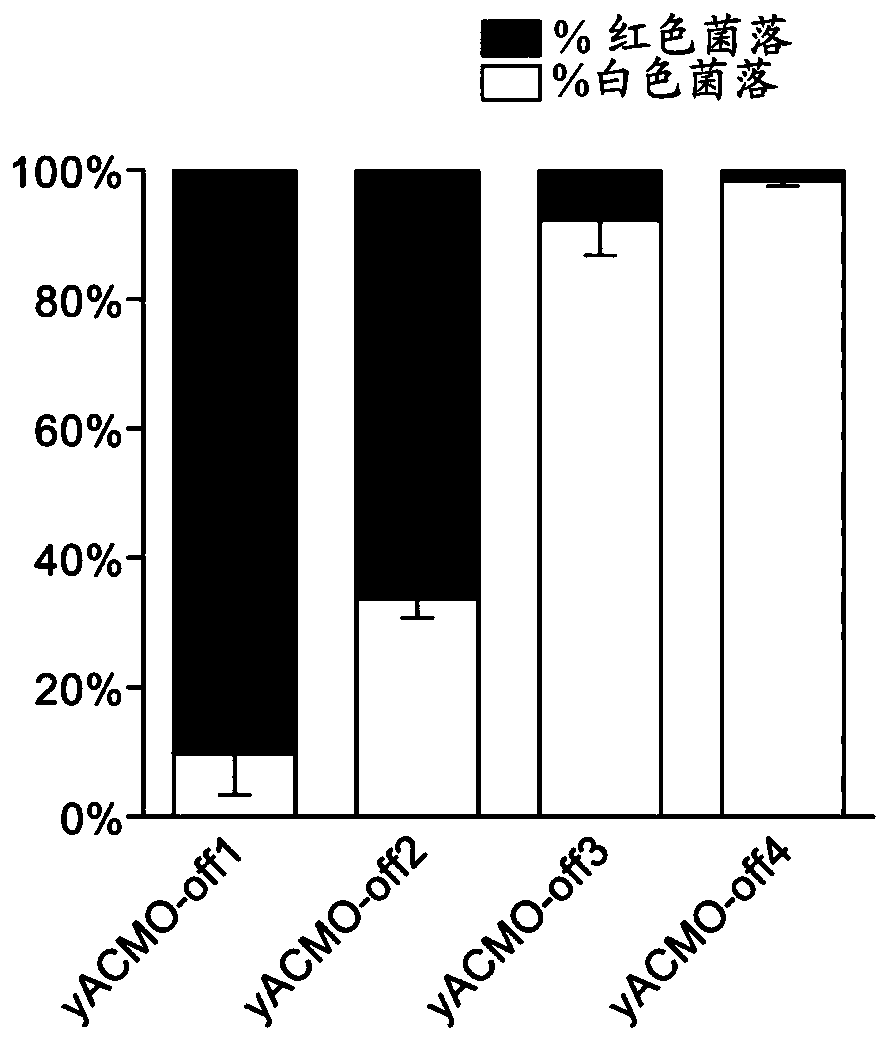
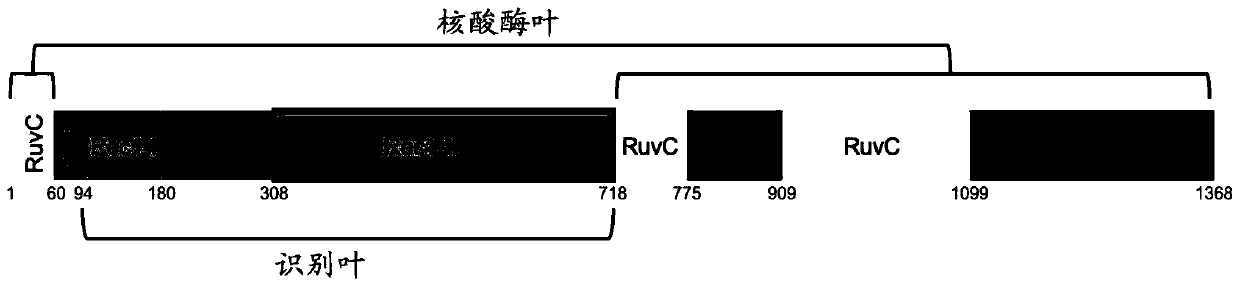
To address the limitations deriving from the unspecific genomic cleavages of the Streptococcus pyogenes Cas9 (SpCas9) and to identify variants with higher cleavage fidelity, the present invention describes a yeast-based assay which allows to simultaneously evaluate the on- and off-target activity towards two engineered genomic targets. The screening of SpCas9 variants obtained by random mutagenesis of the Red-II domain allowed the identification of hits with increased on / off ratios. The best performing nuclease, evoCas9, was isolated through the combination of the identified mutations within a single variant. Side by side analyses with previously reported rationally designed variants demonstrated a significant improvement in fidelity of evoCas9 of the present invention.
Owner:UNIV DEGLI STUDI DI TRENTO
Polyresistin dripping pill and its preparing method for immunodeficiency
The present invention relates to polyresistin dripping pill and its preparation process. Type-A streptococcus hemolyticus strain is deeply cultured and extracted to obtain one kind of refined polysaccharide substance polyresistin with immunoactivity and antitumor activity and named alpha-mannose polypeptide chemically; polyresistin is added into molten matrix via stirring to form pill; and the pill is cooled in cooler and dried to prepare the polyresistin dripping pill. Preparing into dripping pill can raise the stability and biological utilization of the medicine for quick and lasting effect.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Cleaning agent, and preparation method and application thereof
InactiveCN106337028AObvious growth-promoting effectEfficient removalBacteriaMicroorganism based processesBiotechnologyFeces
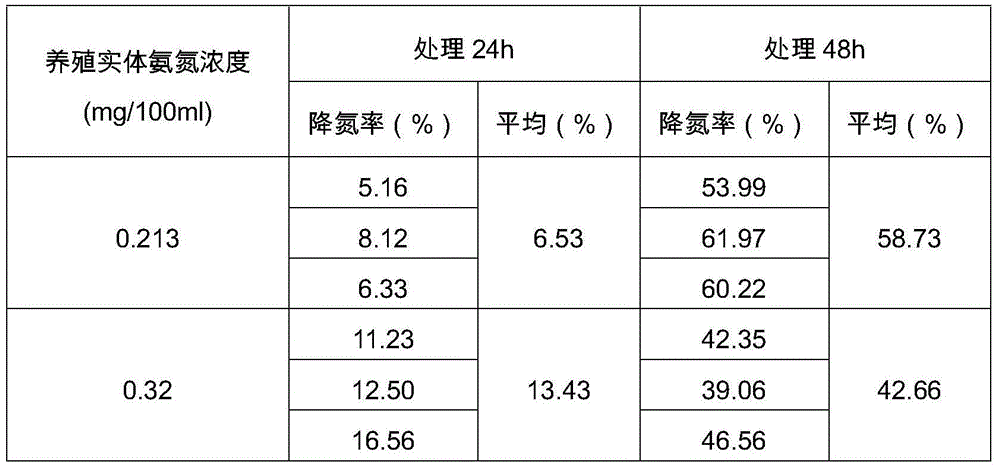
The invention discloses a cleaning agent. The cleaning agent comprises Bacillus natto, Paracoccus denitrificans, Streptococcus faecalis and a nutrient buffer system, wherein the concentration of live bacteria of Bacillus natto is greater than 20 billion per g. The cleaning agent provided by the invention has the beneficial effects that (1) when used in aquaculture, the cleaning agent can efficiently remove ammonia nitrogen and nitrite in water, purify water, eliminate algae, increase transparency, improve water color highly efficiently eliminate contamination, completely decompose residual feeds, manure and the like, enhance the ingestion capacity of aquatic livestock like fish, shrimps, crabs and mussels, decrease stress, substantially promote growth of aquaculture animals and improve the survival rate of aquaculture animals; and (2) when applied to rivers and landscape water bodies, the cleaning agent can efficiently remove ammonia nitrogen and nitrite in water, purify water, eliminate algae, increase transparency and improve water color.
Owner:SHANGHAI OCEAN UNIV
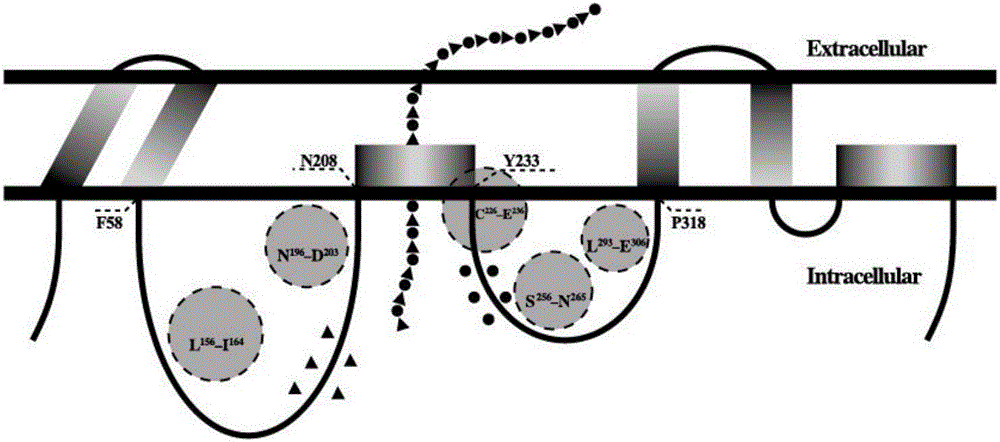
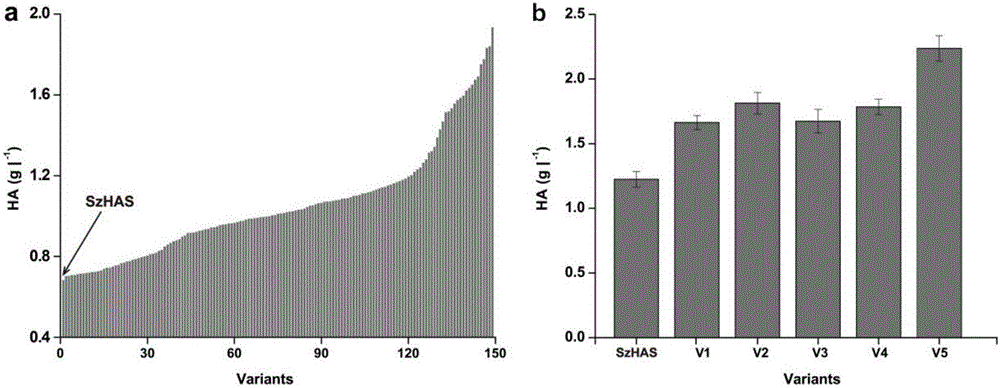
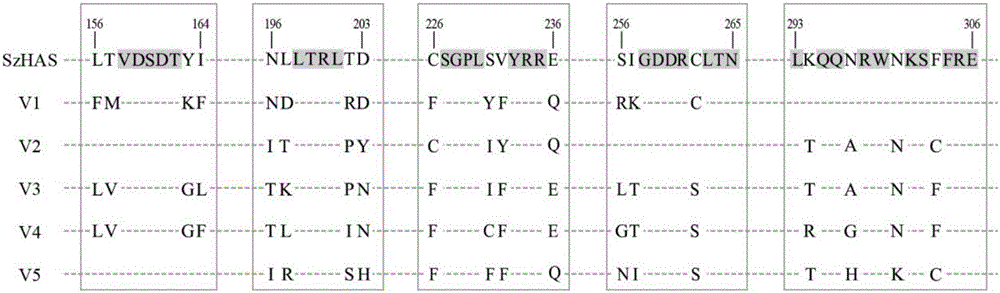
Hyaluronan synthase mutant and application thereof
ActiveCN105838688AIncrease productionIncrease valueBacteriaMicroorganism based processesBiotechnologyStreptococcus zooepidemicus
The invention discloses a hyaluronan synthase mutant and application thereof and belongs to the technical field of bioengineering. The mutant with positive effect in both yield and molecular weight is acquired by subjecting hyaluronan synthase of Streptococcus zooepidemicus origin to intracellular domain mutation in Bacillus subtilis; meanwhile, genes of UDP-GlcUA and UDP-GlcNAc synthetic routes in Bacillus subtilis are subjected to modular assembly expression analysis, and high yield of hyaluronan with wide molecular weight range from Bacillus subtilis is implemented by controlling different UDP-GlcA and UDP-GlcNAc concentrations and ratios. Certain basis is laid for the efficient preparation of hyaluronan from food-grade microorganisms, and this mutant is suitable for industrial production and application.
Owner:JIANGNAN UNIV
Production of Highly Purified Sodium Hyaluronate (HANA) with Controlled Molecular Weight
Disclosed is a process for the production of sodium hyaluronate with a molecular weight of between 60 and 2400 kDa and low polydispersity (1.4 Mw / Mn), which comprises: a) a step of fermentation of Streptococcus equi subsp. zooepidemicus CNCM 1-4645 in a suitable culture medium; b) a step of ultrafiltration of the cell-free filtered solution, by concentrating and diafiltering the solution under differential pressure conditions (ΔP) of 1.0-5.0 barg and transmembrane pressure (TMP) of 0.5-4 barg.
Owner:ALTERGON ITAL
Pneumococcal polysaccharides and their use in immunogenic polysaccharide-carrier protein conjugates
ActiveUS20210038723A1Antibacterial agentsPharmaceutical delivery mechanismCarrier proteinStreptococcus halichoeri
The present invention provides capsular polysaccharides from Streptococcus pneumoniae serotypes identified using NMR. The present invention further provides polysaccharide-protein conjugates in which capsular polysaccharides from one or more of these serotypes are conjugated to a carrier protein such as CRM197. Polysaccharide-protein conjugates from one or more of these serotypes may be included in multivalent pneumococcal conjugate vaccines having polysaccharides from multiple additional Steptococcus pneumoniae serotypes.
Owner:MERCK SHARP & DOHME LLC
High-fidelity cas9 variants and applications thereof
PendingCN110520528AImprove fidelityIncrease in/off ratioFusion with DNA-binding domainPeptide/protein ingredientsStreptococcus pyogenesYeast
To address the limitations deriving from the unspecific genomic cleavages of the Streptococcus pyogenes Cas9 (SpCas9) and to identify variants with higher cleavage fidelity, the present invention describes a yeast-based assay which allows to simultaneously evaluate the on- and off-target activity towards two engineered genomic targets. The screening of SpCas9 variants obtained by random mutagenesis of the Red-II domain allowed the identification of hits with increased on / off ratios. The best performing nuclease, evoCas9, was isolated through the combination of the identified mutations within asingle variant. Side by side analyses with previously reported rationally designed variants demonstrated a significant improvement in fidelity of evoCas9 of the present invention.
Owner:THE UNIV OF TORONTO
Capsule deficiency type Streptococcus equi subsp. zooepidemicus attenuated vaccine strain and preparation method thereof
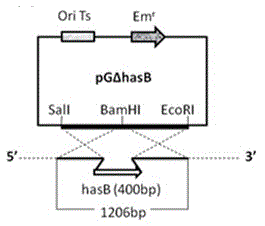
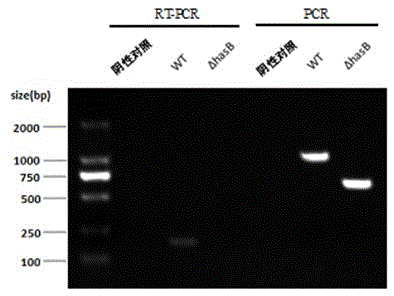
InactiveCN102719389ASimple preparation processLow costAntibacterial agentsBacterial antigen ingredientsDiseaseVaccine Production
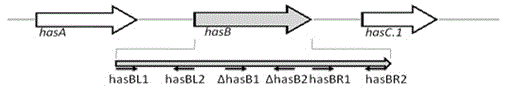
The invention discloses a capsule deficiency type Streptococcus equi subsp. zooepidemicus attenuated vaccine strain and a preparation method thereof, belonging to the technical field of animal vaccine preparation. The capsule deficiency type Streptococcus equi subsp. zooepidemicus attenuated vaccine strain is named Streptococcus equi subsp. zooepidemicus C55138 delta hasB, and was preserved in CCTCC (China Center for Type Culture Collection) on May 14, 2012 with the preservation number of CCTCC NO:M 2012164. The strain is constructed by hasB gene inactivation caused by allele replacing, is attenuated in toxicity, and can be used for preparing vaccine for Streptococcus equi disease. The vaccine production process is simple, the vaccine strain can be cultured on a large scale, the cost is low and the period is short; the vaccine presents cellular immunity and humoral immunity; and the vaccine is low in dosage, high in safety, low in toxicity and convenient for use, and can be popularized and used all over the world.
Owner:SUN YAT SEN UNIV
Novel bacillus subtilis multi-valent vector-based vaccine and application thereof
ActiveCN102949713AHigh temperature resistanceTimely deliveryAntibacterial agentsBacterial antigen ingredientsBacterial diseaseVector vaccine
The invention relates to a novel bacillus subtilis multi-valent vector-based vaccine and application thereof. The novel bacillus subtilis multi-valent vector-based vaccine comprises a bacillus subtilis recombination strain obtained by using a genetic engineering method, wherein the bacillus subtilis recombination strain is classified and named as bacillus subtilis HT5302 and is preserved in the China Center For Type Culture Collection (CCTCC) with a preservation number of CCTCC No:M2012380. The novel bacillus subtilis multi-valent vector-based vaccine is prepared by using the bacillus subtilis recombination strain as a core. A spore with a surface having no a streptococcus agalactiae 3-glyceraldehyde-phosphate dehydrogenase (GAPDH) protein can be generated through inducing the bacillus subtilis recombination strain, and secretes a fusion protein which formed by cell-penetrating peptide and vibrio anguillarum 3-GAPDH when germinating to form a nutrient cell. The novel bacillus subtilis multi-valent vector-based vaccine has a multi-valent protection function on bacterial diseases such as streptococcicosis, vibriosis, edwardsienosis and aeromonas hydrophila.
Owner:马悦 +1
Set of base editing artificial system for paddy
ActiveCN110066824ARealize editingImprove editing efficiencyVector-based foreign material introductionDNA/RNA fragmentationBiotechnologyStreptococcus pyogenes
The invention relates to a set of base editing artificial system for paddy. The system comprises an I adjusting element and an II adjusting element. The I adjusting element can encode a nucleotide sequence of an amino acid sequence I, wherein the amino acid sequence I comprises an amino acid sequence shown in SEQ ID No.1. The II adjusting element comprises an (II-1) nucleotidesequence and an (II-2) nucleotide sequence from the end 5' to the end 3' in sequence. The (II-1) nucleotide sequence comprises a target nucleotide sequence, the (II-2) nucleotide sequencecomprises an sgRNA nucleotide sequence derived from streptococcus pyogenes, and the (II-1) nucleotide sequence and the (II-2) nucleotide sequence are in transcription fusion.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Synthetic peptide and application thereof
ActiveCN108164586ANo effect on growthGood antibacterial effectAntibacterial agentsPeptide/protein ingredientsStreptococcus pneumoniaeStructure analysis
The invention discloses a synthetic peptide. The synthetic peptide comprises: (a) the amino acid sequence of the synthetic peptide is shown as in SEQ ID No.1; (b) one or more amino acids of a peptideis / are deleted, inserted or replaced in the synthetic peptide defined by the (a), and the peptide derived from (a) has the same biological function with the peptide. The synthetic peptide is designedin combination with the structure analysis of Swiss-Model online software according to the known choline combination sequence. The synthetic peptide has the characteristic of combination with the choline molecule on the surface of streptococcus pneumoniae, and has the capabilities of inhibiting growth and promoting autolysis of the streptococcus pneumoniae. The synthetic peptide is non-toxic and has a certain application potential.
Owner:SOUTHWEST MEDICAL UNIVERISTY
Streptococcus zooepidemicus protective antigen Sec_205 and preparation method thereof
InactiveCN107353329AStrong immune responseSignificant passive immune protectionAntibacterial agentsBacterial antigen ingredientsImmune serumsPassive immunity
The invention relates to Streptococcus zooepidemicus protective antigen Sec_205 and a preparation method thereof. The Streptococcus zooepidemicus protective antigen Sec_205 is rSec_205. The antigen preparation method comprises cloning, conversion, inducible expression and purifying. The rSec_205 and an SEZ (Streptococcus zooepidemicus) rehabilitation pig serum show good immune responses; and the mice immunized by the recombinant protein detects high level of antibody titer in mice serum. mice second immune serum has obvious passive immune protection to mice, and an anti-rSec_205 antibody can induce high-level sterilization capability. The immunity protective tests show that after the mice is immunized by the rSec_205, high protection effectiveness is provided. The transcription level of a gene for coding the Sec_205 protein in SEZ-infected mice body is obviously higher than the transcription level of in vitro culture, a flow cytometry result shows that the Sec_205 is distributed on the surface of SEZ cell, which is cell surface protein, in a SEZ adhesion test, Sec_205 can obviously reduce the SEZ adhesion capability for cells, and the inhibition rate is 30.3%.
Owner:HUBEI UNIV
LAMP (Loop-Mediated Isothermal Amplification) primer group, micro-fluidic chip and kit for detecting reproductive tract pathogenic microorganisms
ActiveCN111455075AAccurate detectionHigh detection sensitivityMicrobiological testing/measurementMicroorganism based processesStreptococcus mastitidisReproductive tract
The invention provides a LAMP (Loop-Mediated Isothermal Amplification) primer group, a micro-fluidic chip and a kit for detecting reproductive tract pathogenic microorganisms, and belongs to the technical field of reproductive tract infection detection. The LAMP primer group comprises a streptococcus agalactiae detection primer group, an enterococcus faecalis primer group, a gardnerella vaginosisprimer group, a Candida albicans primer group and a Chlamydia trachomatis primer group. The above LAMP primer group is fixed on the micro-fluidic chip. The LAMP primer group, the micro-fluidic chip and the kit can quickly, sensitively and accurately detect streptococcus agalactiae, enterococcus faecalis, gardnerella vaginosis, Candida albicans and Chlamydia trachomatis by high repeatability.
Owner:TIANJIN CHASE SUN PHARM CO LTD
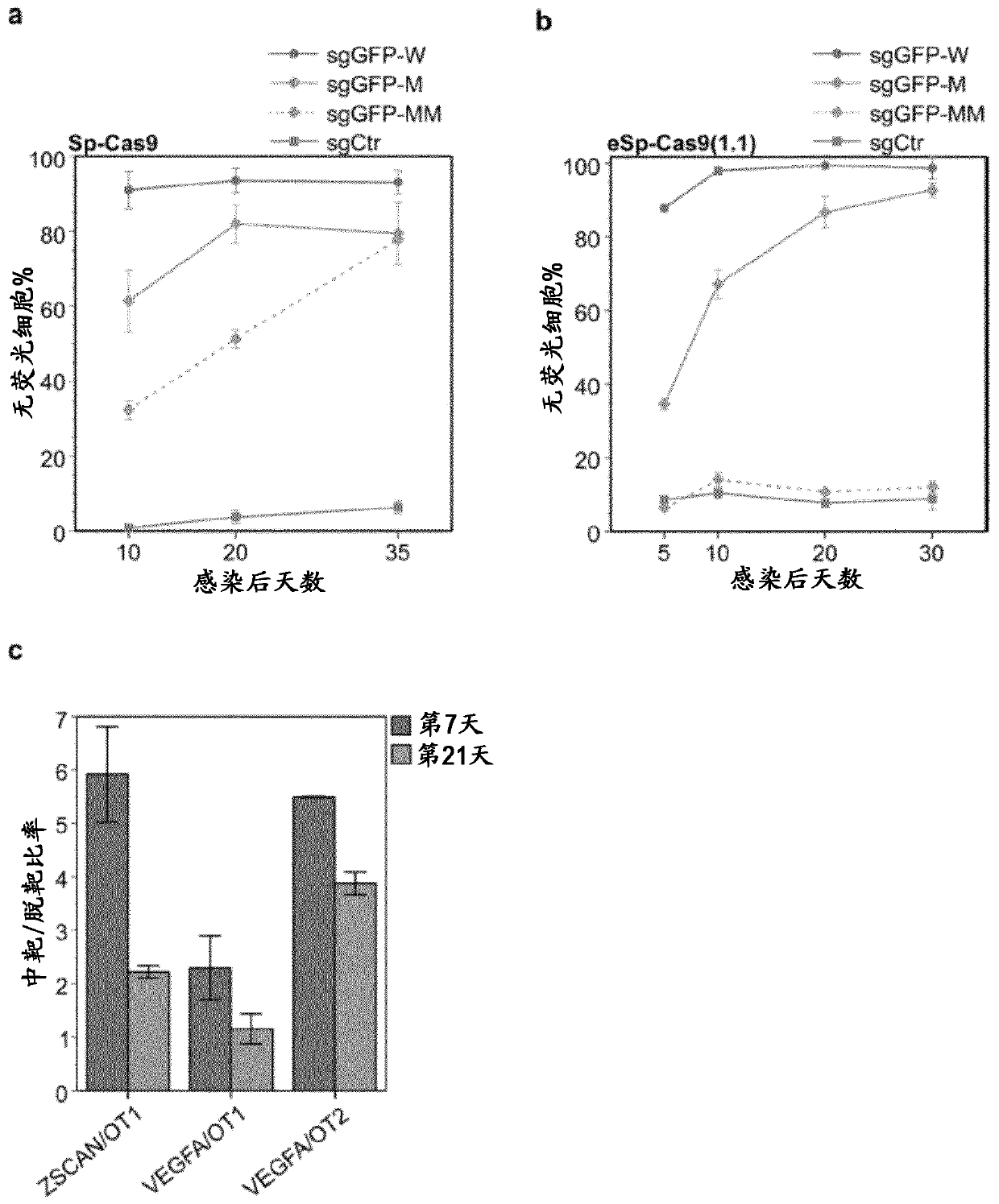
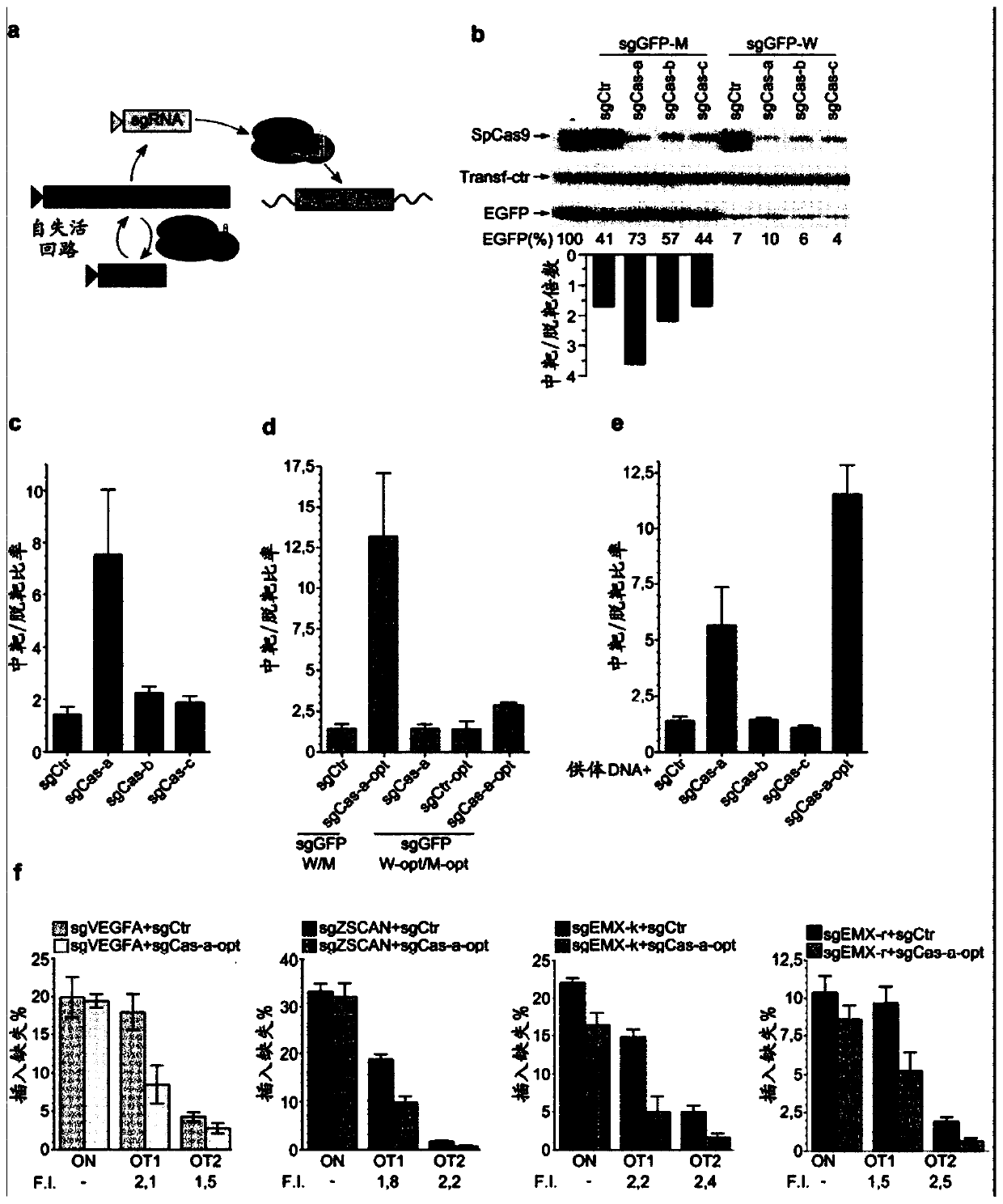
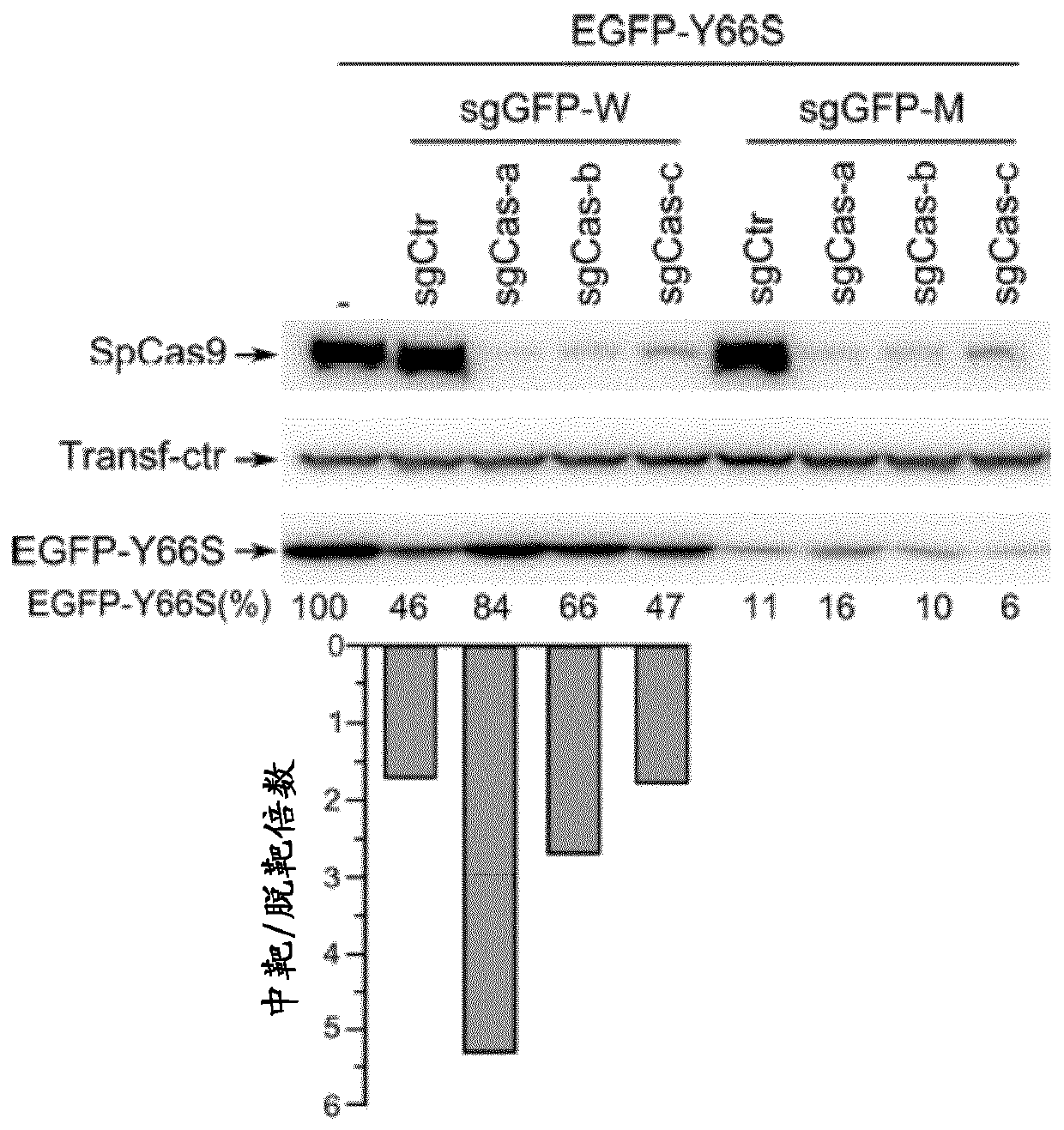
SELF-LIMITING Cas9 CIRCUITRY FOR ENHANCED SAFETY (SLiCES) PLASMID AND LENTIVIRAL SYSTEM THEREOF
InactiveCN110312793APrevent transient propertiesLimited off-target activityAntibacterial agentsAntimycoticsStreptococcus pyogenesGenome editing
The present invention describes a Self-Limiting Cas9 circuitry for Enhanced Safety (SLiCES) which consists of an expression unit for the Streptococcus pyogenes Cas9 (SpCas9), a first Cas9 self-targeting sgRNA and a second sgRNA targeting a chosen genomic locus. The self limiting circuit, by controlling Cas9 levels, results in increased genome editing specificity. For its in vivo utilization, SLiCES was integrated into a lentiviral delivery system (lentiSLiCES) via circuit inhibition to achieve viral particle production. Following its delivery into target cells, the lentiSLiCES circuit is switched on to edit the intended genomic locus while simultaneously stepping up its own neutralization through SpCas9 inactivation. By preserving target cells from residual nuclease activity, the present hit and go system increases safety margins for genome editing.
Owner:ALIA THERAPEUTICS SRL
Group A streptococcus antigen detection test strip and kit and preparation methods thereof
InactiveCN110568187AReduce distractionsRule out false positivesMaterial analysisAntigen testImmunolabeling
The invention discloses a group A streptococcus antigen detection test strip and kit and preparation methods thereof, and relates to the technical field of group A streptococcus detection. The group Astreptococcus antigen test strip is prepared by sequentially attaching a nitrocellulose membrane coated with an anti-group A streptococcus detection antibody, an immune binding pad coated with an anti-group A streptococcus immunolabeled antibody, absorbent paper and a sample pad to a polyvinyl chloride bottom plate; a detection card can detect whether a group A streptococcus antigen is present ina sample to be detected or not by detecting a marker; and the test strip and the detection card comprising the test strip can specifically and quickly detect and diagnose early infection of group A streptococcus, thereby reducing the cost, realizing rapid detection and meeting the requirements of clinical use.
Owner:JIANGSU BIOPERFECTUS TECH CO LTD
Streptococcal vaccine
ActiveUS10821168B2Promotes an increase in inductionEnhance mucosal immunityAntibacterial agentsBacterial antigen ingredientsStreptococcal VaccinesStreptococcus halichoeri
The present invention relates to photon-irradiated streptococcal vaccine preparations and methods for their use.
Owner:GPN VACCINES PTY LTD
Primer and probe group and kit for detecting hemolytic streptococcus through RAA fluorescence method
InactiveCN108103214AHighly specific detectionHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNucleotideFluorescence
The invention relates to a primer and probe group and kit for detecting hemolytic streptococcus through an RAA fluorescence method, and belongs to the technical field of molecular biological detection. The primer and probe group for detecting the hemolytic streptococcus through the RAA fluorescent method comprises an upstream primer, a downstream primer and a probe; the primer and probe group is characterized in that the nucleotide sequence of the upstream primer is shown as SEQ ID NO.1; the nucleotide sequence of the downstream primer is shown as SEQ ID NO.2; and the probe is a substance which includes the 40th-site base modified fluorescence reporter gene, the 42nd-site base modified tetrahydrofuran residue and the 43rd-site base modified quenching group, and the nucleotide sequence of the probe is shown as SEQ ID NO.3. The kit provided by the invention is rapid and sensitive in detection and simple and convenient to operate; the kit is of great significance to spread of the hemolytic streptococcus in the future; and the kit has a relatively high application prospect.
Owner:中华人民共和国浙江出入境检验检疫局 +2
A kind of ceftaroning nano-suspension freeze-dried powder and preparation method thereof
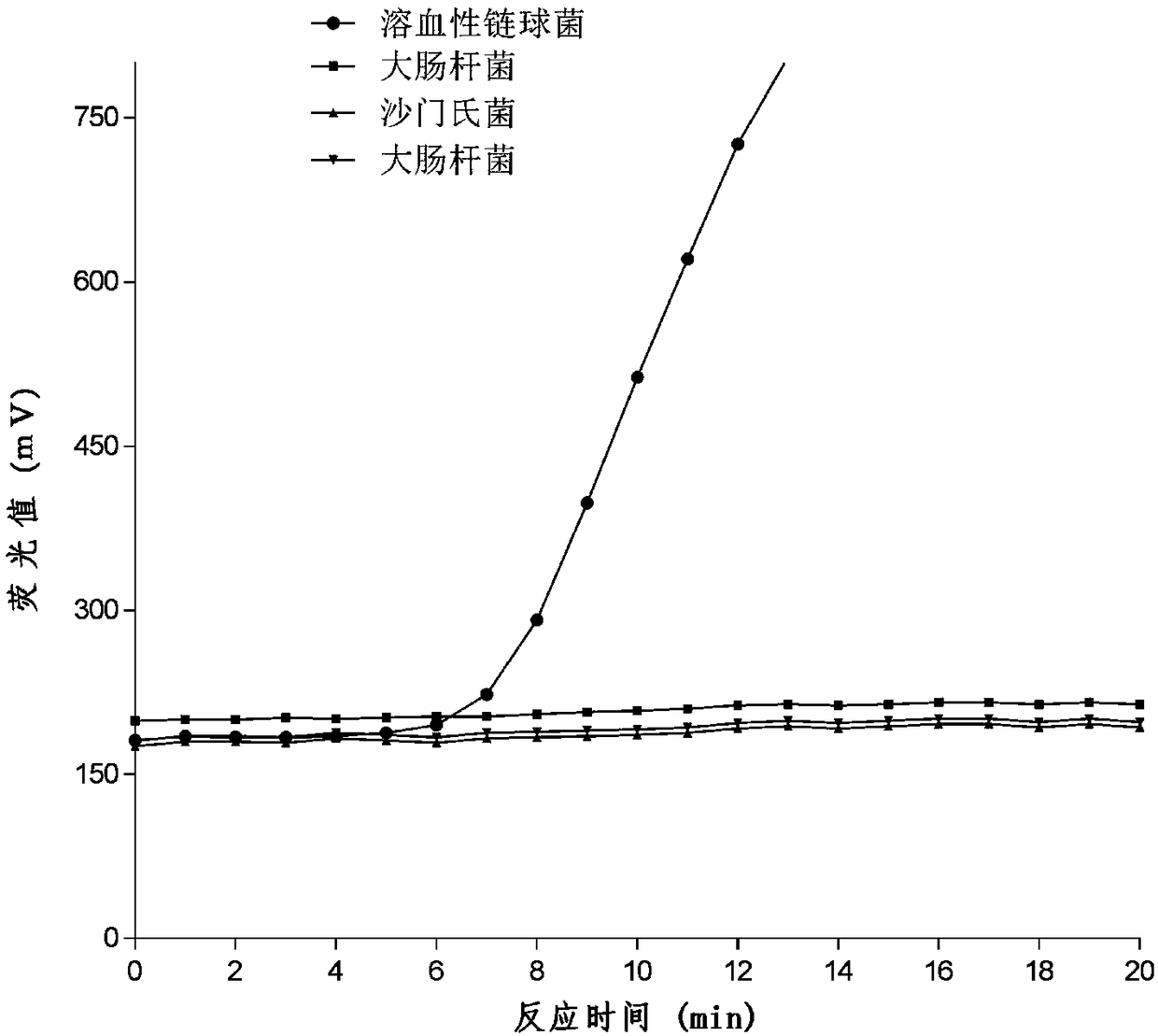
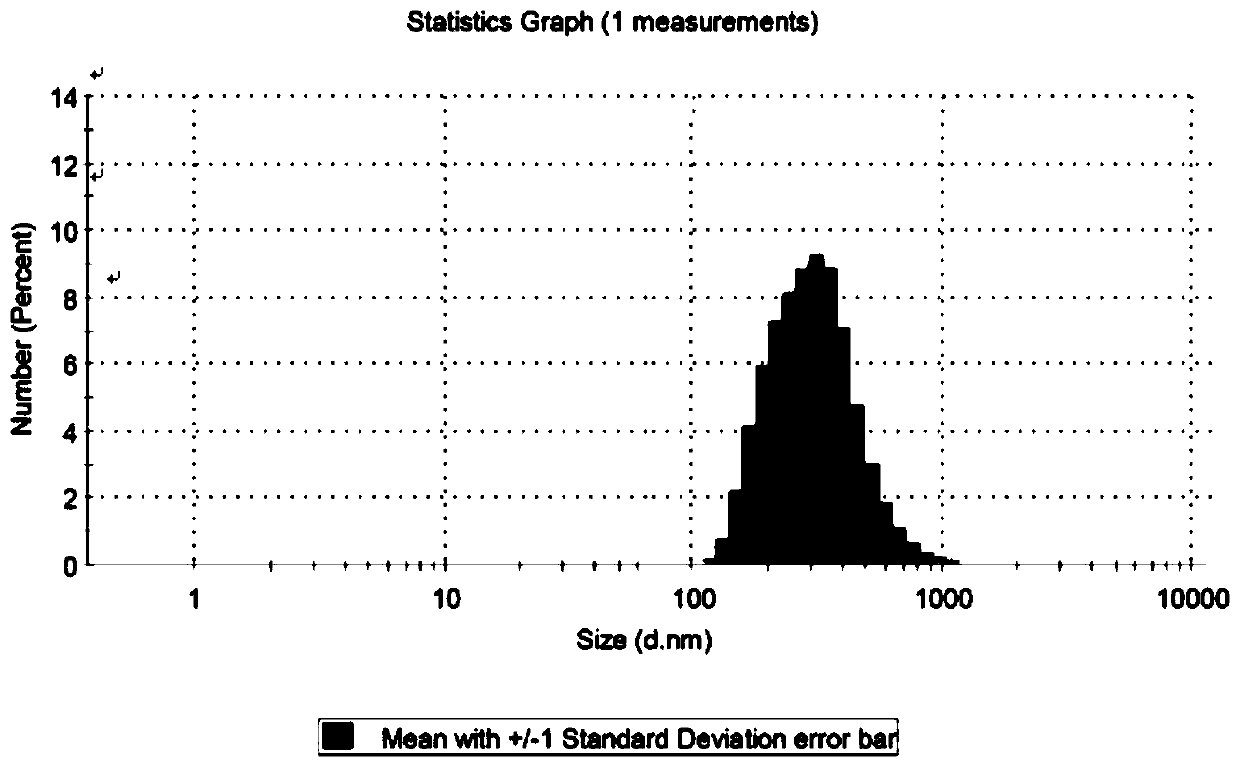
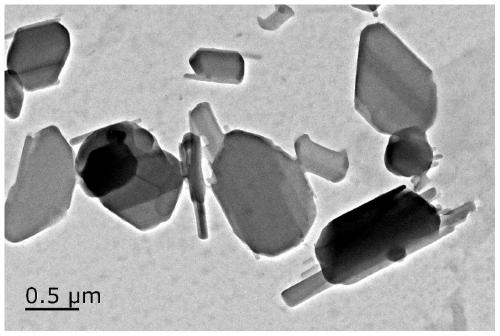
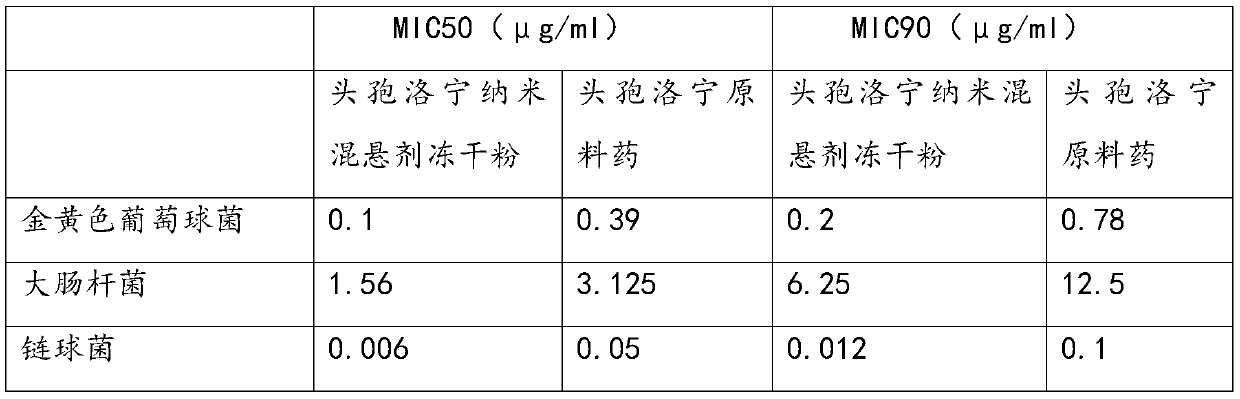
ActiveCN107412170BReduce screening costsSimple prescriptionAntibacterial agentsPowder deliveryBiotechnologyStaphyloccocus aureus
The invention discloses cefalonium nanosuspension freeze-dried powder and a preparation method of the cefalonium nanosuspension freeze-dried powder. The cefalonium nanosuspension freeze-dried powder is prepared from the following components in parts by weight: 1-5 parts of cefalonium raw drug, 0.1-0.5 part of a surfactant, 0.1-0.5 part of a suspending aid, and 1-10 parts of a freeze-drying protective agent. The cefalonium nanosuspension freeze-dried powder prepared by adopting the preparation method disclosed by the invention has good stability, and is uniform in particle size distribution and good in redispersibility, and the solubility and bioavailability of cefalonium can be improved; an organic solvent is not used in a formula, the use of auxiliary materials is little, the toxic and side effects are small, a preparation process is simple, and the popularization and application are convenient. The cefalonium nanosuspension freeze-dried powder disclosed by the invention has good inhibitory activity for clinical isolates of staphylococcus aureus, streptococcus and escherichia coli.
Owner:SOUTH CHINA AGRI UNIV +1
Space efficient microbial strain of 22nd recoverable satellite, preparation and application thereof
The invention relates to an excellent microorganism strain for space mutation, in particular to a good microorganism strain for space mutation, and the preparation method and the application thereof. The discoveries that the bump of a cancer patient infected by hemolytic streptococcus can subsidise naturally and the hemolytic streptococcus and the preparation thereof have a certain of biological activities are made is as early as in the 19th century. In the invention, the special environment of the outer space is utilized to deliver the strains to the outer space through carrying the 22nd recoverable experimental science satellite, the 22nd recoverable experimental science satellite safely returns after traveling for a certain period of time, the suppresormutation strains are eliminated, the forward mutation strains with excellent features are selected out for re-screening, and ultimately the excellent microorganism strains with vigorous life, quick growth speed, high fermentation yield, short fermentation period, high polypeptide content, stable hereditary property, strong productive adaptability and space cumulative effect. The microorganism strain can be applied to the strains for industrialization production, and has the advantages of high output, wide application range, low cost and high effective component content.
Owner:孙卫
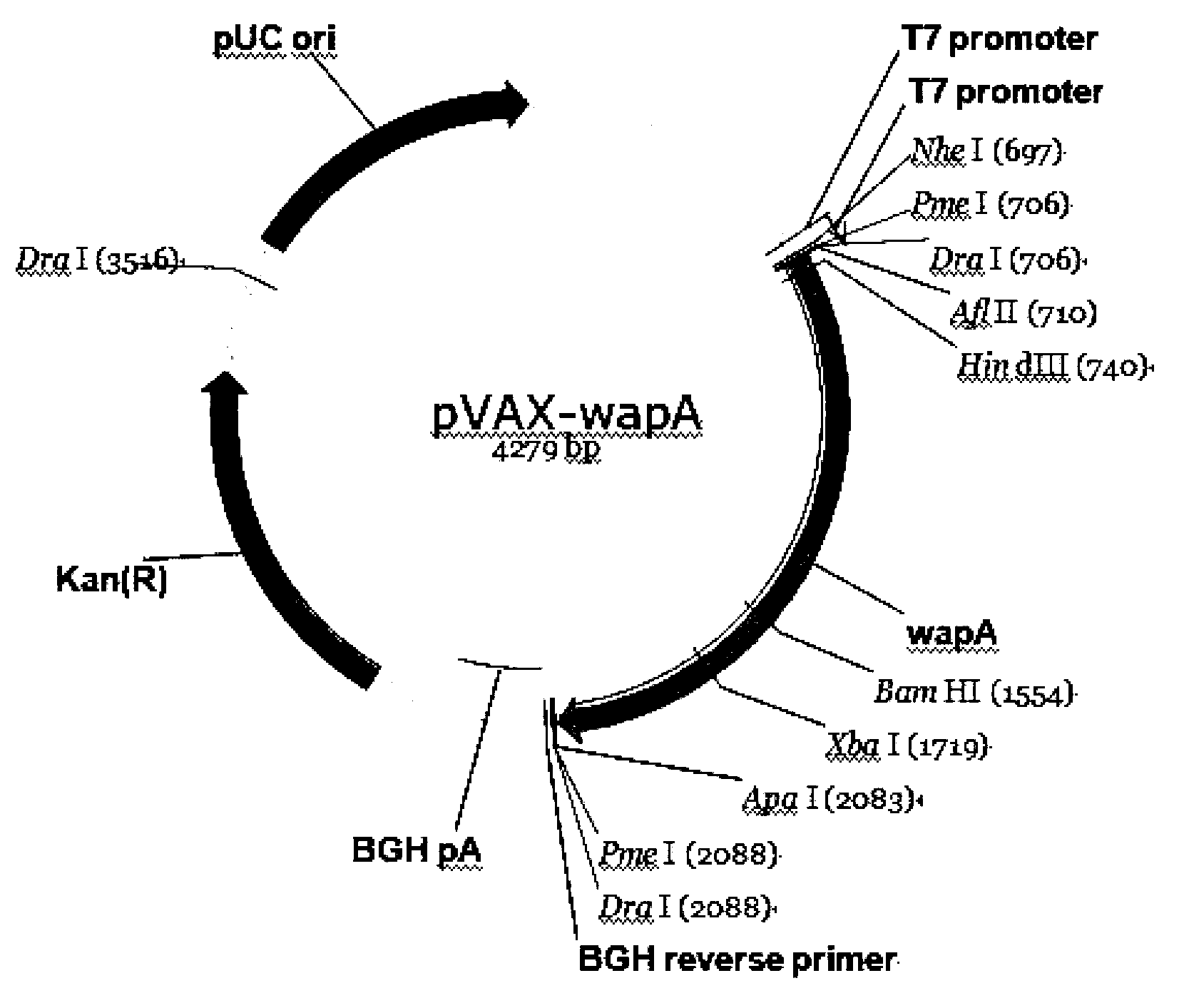
Anti-caries DNA (Deoxyribose Nucleic Acid) vaccine and preparation method and application thereof
InactiveCN103007262AImprove immune efficiencyEffective against cariesAntibacterial agentsBacterial antigen ingredientsAntigen3-deoxyribose
The invention belongs to the technical field of oral preventive medicine, and particularly provides an anti-caries DNA (Deoxyribose Nucleic Acid) vaccine. The anti-caries DNA vaccine is prepared by extracting the protein full-length gene of a wall-connected associated protein A (wapA) from a streptococcus mutans UA159 genome and cloning into a eukaryotic expression vector pVAX1. In order to enhance the immune capacity of the anti-caries DNA vaccine, the anti-caries DNA vaccine is prepared into a chitosan nanometer compound by taking chitosan as a vector. The invention also provides a preparation method and application of the anti-caries DNA vaccine. The anti-caries DNA vaccine provided by the invention has the advantages of high safety, natural antigen expression, lasting immune response and effective caries prevention.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Processes for the formulation of pneumococcal polysaccharides for conjugation to a carrier protein
ActiveUS20200276316A1Desire propertyPromote dissolutionAntibacterial agentsSugar derivativesSucroseStreptococcus pneumoniae conjugated
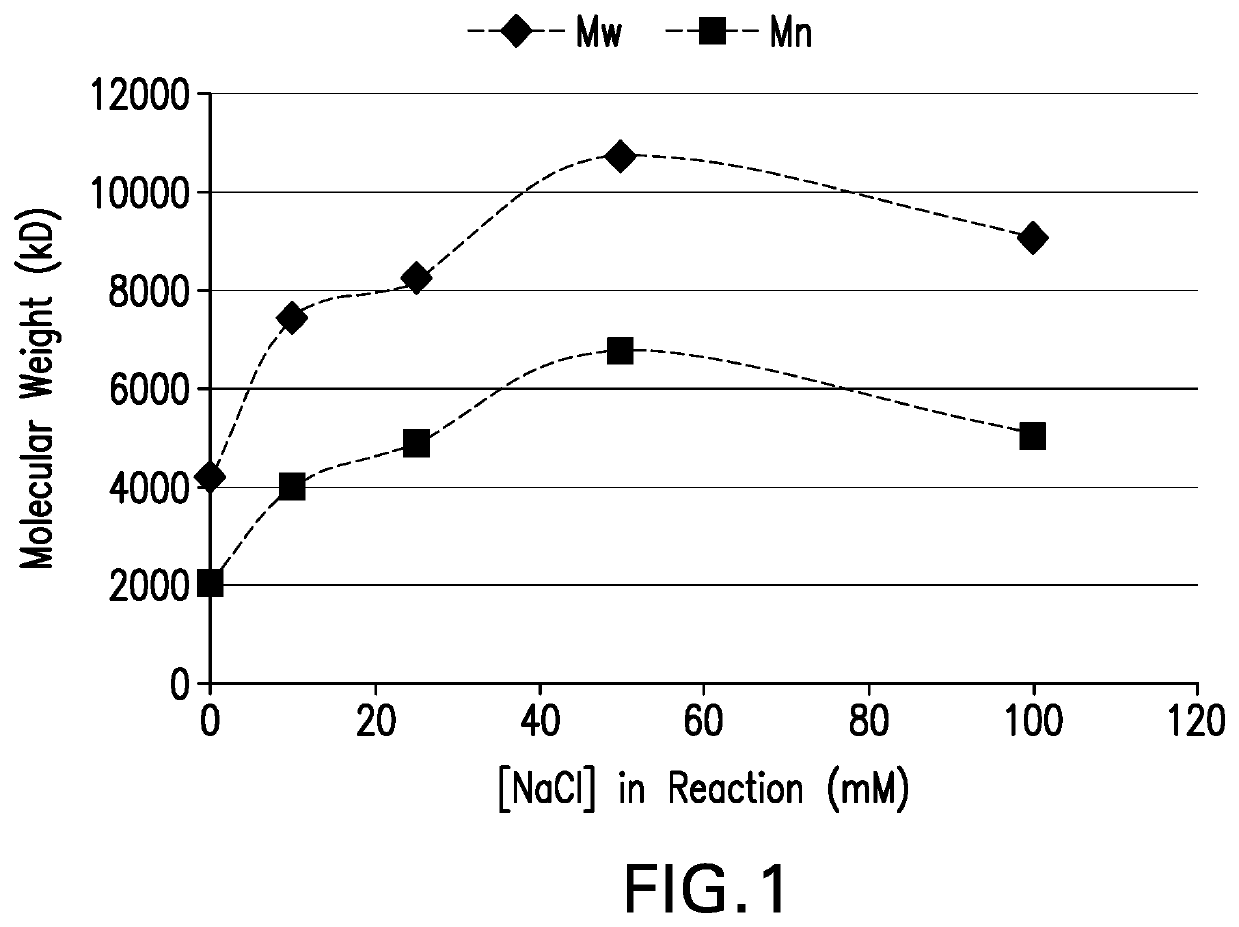
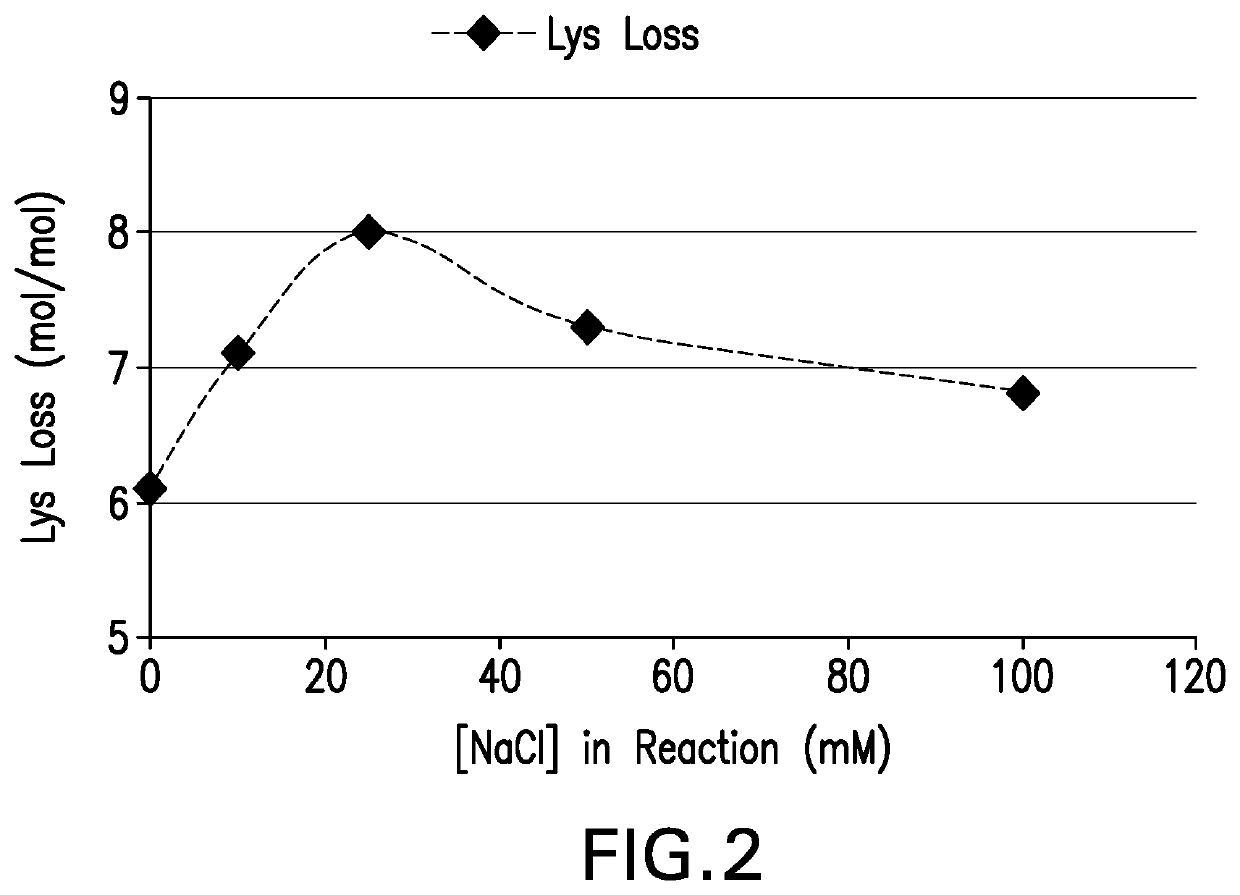
The present invention provides a number of process improvements related to the conjugation of capsular polysaccharides from Streptococcus pneumoniae to a carrier protein. These process are serotype specific and include acid hydrolysis, addition of sodium chloride to the reductive amination reaction, and addition of sucrose to dissolve polysaccharides. Polysaccharide-protein conjugates prepared using the processes of the invention can be included in multivalent pneumococcal conjugate vaccines.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com