Lyase capable of killing streptococcus equi subspecies. Equi and medical application of lyase
A technology of streptococcus equi and lyase, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Expression and purification of lyase
[0030] The primers for amplifying the lyase LysLF1 are:
[0031] Upstream 5'-CGGGATCCATGGCTACATATCAGGAATATAAAAGTC-3';
[0032] Downstream 5'-CGCTCGAGTTATACTTGTGTTGCATTAGAT-3';
[0033] Pass the amplified LysLF1 gene through the specific enzyme cutting site xho I and Bam H I was connected to the Pet28a vector to construct the expression vector pET28a-LysLF1. Transform the constructed vector into Escherichia coli BL21 competent, amplify in 500 mL of fresh LB medium, add 1 / 1000 kanamycin sulfate at the same time, and wait until the bacterial solution OD 600 When the value reached 0.6-0.8, 1 / 1000 IPTG (final concentration 1 mM) was added for induction, the induction temperature was 16°C, and the induction time was 16 h.
[0034] Use Ni-NTA to purify the protein by affinity chromatography: collect the induced bacterial solution (4°C, 10000 × g, 20min), resuspend it with an appropriate amount of Tris-Cl buffer (pH=7.5), and take ...
Embodiment 2
[0042] Determination of Antibacterial Ability of Lyase LysLF1 Solid Plate
[0043] Streptococcus equi subsp. equi 3518 in the logarithmic growth phase was evenly spread on the BHI solid medium plate in the ultra-clean bench, and after drying, 10 µL of the purified lyase LysLF1 was dropped on the plate and marked. In addition, 10 µL of Tris-Cl buffer was dropped on the plate as a control. Place the plate in a 37°C incubator for overnight culture, and observe the formation of the inhibition zone the next day.
[0044] The result is as figure 2 As shown, clear plaques appeared at the markers, indicating that the lyase LysLF1 has lytic activity against Streptococcus equi subsp. equi.
Embodiment 3
[0046] Determination of Bactericidal Activity of Lyase LysLF1 in Vitro
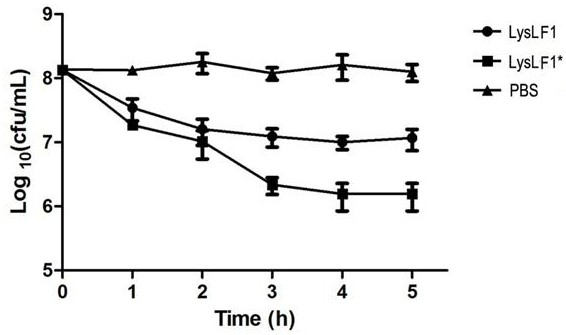
[0047] Streptococcus equi subsp. 600 Adjust the value to around 0.6. Positive control set two groups, LysLF1 treatment group added a single dose of LysLF1 with a final concentration of 100 µg / mL. The LysLF1* treatment group was added with lyase LysLF1 at a final concentration of 100 μg / mL every 10 min, and added 3 times continuously. An equal volume of Tris-Cl buffer was added to the negative control group. The three groups were incubated in a 37°C water bath at the same time, samples were taken every 10 minutes, and colonies were counted by doubling dilution. The experiment was carried out for 60 minutes and repeated three times.
[0048] The result is as image 3 As shown, the number of colonies in the negative control group remained basically unchanged after 60 min, and the number of colonies in the single-dose LysLF1 and multiple-dose LysLF1 treatment groups decreased significantly, and the multi-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com