Anti-caries DNA (Deoxyribose Nucleic Acid) vaccine and preparation method and application thereof
A DNA vaccine and anti-caries technology, which is applied in pharmaceutical formulas, medical preparations with non-active ingredients, digestive system, etc., to achieve the effects of high safety, expanded application range, and easy promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
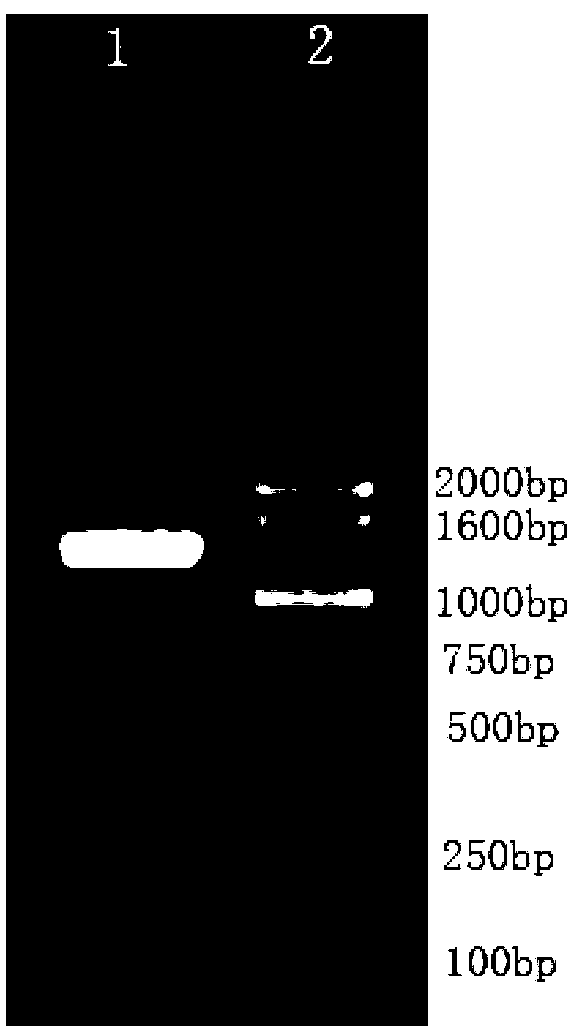
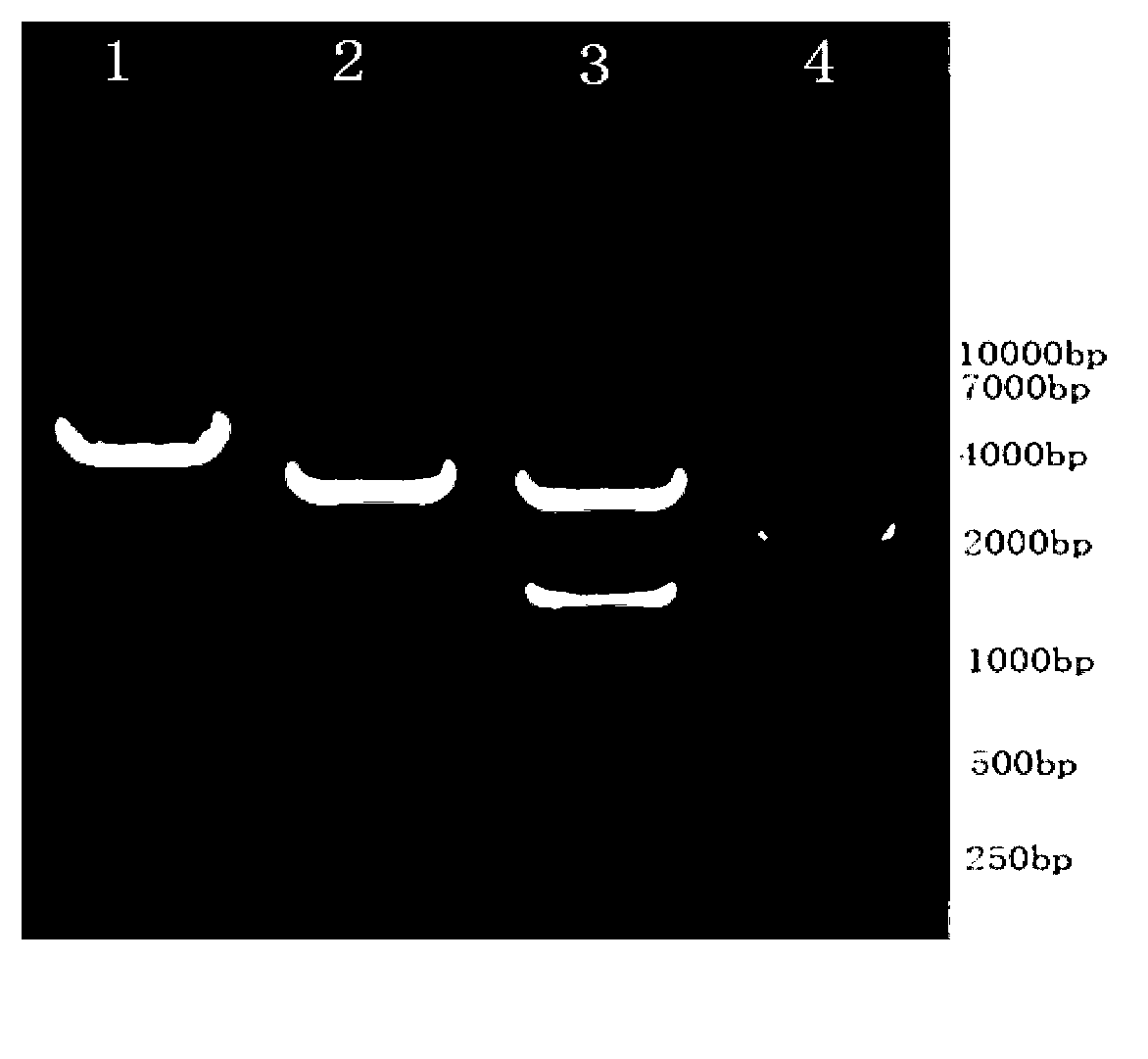
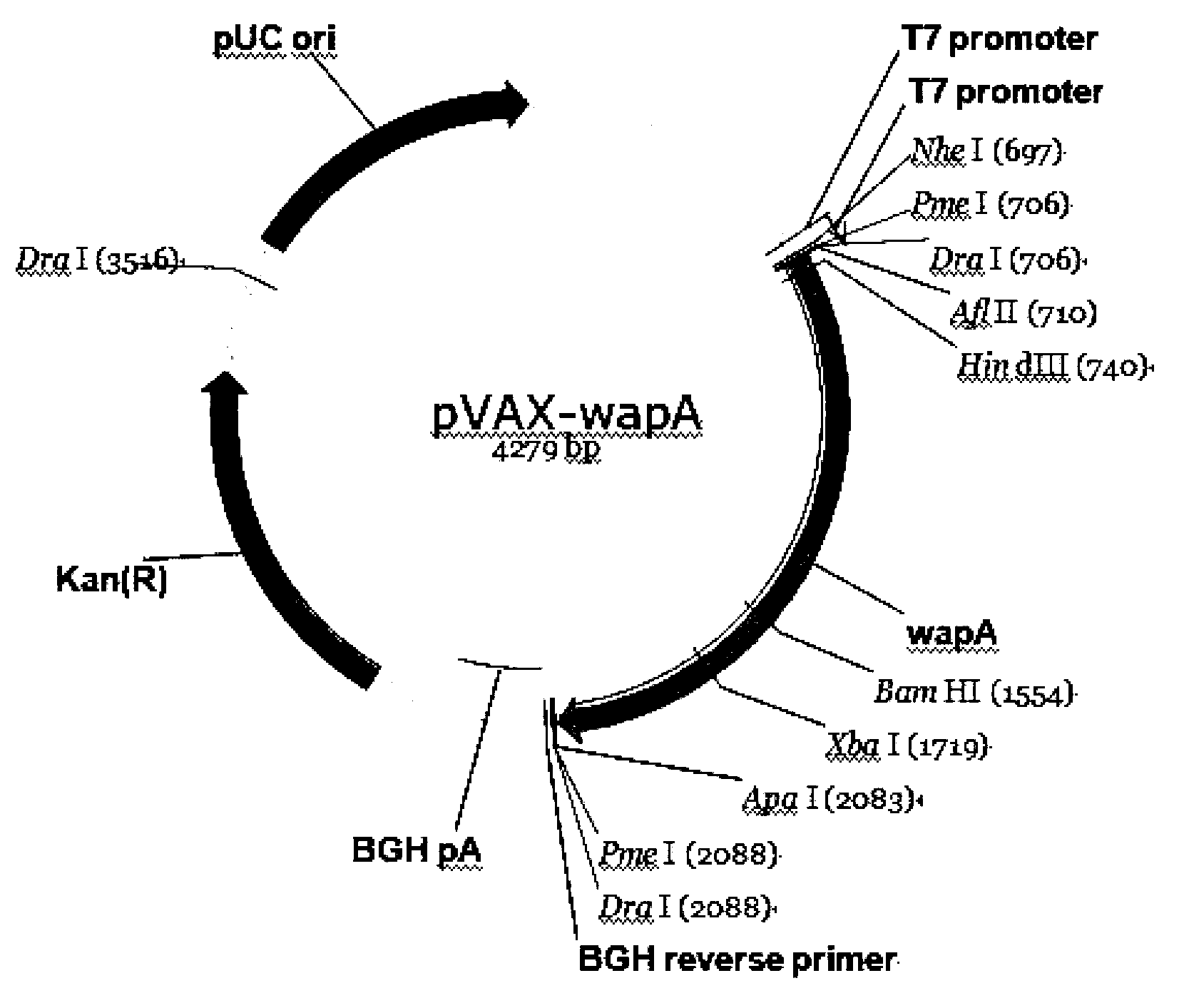
[0058] Example 1: Construction of an anti-caries DNA vaccine——pVAX1-wapA
[0059] 1. Culture of Streptococcus mutans
[0060] The frozen Streptococcus mutans UA159 strain (gifted by the Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, refer to the literature: Huang Zhengwei, Liu Zheng, Ma Rui, Tang Zisheng, Zhu Cailian, Optimization of electric shock transformation conditions for Streptococcus mutans, China Journal of Microecology, Issue 5, 2, 2007, page 404-405) Stretch culture and BHI plate medium (commercial plate medium, purchased from Shanghai Hengyuan Biotechnology Co., Ltd.), the culture condition is 37 ℃ anaerobic culture (80%N 2 10% CO 2 10%H 2 ). Pick the single clone grown on the plate and inoculate it into TY medium (1% tryptone 0.8% yeast extract pH5.0), and culture it with shaking at 37°C under airtight conditions. Bacteria were collected for subsequent experiments.
[0061] 2. Obtaining the genome of Streptococcus m...
Embodiment 2
[0125] Embodiment 2: the preparation of DNA vaccine chitosan nanocomposite
[0126] In order to improve the immunity of DNA vaccine, Na 2 SO 4 As a flocculant, DNA vaccine chitosan nanocomposites were prepared by self-assembly method.
[0127] The assembly steps are: chitosan (CS) is dissolved in NaAc buffer, with Na 2 S0 4 The DNA vaccine solutions were pre-heated to 50-55°C for 10 minutes, mixed quickly in equal volumes, vortexed for 1 minute, and left at room temperature for 30 minutes to obtain the DNA vaccine chitosan nanocomposite. The specific formulation conditions are shown in Table 6. Take 100 μl of nanoparticle suspension, make up 1ml with deionized water, and then use ZetasiserNano ZS laser particle size analyzer (Malvern, UK) to measure the average particle size, particle size distribution and Zeta potential of nanoparticles. The results showed that the grain size of the rice was uniform and the particle size distribution range was narrow. The results are show...
Embodiment 3
[0132] Embodiment 3: pVAX1-wapA and DNA vaccine chitosan nanocomposite eukaryotic expression ability testing process is as follows:
[0133] 1. 293A cell with 1×10 6 Spread 6-well plates at the density of cells / well and wait overnight for the cells to adhere to the wall.
[0134] 2. Transfect 293cells:
[0135] a, prepare pVAX1-wapA / cs, chitosan solution;
[0136] b. Gently mix pVAX1, pVAX1-wapA4μg and lipo10μl with 250μl serum-free medium respectively, and let stand for 5min;
[0137] c. Gently mix the serum-free medium mixed with plasmid and lipo, let stand at room temperature for 15-20min, and the total operation time shall not exceed 25min; Gently mix 500 μl serum-free medium;
[0138] d. Add the mixed solution into the 6-well plate, and change the serum-containing culture solution after 6 hours;
[0139] e. After 48 hours, the TRIzol method was used to extract total RNA. The steps are as follows: ①Add 1ml / well TRIzol directly to the 6-well cell culture plate to lyse ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com