Capsule deficiency type Streptococcus equi subsp. zooepidemicus attenuated vaccine strain and preparation method thereof
A technology for Streptococcus equi and attenuated vaccines, applied in biochemical equipment and methods, methods based on microorganisms, bacteria, etc., can solve problems that hinder the development of attenuated vaccines, and achieve the effects of short cycle, convenient use, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Mutant strain C55138? has B build
[0033] 1. Materials: Streptococcus equi subsp. zooepidemicus C55138, purchased from China Veterinary Drug Control Institute. Plasmid pG + host5 was purchased from Appligene (Illkirch, France).
[0034] 2. Design and synthesis of primers
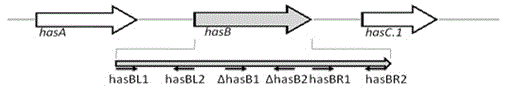
[0035] The genome structure of Streptococcus equi subsp. zooepidemicus C55138 (referred to as strain C55138) is attached figure 1 As shown, using the genomic DNA of strain C55138 as a template, use Primer Premier5.0 to design and amplify the target fragment has B Gene primers, where the hasBL primer pair contains restriction enzymes Sal I and Bam HI restriction site, the hasBR primer pair contains Bam HI and Eco Restriction site for RI. Primers were synthesized by Shanghai Sangong.
[0036] hasBL1: 5'-ATTTCTGTCGACGGCTCAGGATA-3' (SEQ ID NO: 1);
[0037] hasBL2: 5'-AATGGATCCTGACGCATTTAGGT-3' (SEQ ID NO: 2);
[0038] hasBR1: 5'-AACCATTACAATAACGGATCCTTTG-3' (SEQ ID NO: 3);
...
Embodiment 2
[0069] Embodiment 2 mouse virulence experiment
[0070] Twenty BALB / c mice aged 4-6 weeks were randomly divided into two groups, one group was injected with wild bacteria C55138, and the other group was injected with mutant bacteria C55138? has B , used to compare the virulence of the two strains.
[0071] After the bacteria were resuspended in PBS, the bacterial solution was properly diluted to 1×10 5 CFU / mL, mice were injected intraperitoneally with 500 μL, the survival status of the mice was observed, the death time of the mice was recorded, and the results were analyzed. The mice were continuously observed for 12 days.
[0072] See the experimental results Figure 5 : The mice in the wild bacteria group had a mortality rate of 80% within 9 days and showed severe clinical symptoms. The mutant bacteria C55138 ?hasB One group of mice survived 100% within 12 days without any clinical symptoms.
[0073] The experimental results showed that: mutant bacteria ?hasB virule...
Embodiment 3
[0074] Embodiment 3 mutant bacteria ?hasB Active immune protection experiment
[0075] Forty female BALB / c mice aged 4-6 weeks were randomly divided into 4 groups with 10 mice in each group. Group 1 mice were injected intraperitoneally with 500 μL mutant strain C55138 for the first time ?hasB For immunization, the bacterial concentration was 2×10 6 CFU / mL, immunized again with the same dose after 14 days; mice in the second group were used as positive controls to inject formaldehyde inactivated vaccine in the same way for immunization, the inactivated vaccine was emulsified with inactivated wild bacteria and Freund's adjuvant Inactivated vaccine, the first intraperitoneal injection of 500 μL, followed by immunization every 2 weeks. The mice in group 3 were injected with PBS emulsified with the same Freund's adjuvant in the same way as a negative control, and the mice in group 4 were injected with PBS in the same way as a blank control.
[0076] After all the mice in the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com