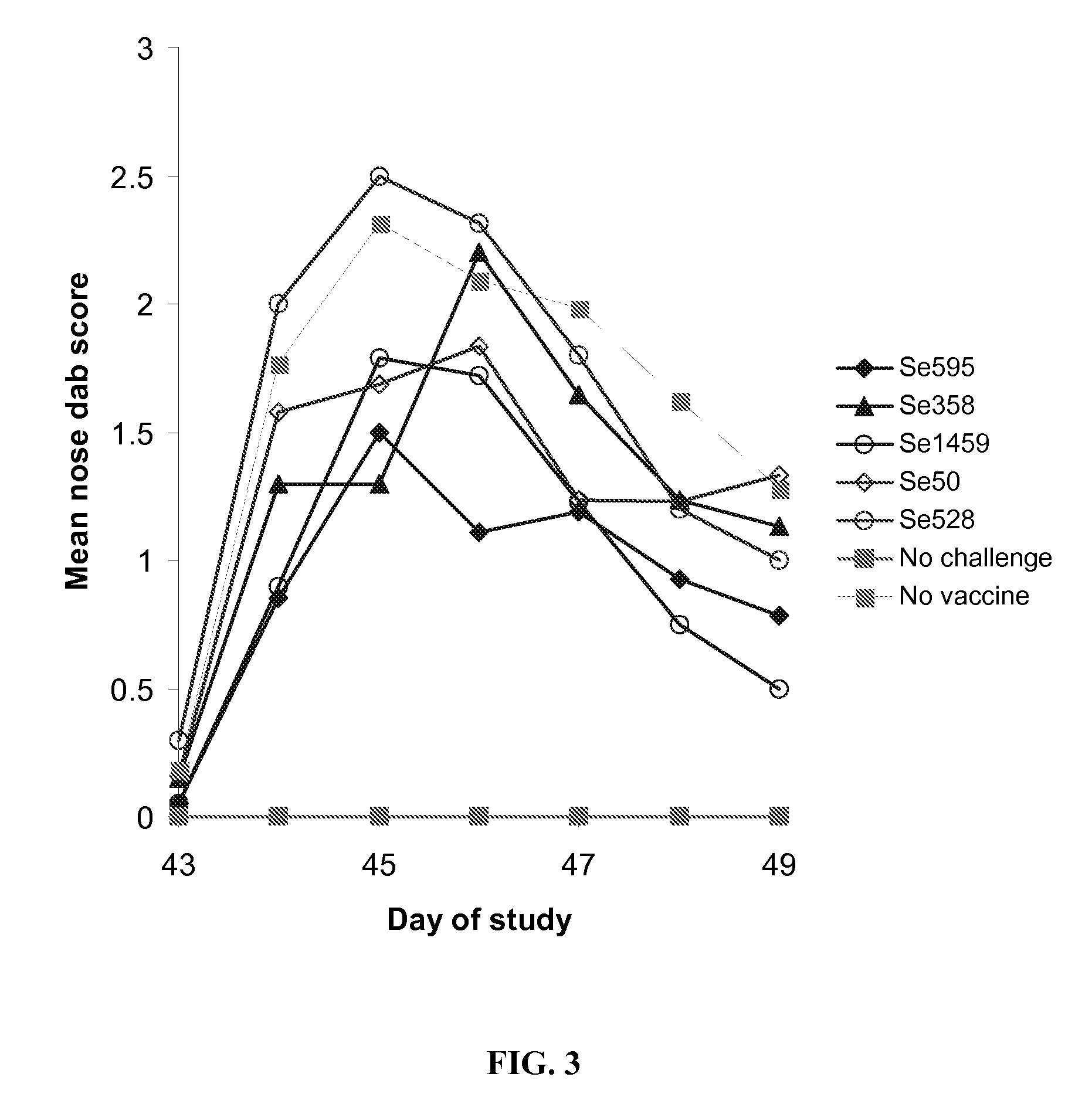
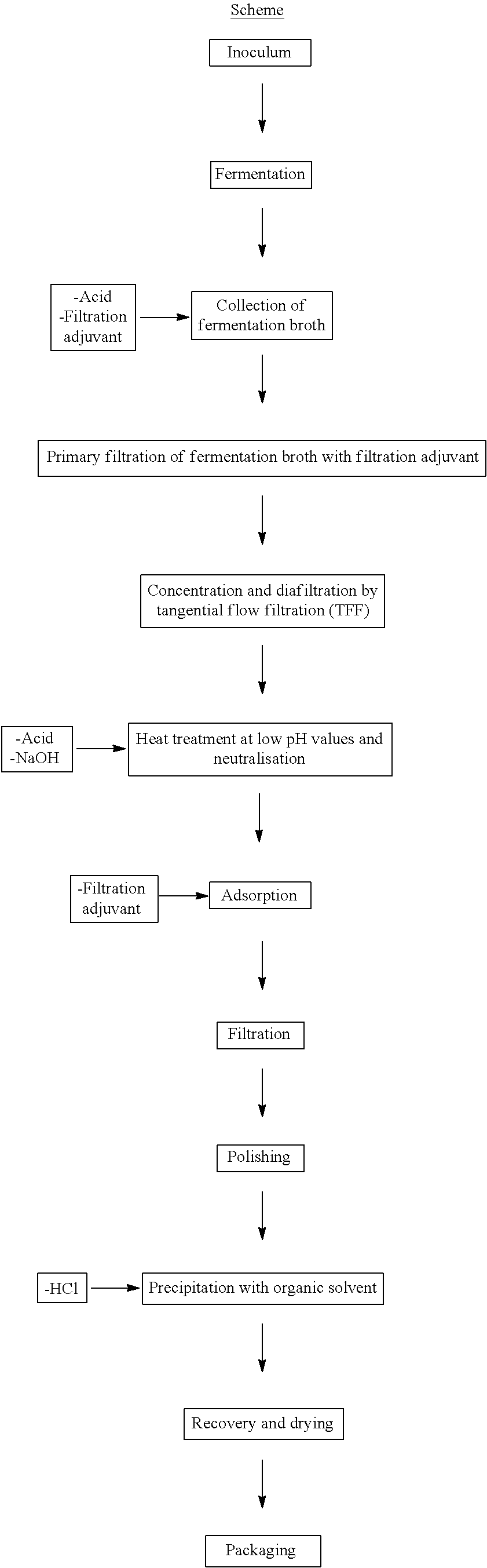
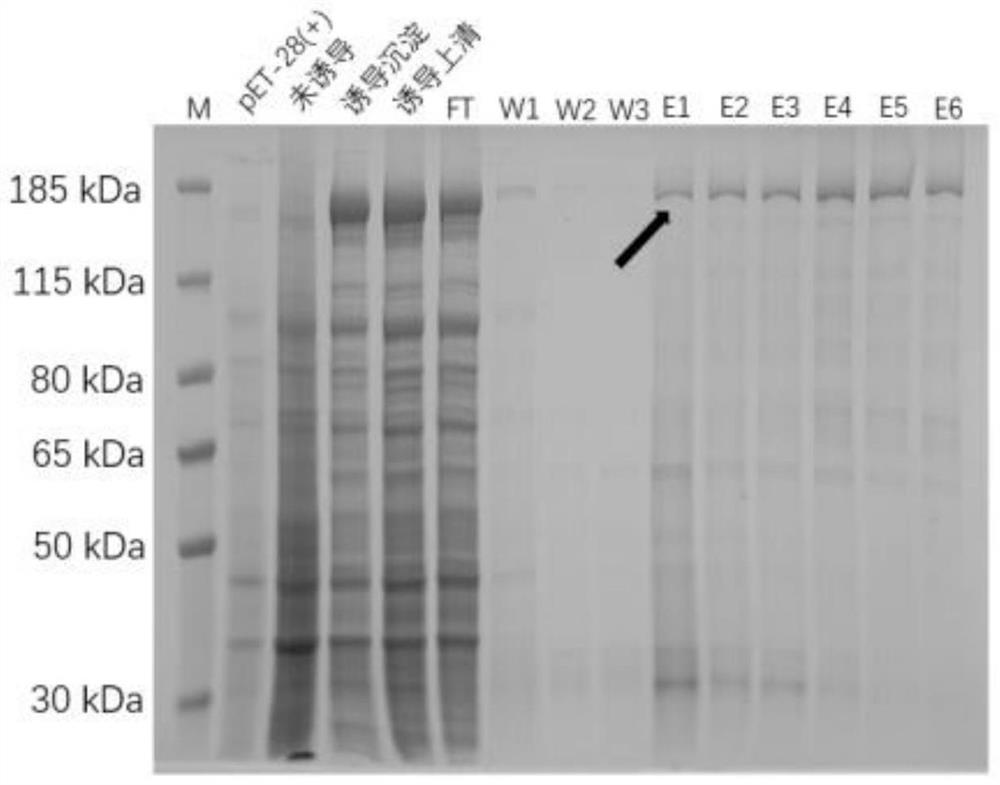
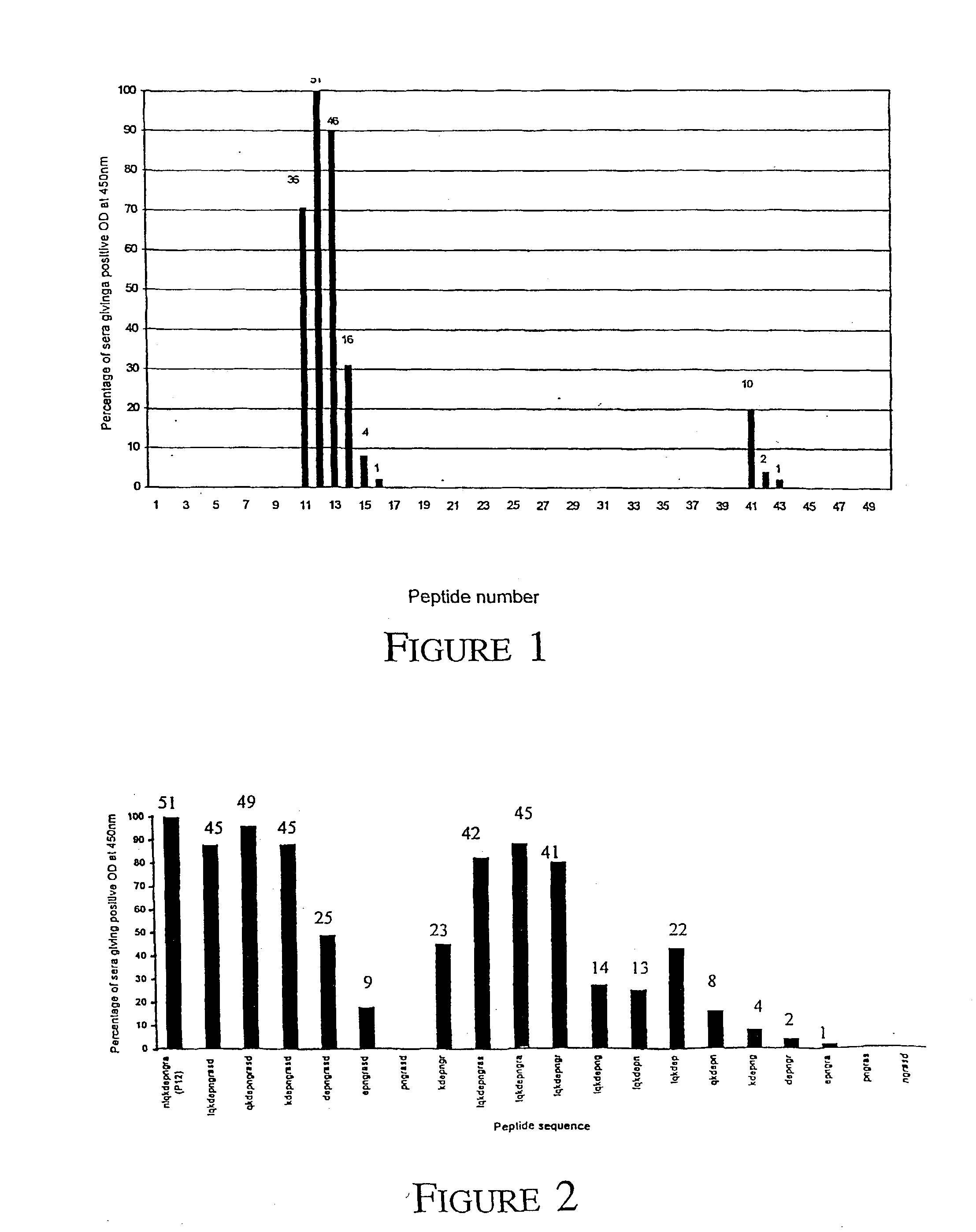
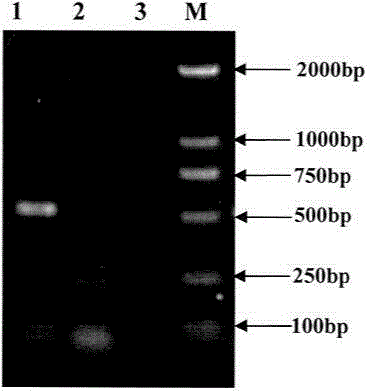
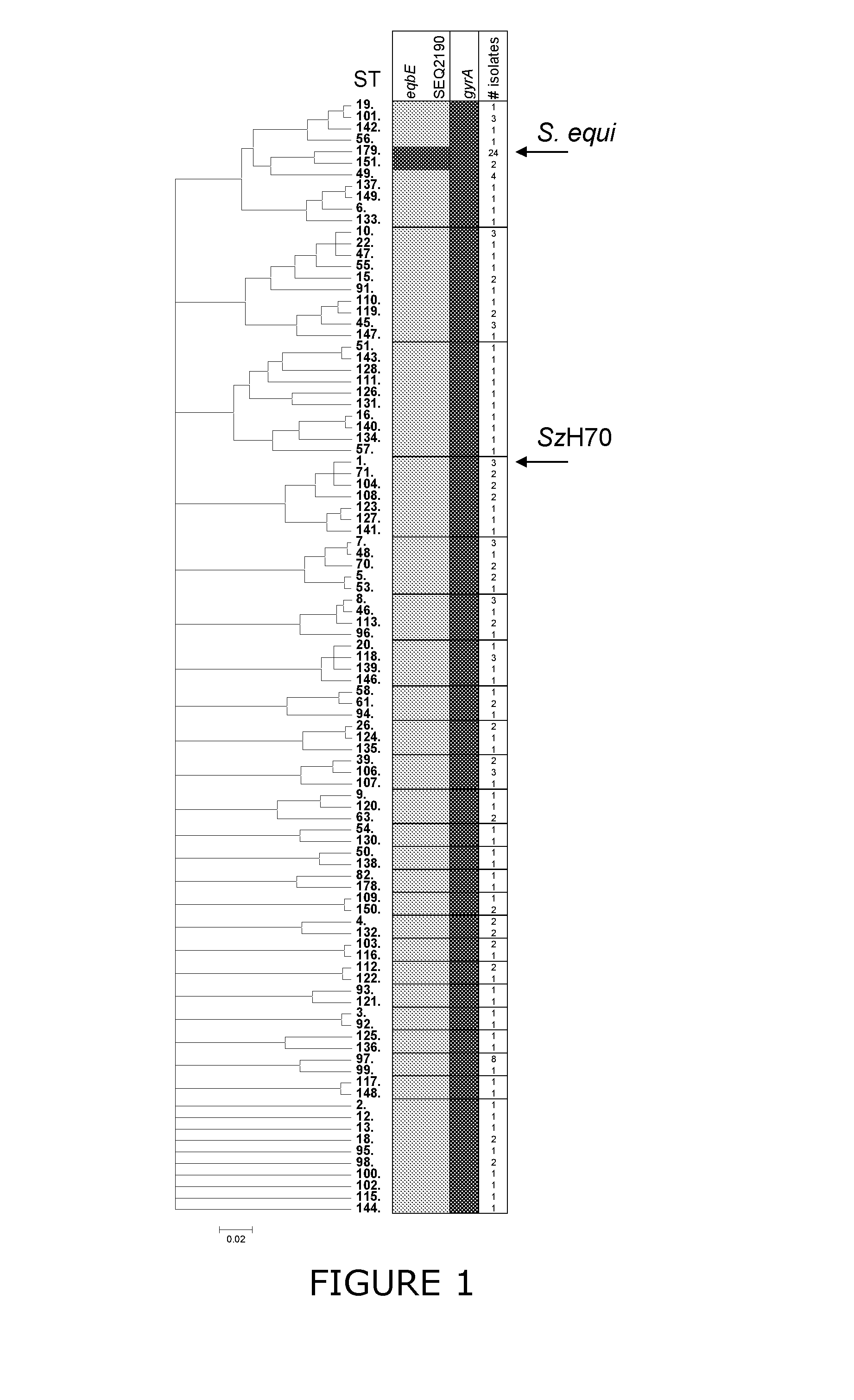
Patents
Literature
55 results about "Streptococcus equi" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A species of facultatively anaerobic, Gram positive, cocci shaped bacteria in the phylum Firmicutes. This species is positive for Lancefield group C, beta hemolysis and arginine deaminase, negative for catalase and can ferment sorbitol. S. equi can be isolated from many animals and is a pathogen that causes equine distemper and human nephritis.
Method for providing hyaluronic acid
InactiveUSRE37336E1Facilitate easeAbility to controlBacteriaSugar derivativesEscherichia coliRecombinant DNA
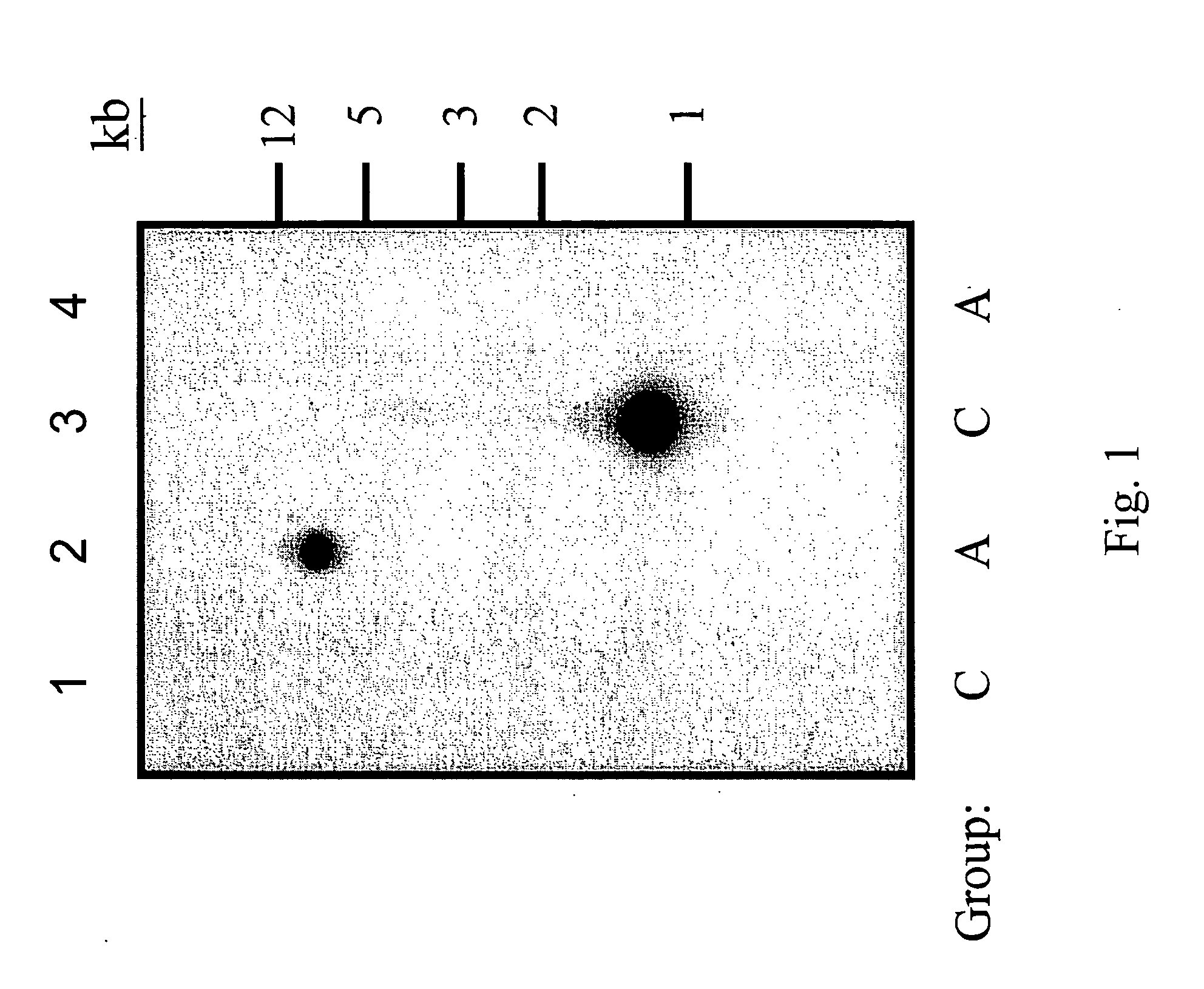
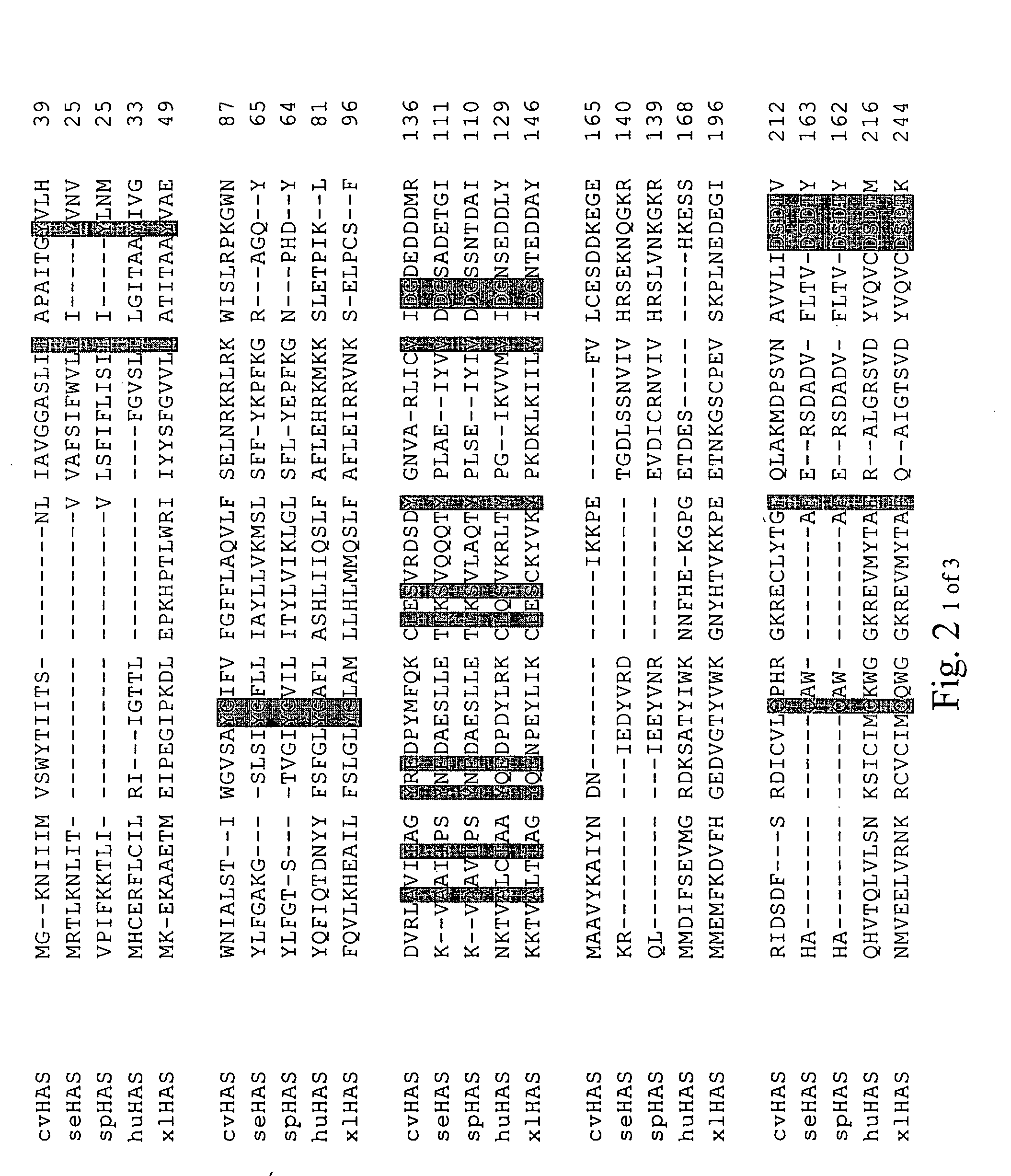
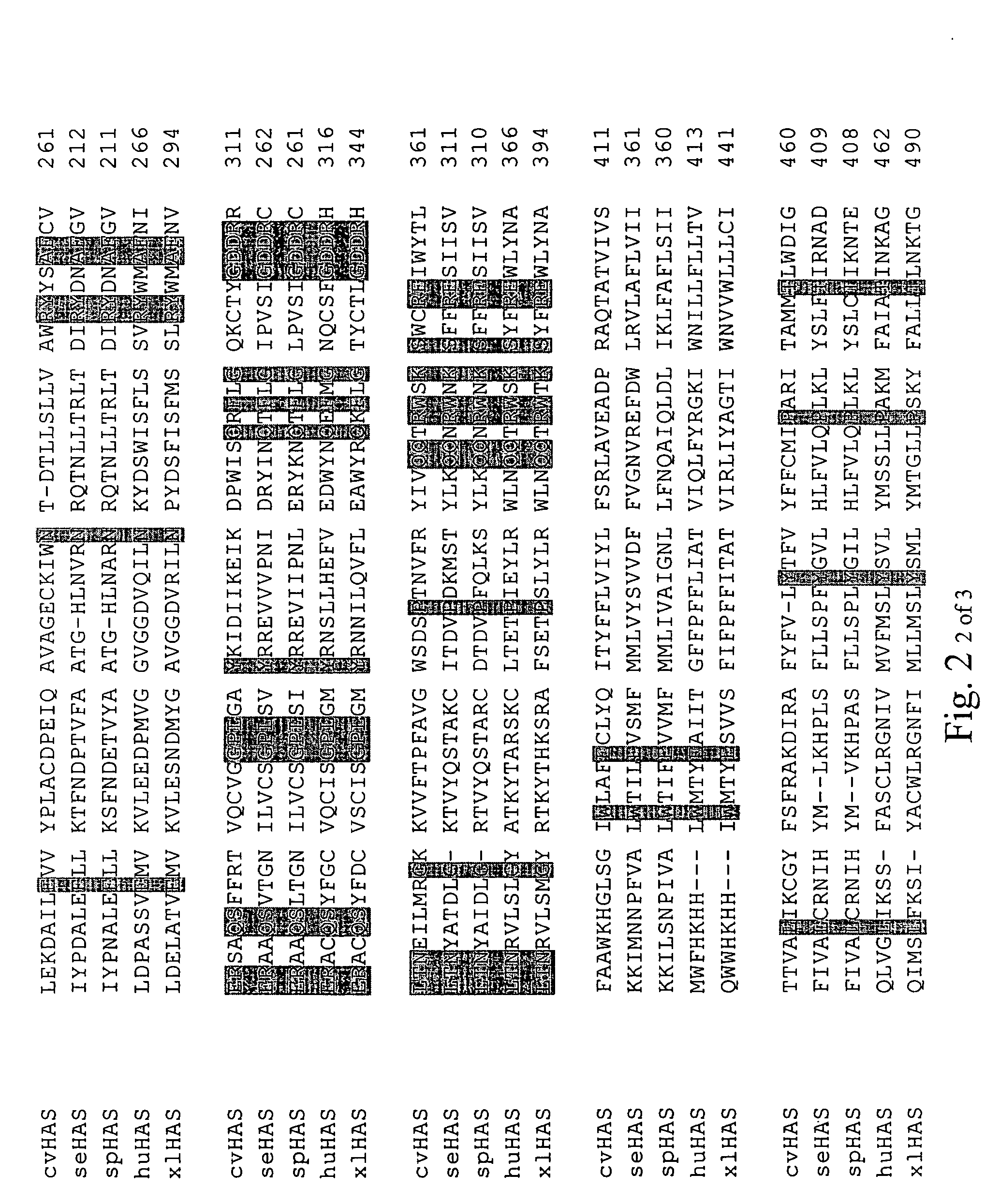
Disclosed are DNA segments encoding <DEL-S DATE="20010821" ID="DEL-S-00001">hyaluronic acid synthase which are employed to construct recombinant cells useful in the production of hyaluronate synthase or hyaluronic acid (HA)<DEL-E ID="DEL-S-00001"> <INS-S DATE="20010821" ID="INS-S-00001">the recombinant DNA segment identified in FIG. 5. <INS-E ID="INS-S-00001">In preferred aspects, chromosomal DNA from Streptococcus equisimilis is partially digested with EcoRI and the resultant fragments are ligated to form recombinant vectors. These vectors are useful in the transformation of host cells such as E. coli and or Streptococcal hosts. <DEL-S DATE="20010821" ID="DEL-S-00002">Resultant transformants are screened by the novel screening assays to identify colonies which have incorporated HA synthase DNA in a form that is being actively transcribed into the corresponding HA synthase enzyme. These colonies may be selected and employed in the production of the enzyme itself or its product, HA.<DEL-E ID="DEL-S-00002"> <INS-S DATE="20010821" ID="INS-S-00002">The recombinant DNA segment identified in FIG. 5 is then inserted into a recombinant Streptococcal host for the production of hyaluronic acid (HA).<INS-E ID="INS-S-00002">
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
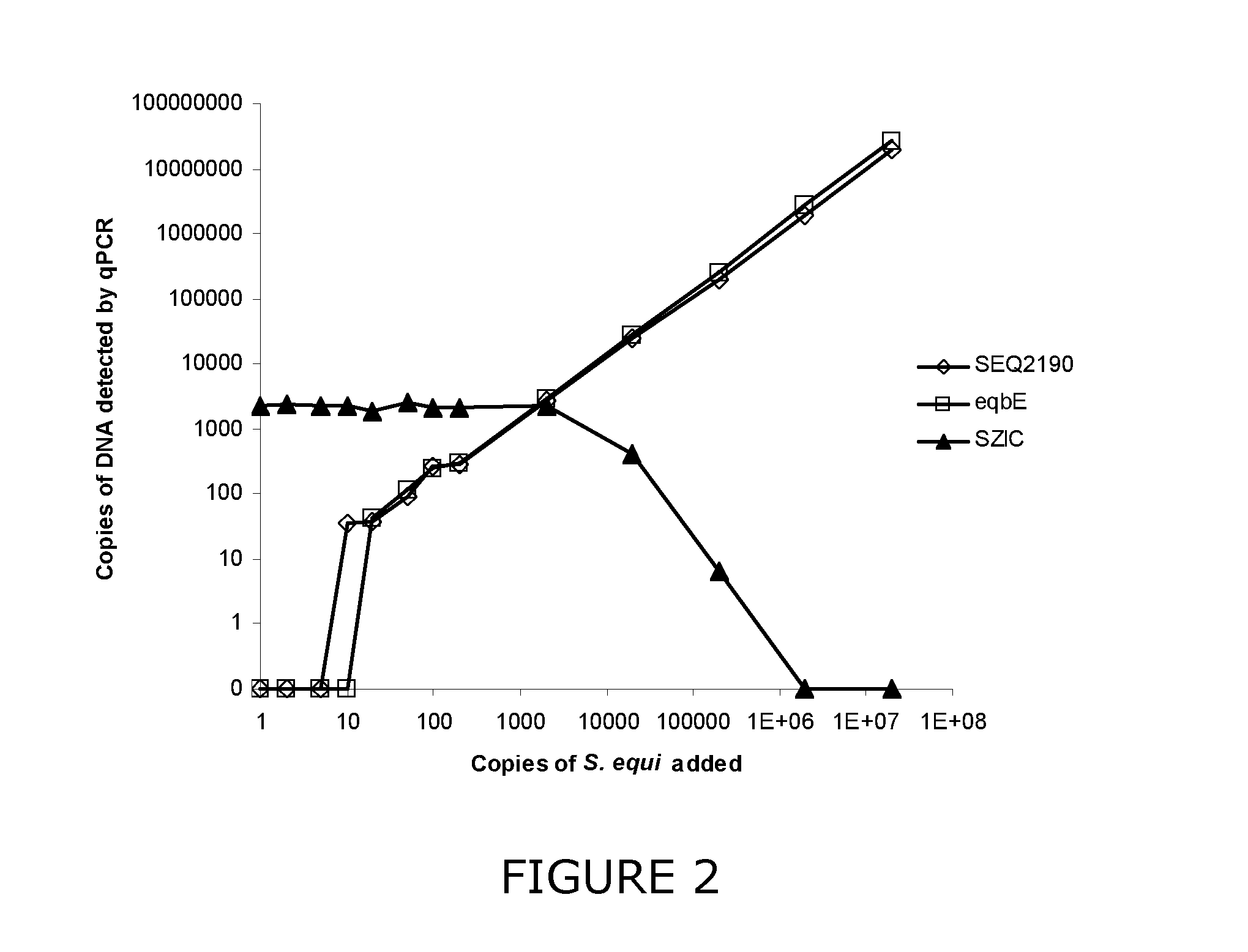
Diagnostic test for streptococcus equi
InactiveUS20110201007A1High sensitivityStrong specificitySugar derivativesMicrobiological testing/measurementDiseaseGene sequence
The invention relates generally to methods and materials concerning diseases caused by Streptococcus equi, and in particular relating to the detection of this pathogen by assessing the presence or absence of the S. equi eqbE gene sequence.
Owner:ANIMAL HEALTH TRUST
Streptococcus equi subsp. Zooepidemicus subspecies SXY36 and application in fermentation production of hyaluronic acid
ActiveCN107338201ANo hemolyticImprove fermentation yieldBacteriaMicroorganism based processesHemolysisGamma ray
Owner:株洲市中建新材料有限公司
Production technology for fermentatively producing sodium hyaluronate by utilizing bacterium
InactiveCN103173507ATo satisfy the market's needsImprove mutagenesis efficiencyMicroorganism based processesFermentationStreptococcus zooepidemicusHydrogen
The invention relates to a production technology for fermentatively producing sodium hyaluronate by utilizing bacterium. The production technology comprises the following steps of: using streptococcus equi subsp. zooepidemicus (Streptococcus Zooepidemicus) as an original strain; mutagenizing by virtue of nitrosoguanidine, and also mutagenizing by virtue of a protoplast in the presence of ultraviolet rays; introducing a special culture medium; carrying out submerged fermentation to produce high-output hyaluronic acid; and then carrying out a relative separation and purification technology to obtain a high-purity sodium hyaluronate product. According to the production technology for fermentatively producing the sodium hyaluronate by utilizing the bacterium, under the culture conditions that the culture temperature is 32 to 38 DEG C, the pH (Potential Of Hydrogen) of fermentation liquor is maintained within the range of 5.0 to 9.0, and a fermentation tank is at speed of stirring of 150 to 600rev / min, the output of the hyaluronic acid is greatly increased; and the production technology is suitable for being applied to industrial production.
Owner:宁波林叶生物科技有限公司
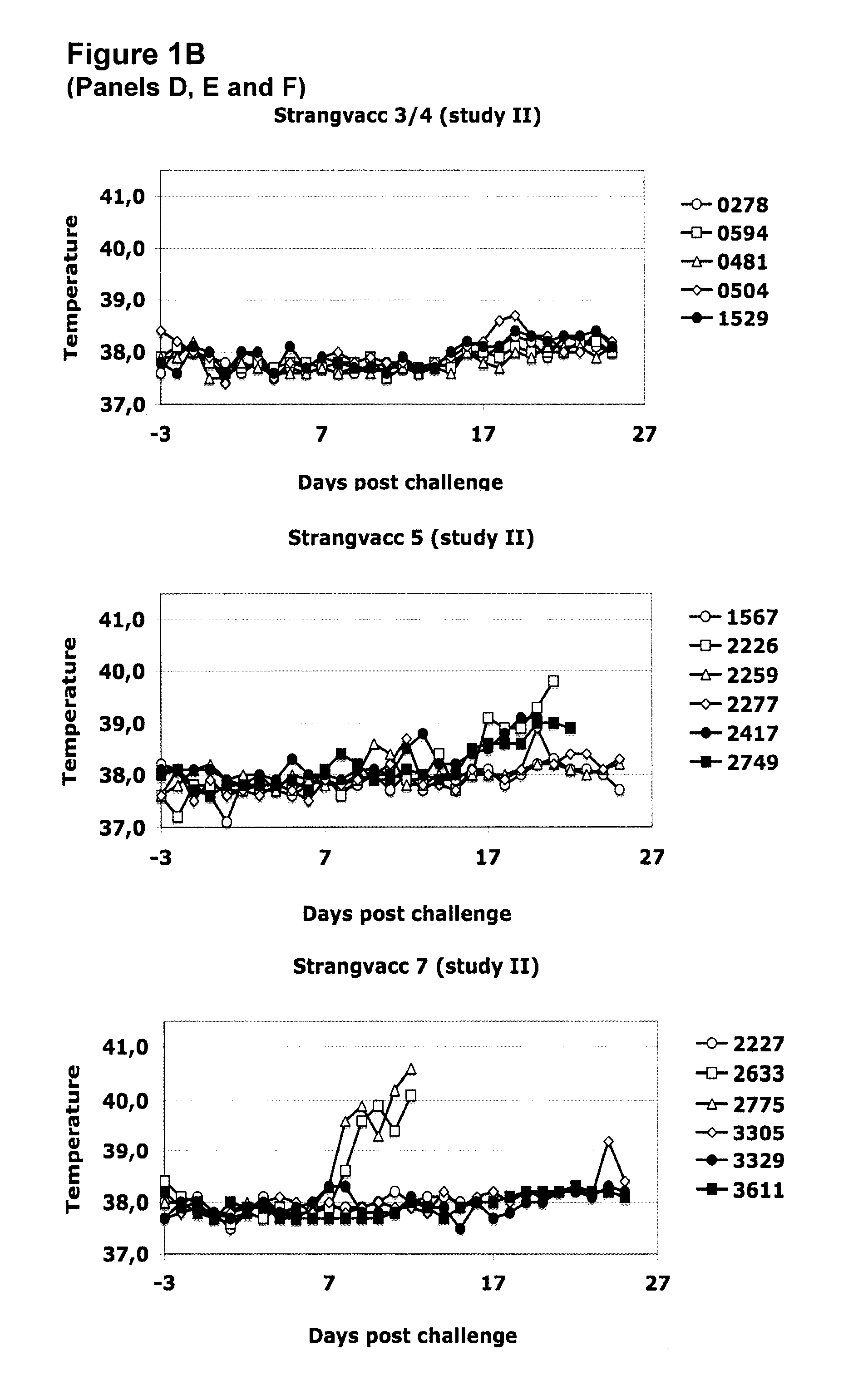
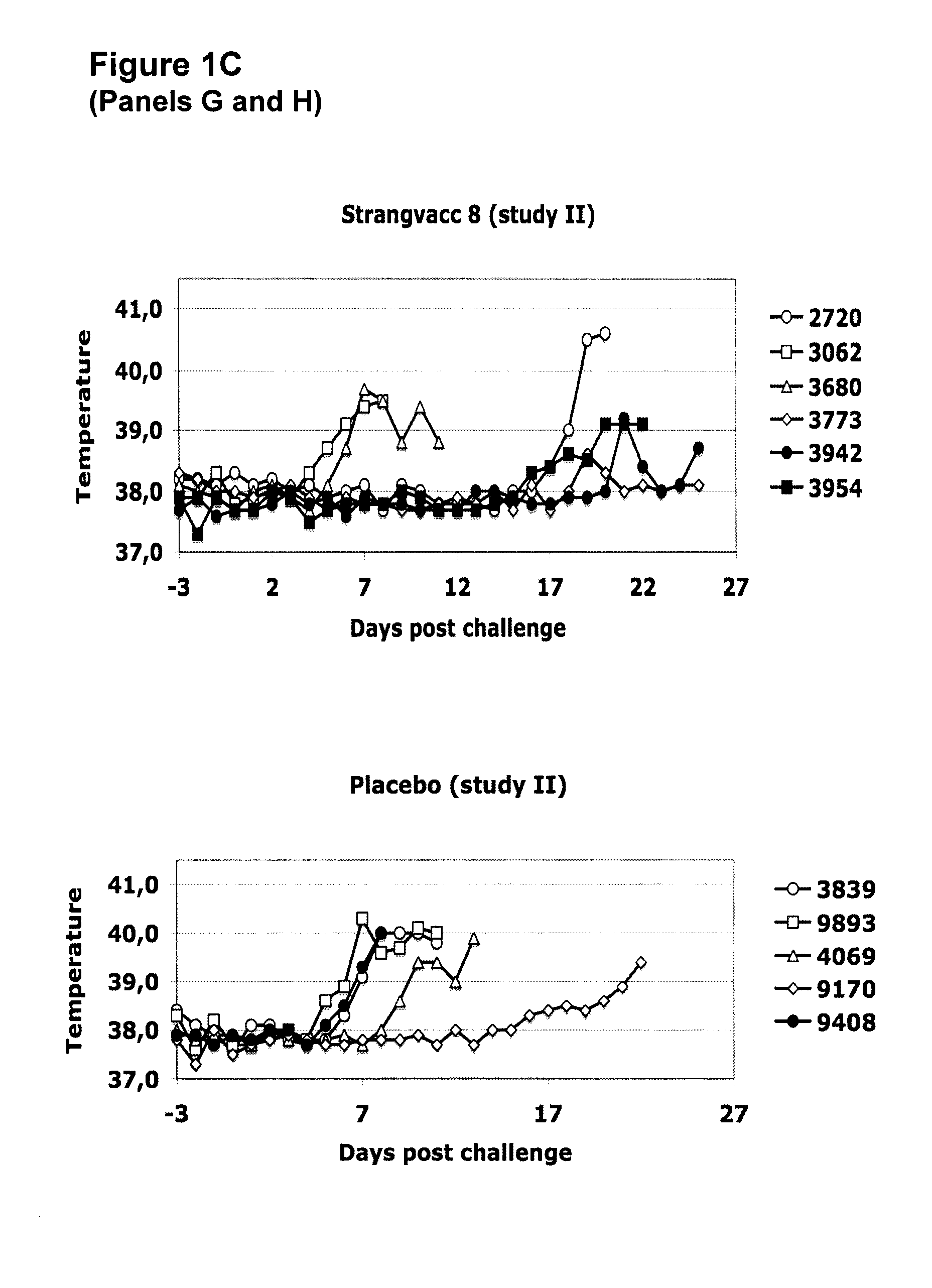
Vaccine against streptococci
The invention relates to subunit immunogenic or vaccine compositions which may comprise at least one polypeptide of Streptococcus equi and methods for preparing and / or formulating such compositions. The invention also relates to the use of such subunit compositions, such as a method for eliciting an immunogenic response or a protective immune response, which may comprise administering the composition to a mammal susceptible to streptococcal infection.
Owner:MERIAL INC
Swine Streptococcosis trivalent inactivated vaccine and preparation method thereof
ActiveCN102949714AImprove survival rateReduce morbidityAntibacterial agentsBacterial antigen ingredientsStreptococcus suis serotypeAnimals vaccines
The invention belongs to the technical field of preparation of animal vaccines and particularly relates to a trivalent inactivated vaccine for Streptococcus equi subsp. zooepidemicus, Streptococcus suis serotype 2 and Streptococcus suis serotype 7, as well as a preparation method and application thereof. The inactivated vaccine disclosed by the invention is mainly applicable to prevention and treatment of swine Streptococcosis for sows, boars and piglets. The invention discloses separation, identification and application of three strains special for preparing the swine Streptococcosis trivalent inactivated vaccine, wherein the three strains for the vaccine are respectively a Streptococcus suis serotype 2-LT strain (with the collection number being CCTCC NO:M2011282), a Streptococcus suis serotype 7-YZ strain (with the collection number being CCTCC NO:M2011160) and a Streptococcus equi subsp. Zooepidemicus XS strain (with the collection number being CCTCC NO:M2011405). The inactivated vaccine disclosed by the invention can achieve multiple prevention effects with one injection, so that the stresses of swine are reduced, and the inactivated vaccine is safe and reliable and is free from the potential hazards of toxin spreading.
Owner:HUAZHONG AGRI UNIV +1
Methods of producing hyaluronic acid using a recombinant hyaluronan synthase gene
InactiveUS20050202540A1Organic active ingredientsBacteriaStreptococcus equisimilisHyaluronan synthase
The present invention relates to a nucleic acid segment having a coding region segment encoding enzymatically active Streptococcus equisimilis hyaluronate synthase (seHAS), and to the use of this nucleic acid segment in the preparation of recombinant cells which produce hyaluronate synthase and its hyaluronic acid product. Hyaluronate is also known as hyaluronic acid or hyaluronan.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Production of highly purified sodium hyaluronate (HANa) with controlled molecular weight
Disclosed is a process for the production of sodium hyaluronate with a molecular weight of between 60 and 2400 kDa and low polydispersity (1.4 Mw / Mn), which comprises: a) a step of fermentation of Streptococcus equi subsp. Zooepidemicus CNCM 1-4645 in a suitable culture medium; b) a step of ultrafiltration of the cell-free filtered solution; by concentrating and diafiltering the solution under differential pressure conditions (ΔP) of 1.0-5.0 bar(g) and transmembrane pressure (TMP) of 0.5-4 bar(g).
Owner:ALTERGON ITAL
Production of Highly Purified Sodium Hyaluronate (HANA) with Controlled Molecular Weight
Disclosed is a process for the production of sodium hyaluronate with a molecular weight of between 60 and 2400 kDa and low polydispersity (1.4 Mw / Mn), which comprises: a) a step of fermentation of Streptococcus equi subsp. zooepidemicus CNCM 1-4645 in a suitable culture medium; b) a step of ultrafiltration of the cell-free filtered solution, by concentrating and diafiltering the solution under differential pressure conditions (ΔP) of 1.0-5.0 barg and transmembrane pressure (TMP) of 0.5-4 barg.
Owner:ALTERGON ITAL
Serum-free anaerobic high-density fermentation culture process for Streptococcus equi subsp. zooepidemicu
ActiveCN105925517AEasy to achieve large-scale high-density cultureImproving immunogenicityBacteriaMicroorganism based processesVaccine ImmunogenicityFermentation
The invention discloses serum-free anaerobic high-density fermentation culture process for Streptococcus equi subsp. Zooepidemicu and belongs to the field of agricultural microbiology. Streptococcus equi subsp. Zooepidemicu fermented high-density antigens are obtained through the sequential steps of resuscitating lyophilized seeds with TSA, carrying out primary culture on a slant of TS agar, carrying out secondary seed culture on a TSB medium, and anaerobically fermenting in enriched trypsin-casein medium free of serum and glucose. Study on industrial enlarged production is carried out through the method of anaerobic fermentation culture without adding any serum or glucose, the content of fermented antigens is not reduced, three is no need for ventilation regulation and dissolved oxygen control during fermentation, the antigen content is high, process parameters are easy to control, and supplements are reduced; the process is stable and high in growth speed compared with the existing fermentation process and is easy for implementing large-scale high-density culture of Streptococcus equinus antigens; immunogenicity of a strain is improved, and protection for vaccines is improved.
Owner:山东滨州沃华生物工程有限公司
Streptococcus equi subsp and production technology for preparing hyaluronic acid through streptococcus equi subsp
ActiveCN106434444AIncrease productivityHigh yieldBacteriaMicroorganism based processesFermentationHyaluronic acid
The invention discloses a streptococcus equi subsp and a production technology for preparing hyaluronic acid through the streptococcus equi subsp. According to the novel screened streptococcus equi subsp, the improved fermentation technology is combined, the output rate of a hyaluronic acid product is high, and can be 9.5 g / L. The fermented streptococcus equi subsp is easy to separate and purify, and the production cycle is short; production is easy to control, the streptococcus equi subsp is suitable for industrial mass production, the yield is high, and the production cost is greatly reduced.
Owner:东营佛思特生物工程有限公司
Broad Spectrum of Streptococcus Lyase and Use Thereof
ActiveUS20180104316A1High activityIncrease enzyme activityAntibacterial agentsMilk preparationEscherichia coliBacteroides
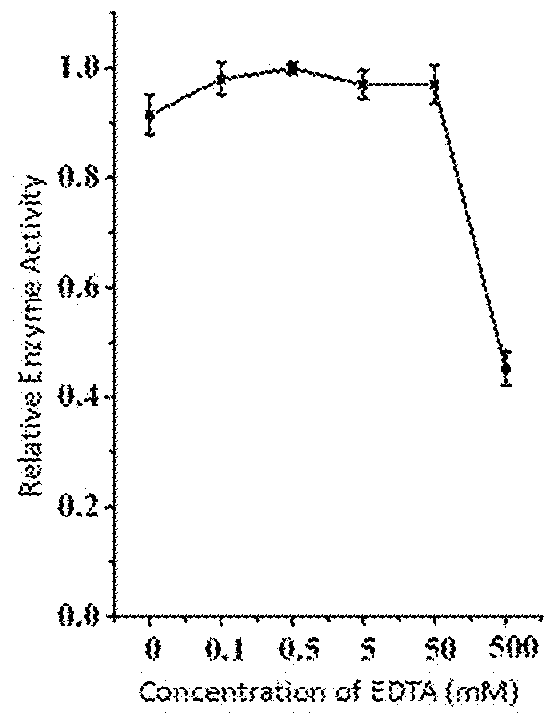
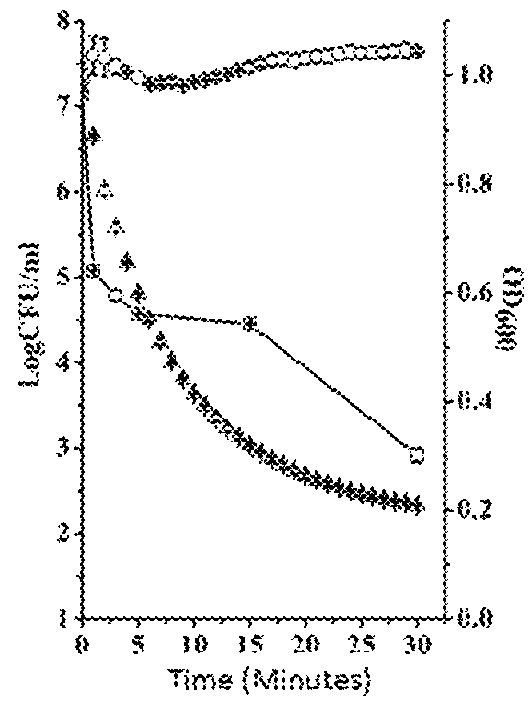
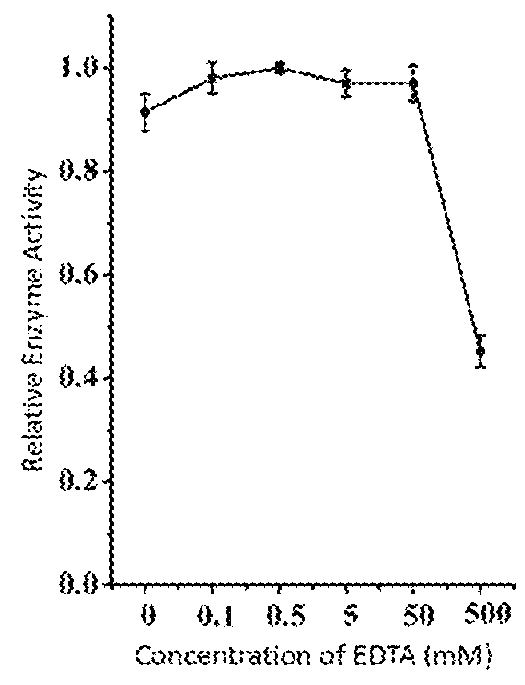
This invention discloses a lysin that can kill many species of Streptococci. A new lysin, ClyR, was constructed by the gene splicing method. The ClyR can effectively kill different species of Streptococci, including Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus suis, Streptococcus uberis, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus mutans, Streptococcus equi, and various Enterococci and Staphylococcus aureus. ClyR shows good stability and is not sensitive to EDTA and high concentration of NaCl. Moreover, the ClyR is active in a wide range of pH and maintains high activity in pH 5-11. Recombinant protein ClyR is well expressed in E. coli stain BL21 (DE3). High doses of ClyR showed no apparent toxicity in mice. Furthermore, administration of 0.8 mg per mouse once is able to completely protect the mouse infected with lethal doses of Group B Streptococci. The ClyR can be used alone or in combination with different forms of reagents and solutions, for the control of a variety of Streptococci and for the treatment of infections caused by these bacteria. It has a broad application prospect.
Owner:PHAGELUX INC
Lyase capable of killing streptococcus equi subspecies. Equi and medical application of lyase
PendingCN112501189AEfficient killingBroad cracking spectrumAntibacterial agentsBacteriaLyaseStreptococcus equi subsp equi
The invention provides lyase capable of killing streptococcus equi subspecies. Equi and medical application, and provides a new bacteriophage lyase which can be independently used or compounded with other substances for use, has strong lytic activity and a wide lytic spectrum, can effectively kill streptococcus equi subsp. Equi and streptococcus suis. The invention provides a novel medicine for preventing and treating diseases caused by streptococcus equi subsp. Equi and streptococcus suis infection.
Owner:JILIN UNIV
Capsule deficiency type Streptococcus equi subsp. zooepidemicus attenuated vaccine strain and preparation method thereof
InactiveCN102719389ASimple preparation processLow costAntibacterial agentsBacterial antigen ingredientsDiseaseVaccine Production
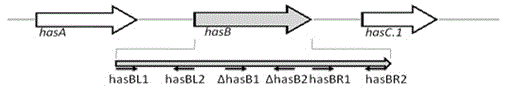
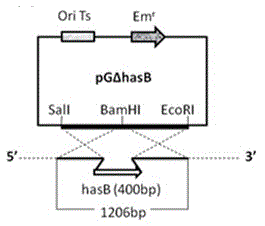
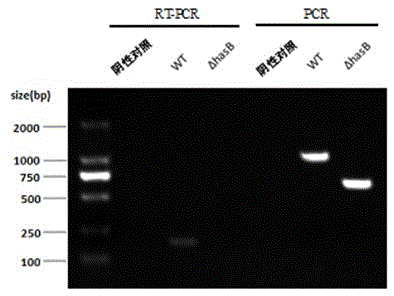
The invention discloses a capsule deficiency type Streptococcus equi subsp. zooepidemicus attenuated vaccine strain and a preparation method thereof, belonging to the technical field of animal vaccine preparation. The capsule deficiency type Streptococcus equi subsp. zooepidemicus attenuated vaccine strain is named Streptococcus equi subsp. zooepidemicus C55138 delta hasB, and was preserved in CCTCC (China Center for Type Culture Collection) on May 14, 2012 with the preservation number of CCTCC NO:M 2012164. The strain is constructed by hasB gene inactivation caused by allele replacing, is attenuated in toxicity, and can be used for preparing vaccine for Streptococcus equi disease. The vaccine production process is simple, the vaccine strain can be cultured on a large scale, the cost is low and the period is short; the vaccine presents cellular immunity and humoral immunity; and the vaccine is low in dosage, high in safety, low in toxicity and convenient for use, and can be popularized and used all over the world.
Owner:SUN YAT SEN UNIV
Streptococcus equi compositions and methods of use
Owner:WYETH LLC
Streptococcus equi strain XJMSY16-1 and application thereof in streptococcus equi vaccine
ActiveCN108220182AReduce manufacturing costEquine Streptococcus PreventionAntibacterial agentsBacterial antigen ingredientsDiseaseStreptococcus zooepidemicus
The invention relates to Streptococcus equi (also named as Streptococcus equi subsp.zooepidemicus) XJMSY16-1 with the preservation number of CGMCC No.12428. The strain has 16S rRNA gene sequences in asequence table 1. The stain is separated from sick horses suffering from Streptococcus equi and is a positive Gram bacterium, cells of the strain take the shapes of spheres and are arranged in pairs,or take the shapes of bent long chains, and are low in growth property in common meat soup and panels; beta-type hemolysis is caused on a panel with 5% fresh sheep blood, completely transparent hemolysis zones are formed around small dewdrop shaped bacterial colonies, and the width of the hemolysis zones can be up to 2.5-3.2 mm. The strain grows in a facultative anaerobic manner, the growth temperature is 26-40 DEG C, the most appropriate temperature is 37 DEG C, and the most appropriate growth pH value is 7.4-7.5. Glucose can be fermented to generate acids, and lactose, mannitol and sorbitolcannot be fermented with the strain. The strain can be used for preparing streptococcus equi deactivation vaccines. The vaccines prepared from the strain are high in disease specificity, low in cost,high in security and good in protection effect.
Owner:XINJIANG AGRI UNIV
Streptococcus equi subsp. zooepidemicus protective antigen HP0623 and preparation method thereof
InactiveCN107129527AImproving immunogenicityAvoid stickingAntibacterial agentsBacterial antigen ingredientsProtective antigenProtein purification
The invention relates to a streptococcus equi subsp. zooepidemicus (SEZ) protective antigen HP0623 and A preparation method thereof. The SEZ protective antigen HP0623 is a SEZ HP0623 recombinant protein, is composed of 429 amino acid residues, and has a molecular weight of 47.04 kDa. The preparation method comprises steps of gene clone, enzyme cutting and enzyme linking, inducible expression, and protein purification. The HP0623 can be used to prepare vaccines. The provided SEZ protective antigen HP0623 expresses good immunogenicity in mouse experiments; the generated antibody can provide a strong protective force and induce a high level sterilizing performance; the SEZ protective antigen HP0623 is located on the surface of SEZ, and rHP0623 can inhibit the adhesion of SEZ on Hep-2 cells.
Owner:HUBEI UNIV
Streptococcus equi vaccine compositions and method of use
InactiveUS20060110411A1Bacterial antigen ingredientsVeterinary vaccineProtection sexProtective immunity
This invention relates to compositions comprising live, attenuated Streptococcus equi (S. equi), or a fractional extract of S. equi, in combination with at least one immunostimulant for stimulating mucosal immunity, such as saponin. The invention also relates to methods of preparation and dosage forms containing the composition of the invention as well as methods of use for stimulating the immune system of an equine and inducing an immune response to S. equi by contacting the cells of nasopharyngeal mucosa with the composition of the invention. Furthermore, the invention relates to a method of immunizing an equine to induce protective immunity against S. equi.
Owner:WYETH LLC
Streptococcus equi subsp. Equi HLJ2018D-LX strain and application thereof in preparation of streptococcus equi subsp. Equi inactivated vaccine
ActiveCN112011479AImproving immunogenicityGood cultivation characteristicsAntibacterial agentsBacterial antigen ingredientsStreptococcus equi subsp equiImmunogenicity
The invention discloses a donkey-derived streptococcus equi subsp. Equi HLJ2018D-LX strain and application thereof in preparation of a streptococcus equi subsp. Equi inactivated vaccine, and belongs to the field of biological vaccines. The donkey-derived streptococcus equi subsp. Equi HLJ2018D-LX strain has the advantages of good immunogenicity, good culture characteristics and the like, and the inactivated vaccine is prepared by inoculating the streptococcus equi subsp. Equi HLJ2018D-LX strain into a streptococcus enrichment liquid, performing culturing, and performing inactivating with formaldehyde (the final concentration is 0.2% v / v). Experimental proves that the streptococcus equi subsp. Equi inactivated vaccine prepared by the invention is used for immunizing mice, the streptococcusequi subsp. Equi inactivated vaccine can prevent mice from being attacked by virulent streptococcus equi subsp. Equi, and greatly reduces the morbidity in donkey body tests. The vaccine has the advantages of safety, low-dose immunization and the like, is the first streptococcus equi subsp. Equi inactivated vaccine for donkeys, and can be used for preventing strangles caused by streptococcus equi subsp. Equi.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
ELISA (enzyme-linked immuno sorbent assay) detection kit and method both for detecting streptococcus equi subsp. zooepidemicus
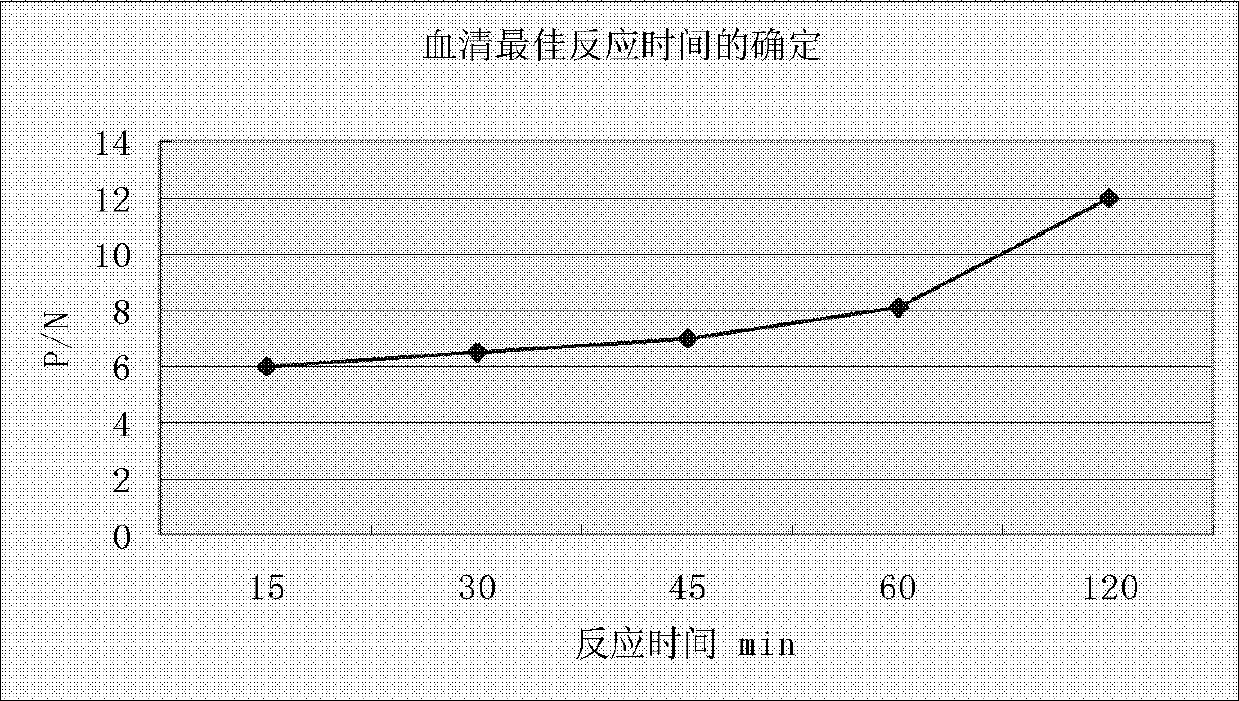
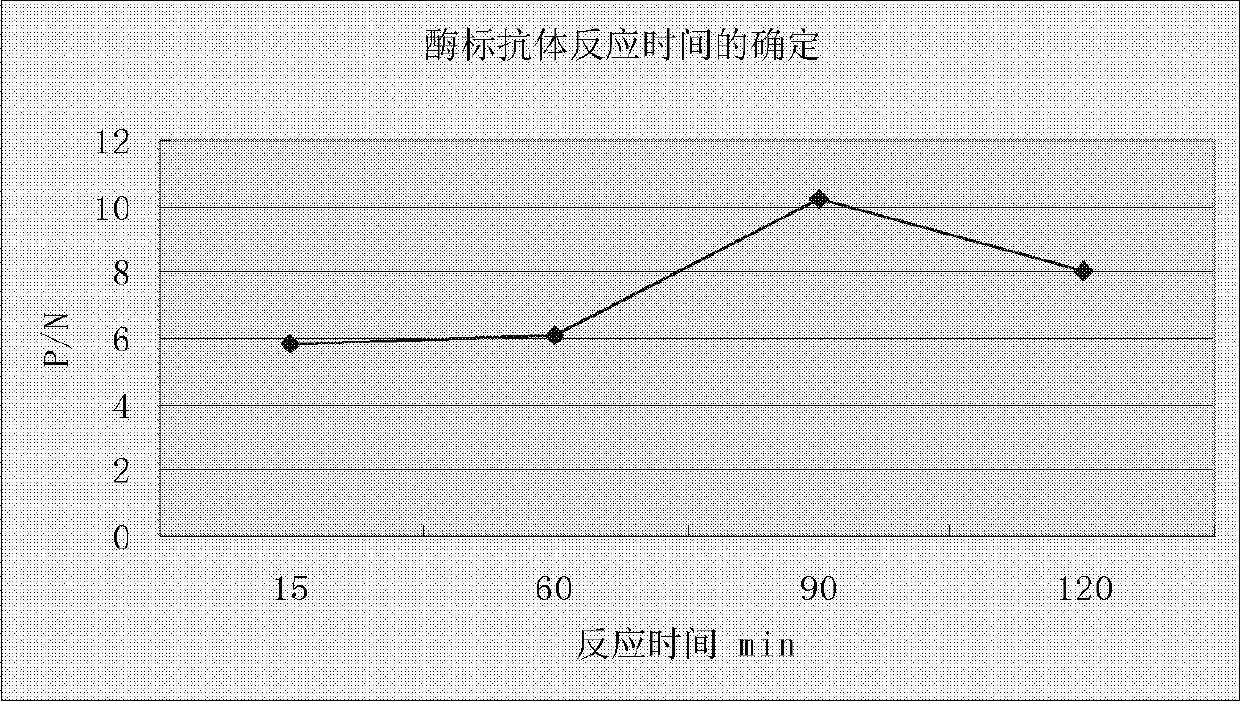
The invention belongs to the field of eqidemiology and sanitation detection, and discloses an ELISA (enzyme-linked immuno sorbent assay) quick detection kit and a method both for quickly detecting streptococcus equi subsp. zooepidemicus; particularly, the kit provided by the invention includes a recombined streptococcus equi subsp. zooepidemicus type M protein antigen and an ELIAS secondary antibody. The invention also discloses an indirect ELISA method for detecting streptococcus equi subsp. zooepidemicus. The method is quick, simple and convenient, and can be used for directly detecting to-be-detected blood serum. The detection kit and the detection method provided by the invention have high sensitiveness and strong specificity.
Owner:NANJING AGRICULTURAL UNIVERSITY +1
Recombinant fusion protein of 8 proteins of streptococcus equi subsp.equi as well as preparation method and application of recombinant fusion protein
PendingCN114437235AGood antigenicityImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsMycoproteinStreptococcus equi subsp equi
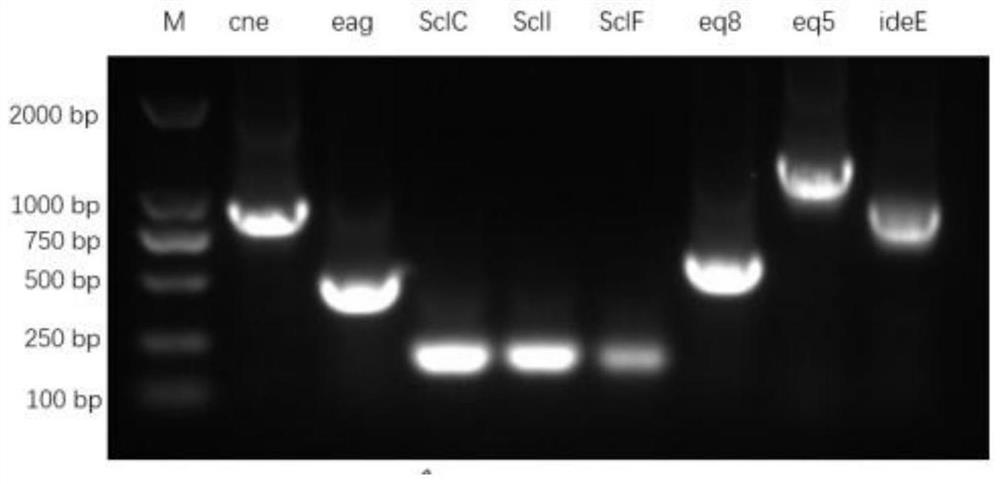
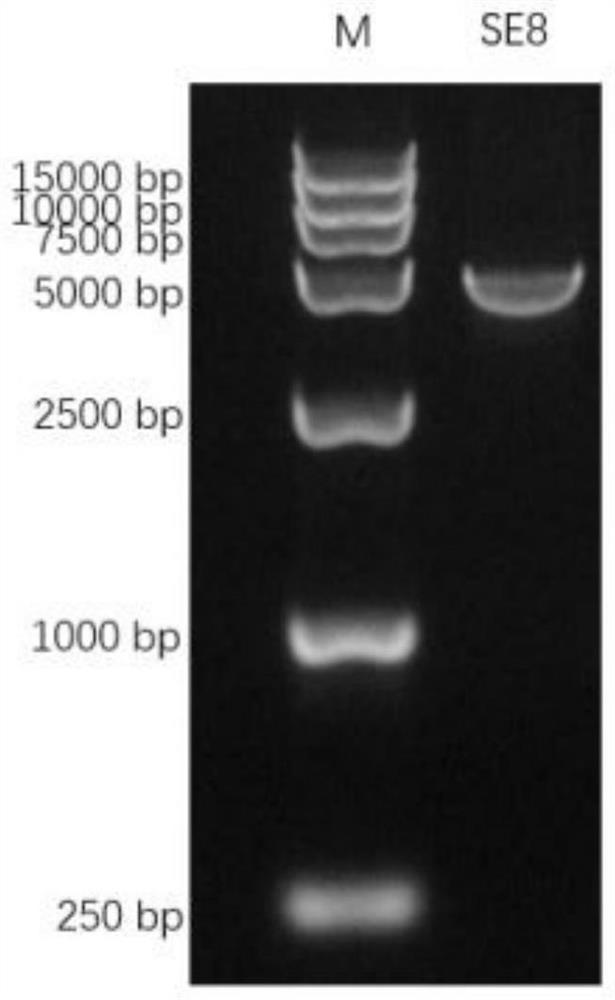
The invention discloses a recombinant fusion protein of 8 proteins of streptococcus equi subsp.equi as well as a preparation method and application of the recombinant fusion protein. The recombinant fusion protein is obtained by sequentially connecting eight proteins of cne, eag, SclC, SclI, SclF, eq5, eq8 and ideE of streptococcus equi subsp.equi through protein connectors Gly-Gly-Gly. Preferably, the recombinant fusion protein is named as SE8, and the amino acid sequence of the recombinant fusion protein is as shown in SEQ ID NO. 2. Experiments prove that the recombinant fusion protein SE8 prepared by the invention has good antigenicity and immunogenicity, an immunized mouse can effectively induce an organism to generate a high-level specific antibody, and the recombinant fusion protein SE8 plays a role in protecting an S.equi infected mouse. In addition, the recombinant fusion protein is a mycoprotein prepared by purifying escherichia coli, does not contain other components, has high safety, and avoids the phenomenon of infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Antigenic peptide fragments of vapa protein, and uses thereof
InactiveUS20030170260A1Strong specificityLess false positivesBacteriaImmunoglobulins against bacteriaRhodococcus equiAntigen binding
An isolated peptide fragment of the VapA protein that binds antibodies specific for Rhodococcus equi and the VapA protein. In a preferred form the peptide contains an amino acid sequence of 5 or more amino acid residues that is identical to or homologous to the amino acid sequence of at least one region of the VapA protein that is responsible for immunological recognition. Methods of diagnosing a vertebrate for the presence of R. equi using the peptide and methods of vaccinating a vertebrate against R. equi using the peptide are also claimed.
Owner:RURAL INDS RES & DEV
PCR detection kit and detection method for Streptococcus equi
InactiveCN103555819ASimple and fast operationThe detection process is fastMicrobiological testing/measurementMicroorganism based processesElectrophoresisStreptococcus equi subsp equi
The present invention relates to a PCR detection kit for detecting Streptococcus equi (further called Streptococcus equi subsp equi). The detection kit comprises primers, dNTP, a buffer and DNA polymerase, wherein the upstream primer is 5'-CTATTAAAGTCTCCATTG-3', and the downstream primer is 5'-AATGTTGTTCAAGCAAATTC-3'. The PCR detection method comprises: providing a sample template requiring detection; adding dNTP, a 10*buffer, primers, DNA polymerase, a sample requiring detection and ddH2O to a PCR thin-walled tube, and uniformly mixing; amplifying the mixture in the thin-walled tube through a PCR instrument; carrying out electrophoresis on the amplification product in electrophoresis equipment; and analyzing and judging the result. According to the present invention, the PCR kit preparation method is simple and is suitable for industrial production; the PCR detection method has characteristics of high Streptococcus equi detection sensitivity, short detection period, rapidness, and strong operability, wherein detection accuracy can achieve template DNA of 80 bacterial while a detection cost is relatively low; and the detection kit and the detection method have important application values in clinical diagnosis of the disease.
Owner:XINJIANG AGRI UNIV
Diagnostic test for bacterial pathogens using internal control bacterial strain
The invention relates to a method for detecting the presence or absence of a bacterial pathogen in a biological sample obtained from a human or animal subject, using an internal control. In particular, the invention relates to a method for detecting the presence or absence of Streptococcus equi in an equine sample using a control bacterial strain as internal control for DNA extraction and PCR. The invention also relates to host cells (such as bacterial cells) and nucleic acids for use as internal standard in said method in addition to diagnostic kits comprising said host cells and nucleic acids.
Owner:ANIMAL HEALTH TRUST
Method of producing macedocin by culturing Streptococcus macedonicus
InactiveUS7449311B2Broad inhibitory activity profileEfficient productionBiocideBacteriaBiotechnologyReady to eat
Owner:VRIJE UNIV BRUSSEL
Broad spectrum of Streptococcus lyase and use thereof
ActiveUS9993532B2Increase enzyme activityHigh activityAntibacterial agentsMilk preparationEscherichia coliBacteroides
This invention discloses a lysin that can kill many species of Streptococci. A new lysin, ClyR, was constructed by the gene splicing method. The ClyR can effectively kill different species of Streptococci, including Streptococcus pneumoniae, Streptococcus pyogenes, Streptococcus suis, Streptococcus uberis, Streptococcus agalactiae, Streptococcus dysgalactiae, Streptococcus mutans, Streptococcus equi, and various Enterococci and Staphylococcus aureus. ClyR shows good stability and is not sensitive to EDTA and high concentration of NaCl. Moreover, the ClyR is active in a wide range of pH and maintains high activity in pH 5-11. Recombinant protein ClyR is well expressed in E. coli stain BL21 (DE3). High doses of ClyR showed no apparent toxicity in mice. Furthermore, administration of 0.8 mg per mouse once is able to completely protect the mouse infected with lethal doses of Group B Streptococci. The ClyR can be used alone or in combination with different forms of reagents and solutions, for the control of a variety of Streptococci and for the treatment of infections caused by these bacteria. It has a broad application prospect.
Owner:PHAGELUX INC
Antigenic peptide fragments of VapA protein, and uses thereof
InactiveUS7169393B2Strong specificityLess false positivesBacteriaImmunoglobulins against bacteriaRhodococcus equiAntigen binding
An isolated peptide fragment of the VapA protein that binds antibodies specific for Rhodococcus equi and the VapA protein. In a preferred form the peptide contains an amino acid sequence of 5 or more amino acid residues that is identical to or homologous to the amino acid sequence of at least one region of the VapA protein that is responsible for immunological recognition. Methods of diagnosing a vertebrate for the presence of R. equi using the peptide and methods of vaccinating a vertebrate against R. equi using the peptide are also claimed.
Owner:RURAL INDS RES & DEV
Streptococcus equisimilis hyaluronan synthase gene and expression thereof in bacillus subtilis
The present invention relates to a nucleic acid segment having a coding region segment encoding enzymatically active Streptococcus equisimilis hyaluronate synthase (seHAS), and to the use of this nucleic acid segment in the preparation of recombinant cells which produce hyaluronate synthase and its hyaluronic acid product. Hyaluronate is also known as hyaluronic acid or hyaluronan.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Vaccine against streptococcal infections based on recombinant proteins
ActiveUS20130236469A1Organic active ingredientsBacterial antigen ingredientsAntigenStreptococcus mitis
An antigenic composition comprises several antigenic components derived from antigens of Streptococcus equi subsp. equi or subsp. zooepidemicus, wherein at least one component is a fusion protein or polypeptide comprising two or more such antigens or fragments thereof. The antigenic composition may be used for immunization of mammals against S. equi subsp. equi and / or subsp. zooepidemicus. A vaccine composition comprising the antigenic composition as immunizing component is also disclosed.
Owner:INTERVACC
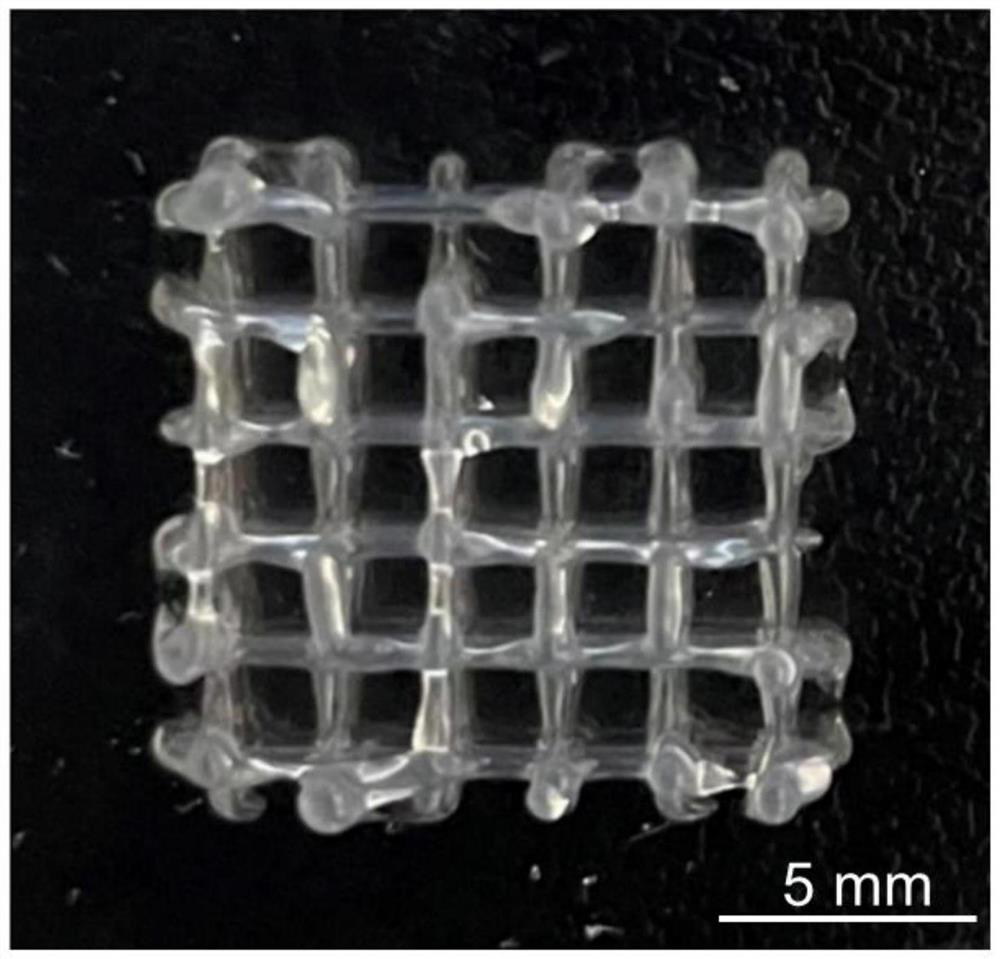
Method for immobilizing microorganism high-yield hyaluronic acid by using 3D printing technology
PendingCN114438067AImproves antioxidant activityIncrease productionBacteriaMicroorganism based processesBiotechnologyMethacrylate
The invention belongs to the technical field of hyaluronic acid preparation, and particularly relates to a method for high-yield hyaluronic acid by immobilizing microorganisms through a 3D printing technology, GelMA is prepared through a reaction of gelatin and a methacrylic acid esterification reagent, then Gel, GelMA and a photoinitiator are mixed to prepare bio-ink based on Gel-GelMA, and the bio-ink is applied to preparation of high-yield hyaluronic acid. Then, streptococcus equi subspecies for producing hyaluronic acid are uniformly loaded into bio-ink based on Gel-GelMA, then, an immobilized reactor loaded with microorganisms is prepared through a 3D printing technology, and finally, hyaluronic acid is produced in fermentation liquor containing carbon source and nitrogen source substances. The method provided by the invention can solve the defects of low yield, high cost and the like of the current planktonic microorganism direct fermentation method, improves the yield of hyaluronic acid, and can easily separate hyaluronic acid from surrounding media by utilizing a structure containing immobilized bacteria, thereby simplifying the acquisition process of hyaluronic acid.
Owner:SUN YAT SEN UNIV SHENZHEN +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com