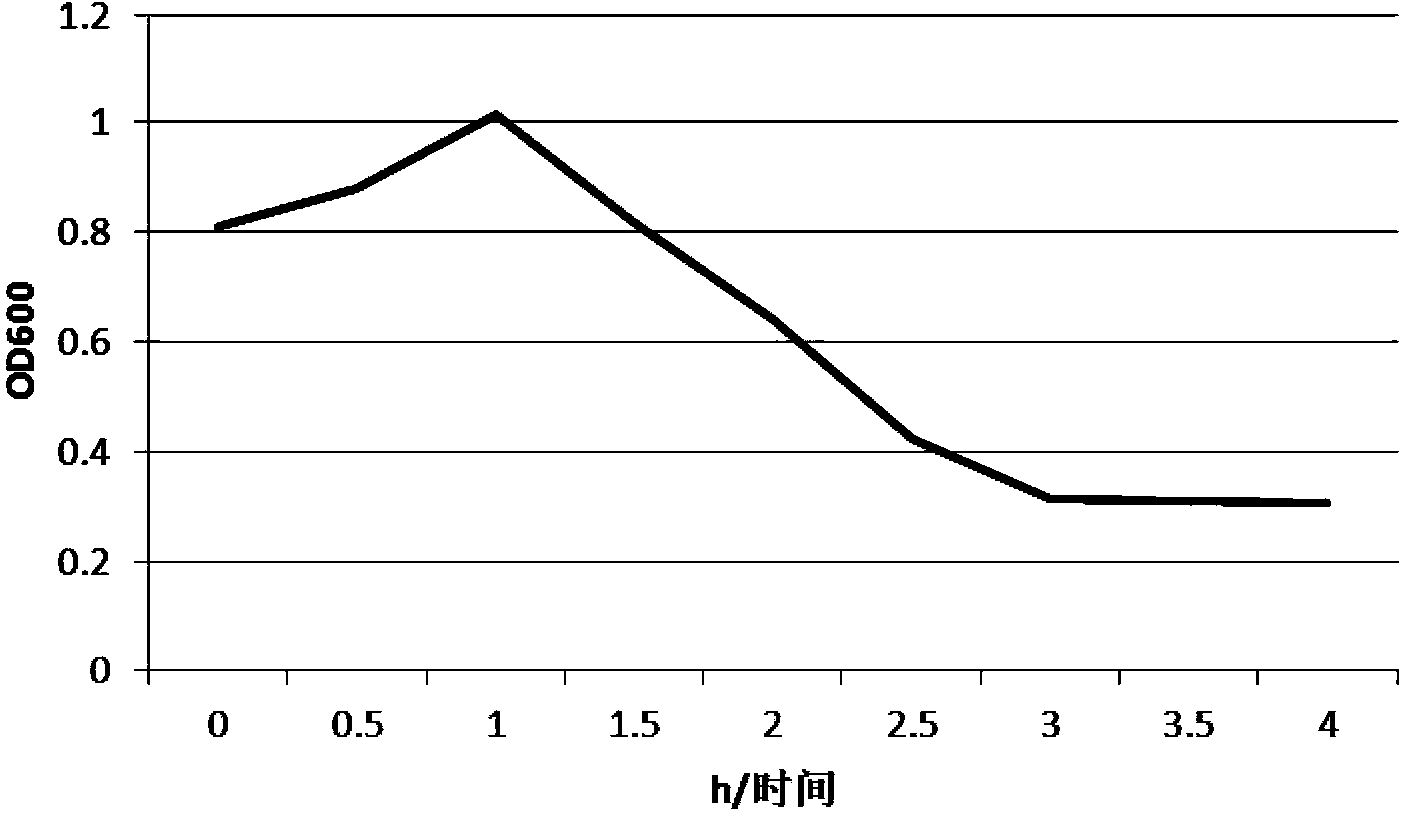
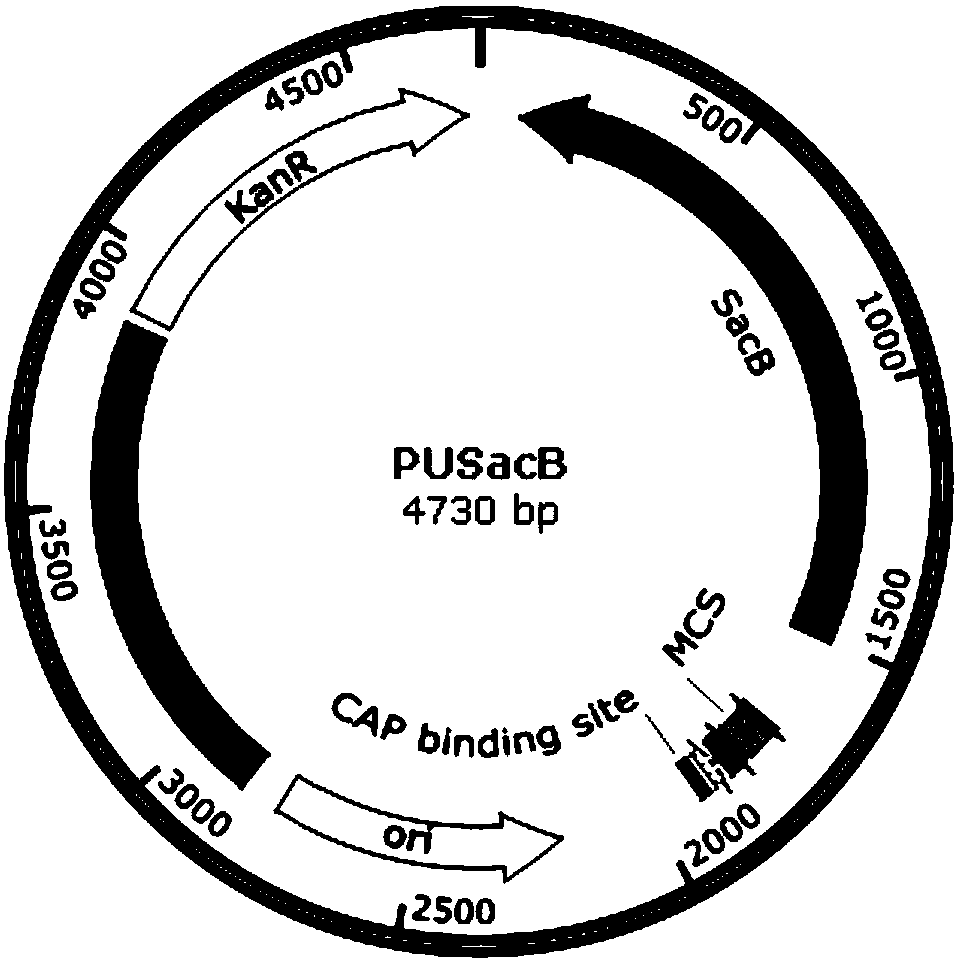
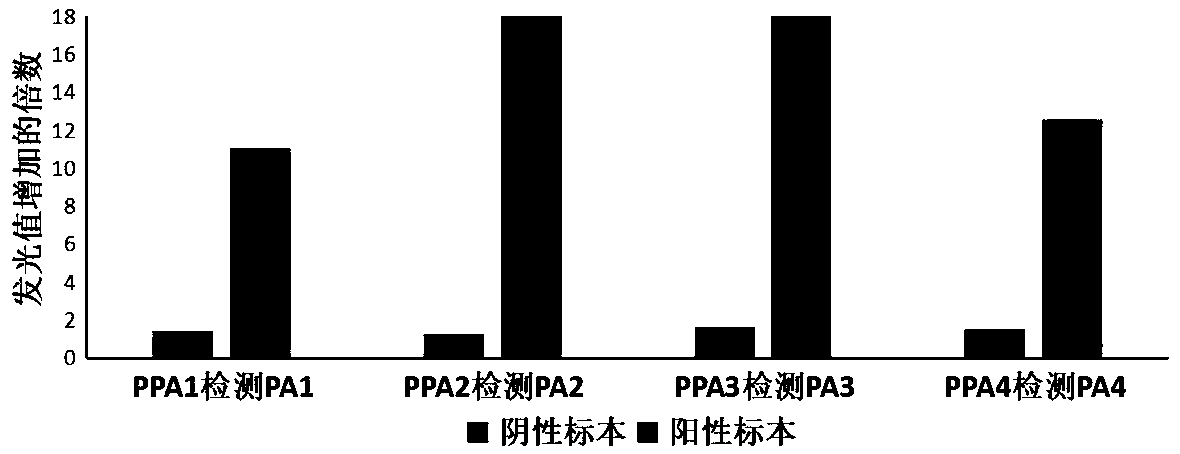
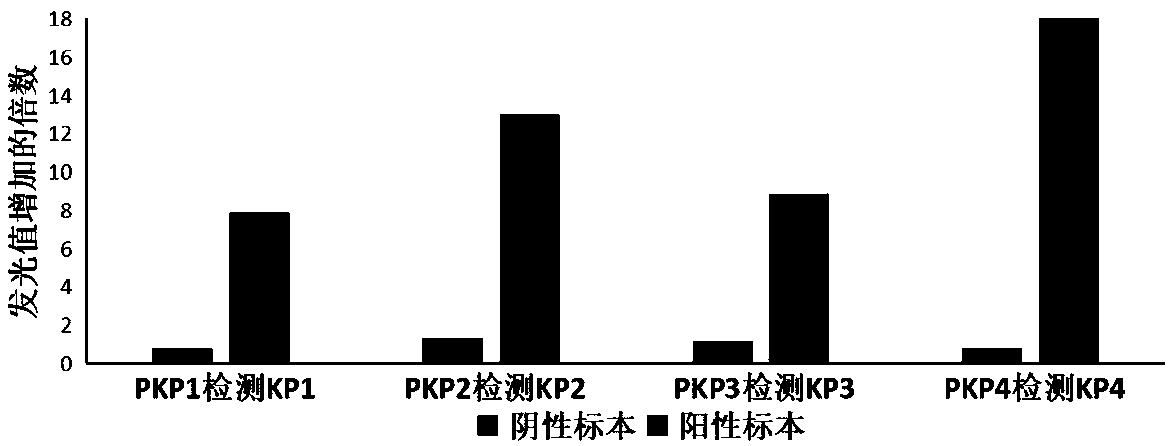
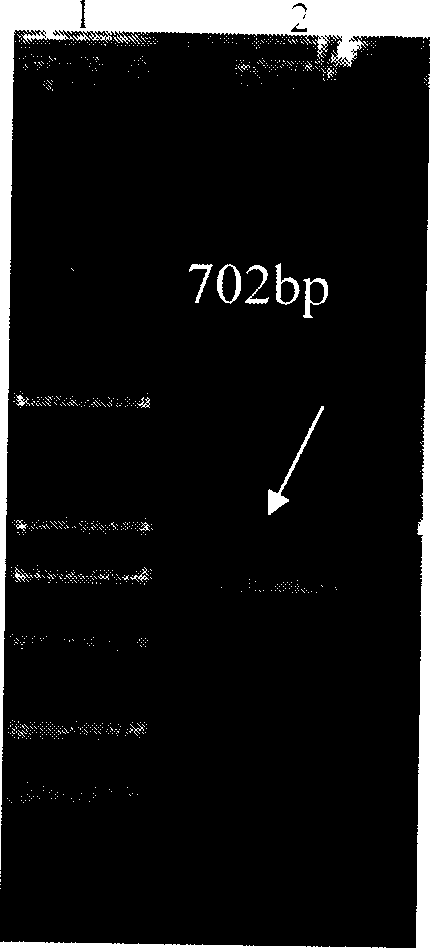
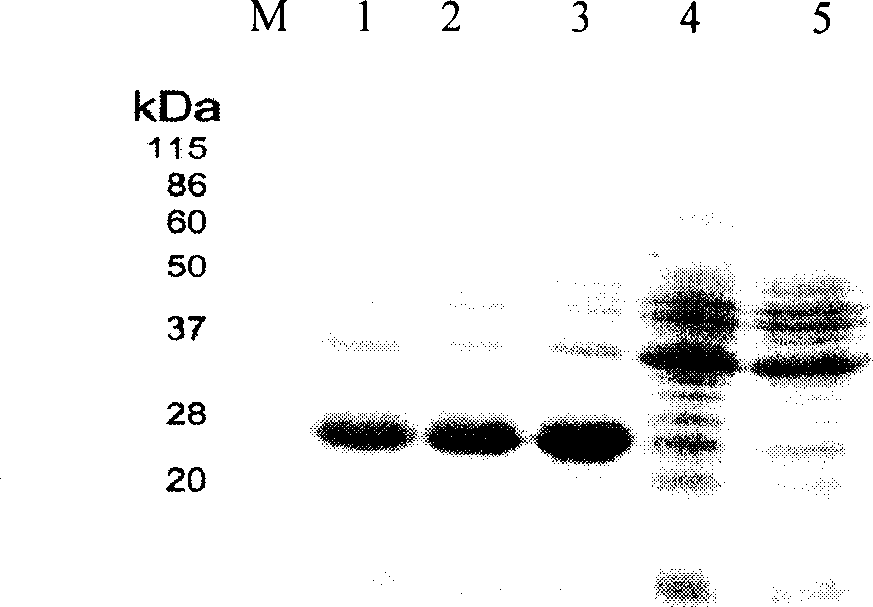
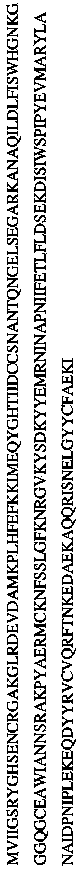Patents
Literature
72 results about "Phage lysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
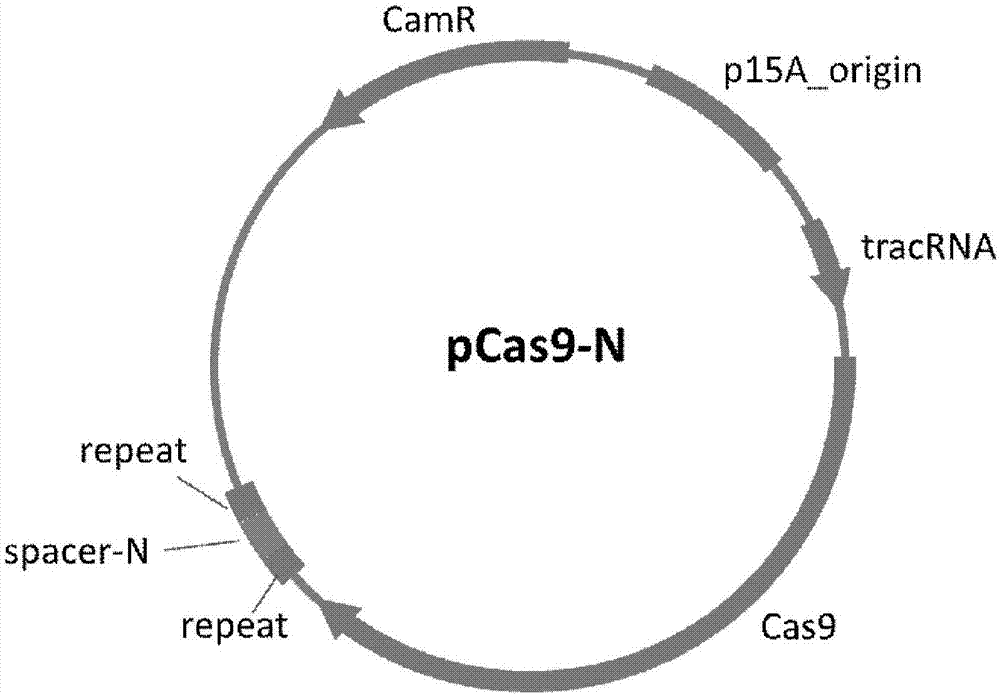
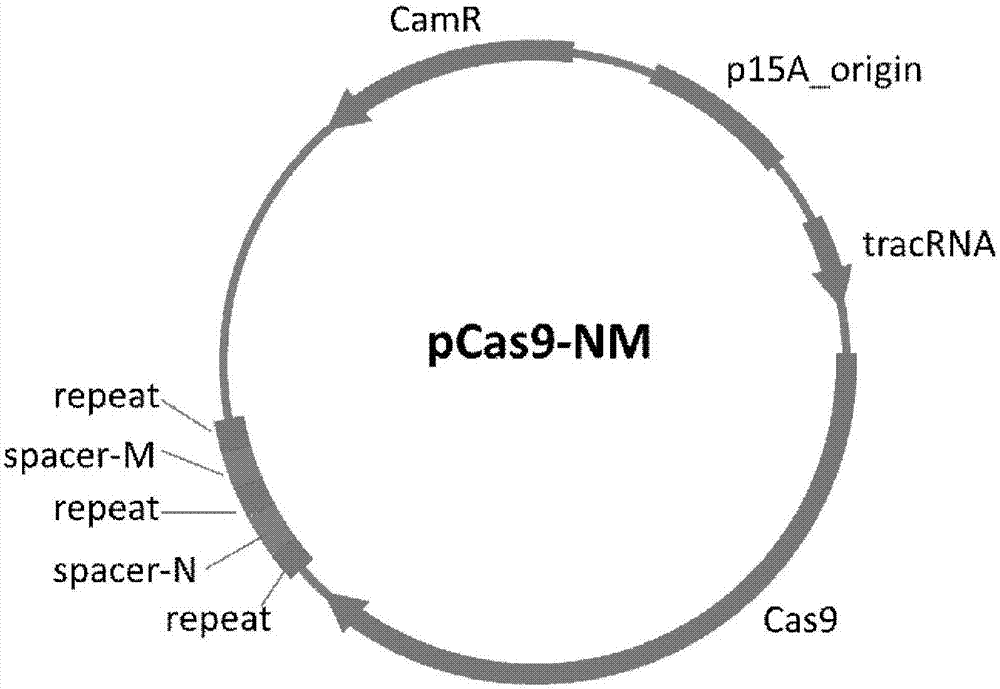
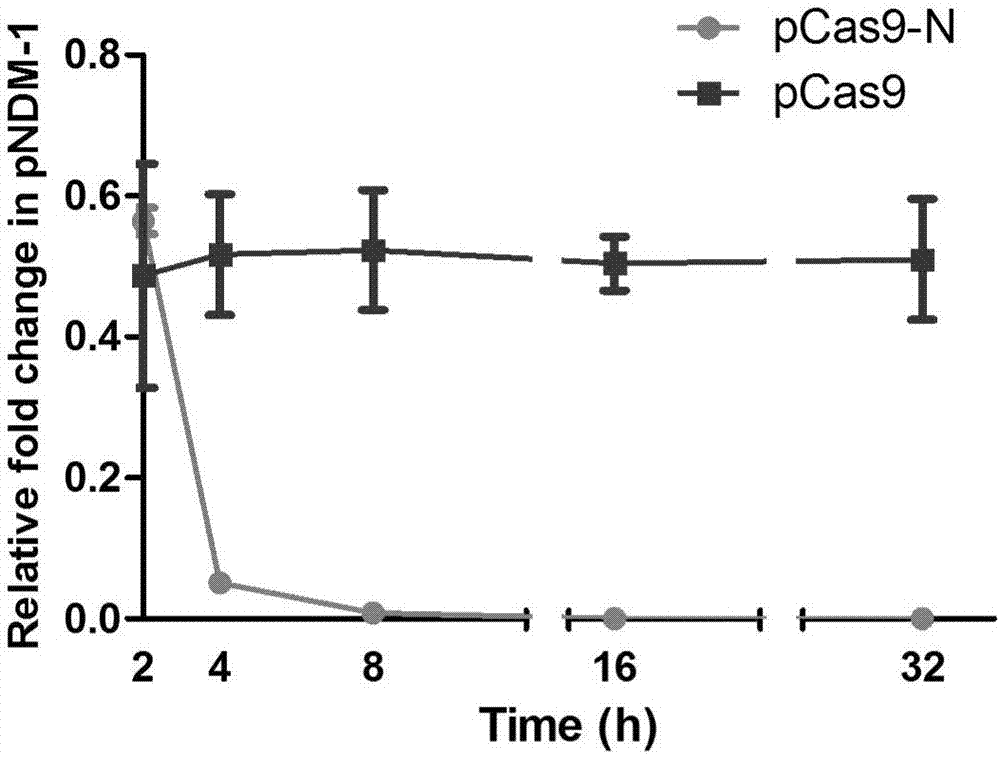
Method of packaging CRISPR-Cas9 (clustered regularly interspaced short palindromic repeat-associated 9) system by using temperate phage vector
ActiveCN107365804ADelay drug resistanceBlock horizontal transferHydrolasesStable introduction of DNAEscherichia coliPhage lysis
The invention discloses a method of packaging a CRISPR-Cas9 (clustered regularly interspaced short palindromic repeat-associated 9) system by using a temperate phage vector. The method comprises the steps of: (1) constructing suicide genes, target spot sequences bound with specific gRNA (guide ribonucleic acid), and downstream PAM (protospacer adjacent motif) sequences into pSTK (protein serine threonine kinase) plasmids, (2) transforming the pSTK plasmids into escherichia coli host bacteria, (3) transforming CRISPR-Cas9 sequence recombination template double-chain DNA (deoxyribonucleic acid) linear fragments carrying phage sequence homologous arms at the two ends into the host bacteria, (4) inducing expression of homologous recombination related enzymes and the suicide genes SacB, (5) screening the host bacteria subjected to homologous recombination, and (6) inducing temperate phages to crack the host bacteria, and harvesting the recombined temperate phages packaging the CRISPR-Cas9 system, wherein chromosomes of the escherichia coli host bacteria are integrated with the temperate phages; and plasmids capable of expressing the homologous recombination related enzymes are transformed into the escherichia coli host bacteria. According to the packaging method, a secondary recombination step of deleting a resistance marker is removed, and the technical support is provided for the phage vector presenting CRISPR-Cas9 system to resist drug-resistance bacteria efficiently and quickly.
Owner:INST OF PLA FOR DISEASE CONTROL & PREVENTION
Phage lytic enzymes as well as gene, gene recombinant expression vector and application thereof
ActiveCN112143747AGood lytic activityPromote infectionAntibacterial agentsBacteriaOrganomercurial lyaseNucleotide
The invention discloses phage lytic enzymes as well as a gene recombinant expression vector and application thereof. The phage lytic enzyme expression gene is a nucleotide sequence shown as SEQ ID NO:1, and the nucleotide sequence can express phage lytic enzymes in a host cell by constructing a recombinant vector. The phage lytic enzymes obtained through purification have relatively high in-vitroand in-vivo bactericidal activity, acts quickly, is safe and harmless to organisms, can well treat multi-drug-resistance shigella infection, and also has a certain inhibition effect on staphylococcusaureus, vibrio parahaemolyticus and other pathogenic bacteria. The phage lytic enzymes have good lytic thallus activity within the range of 28-42 DEG C, and due to the characteristics, the phage lytic enzymes have a wide prospect in the aspect of preparing drug-resistant infectious disease medicaments and can be used as a substitute or supplement of conventional antibiotics.
Owner:KUNMING UNIV OF SCI & TECH
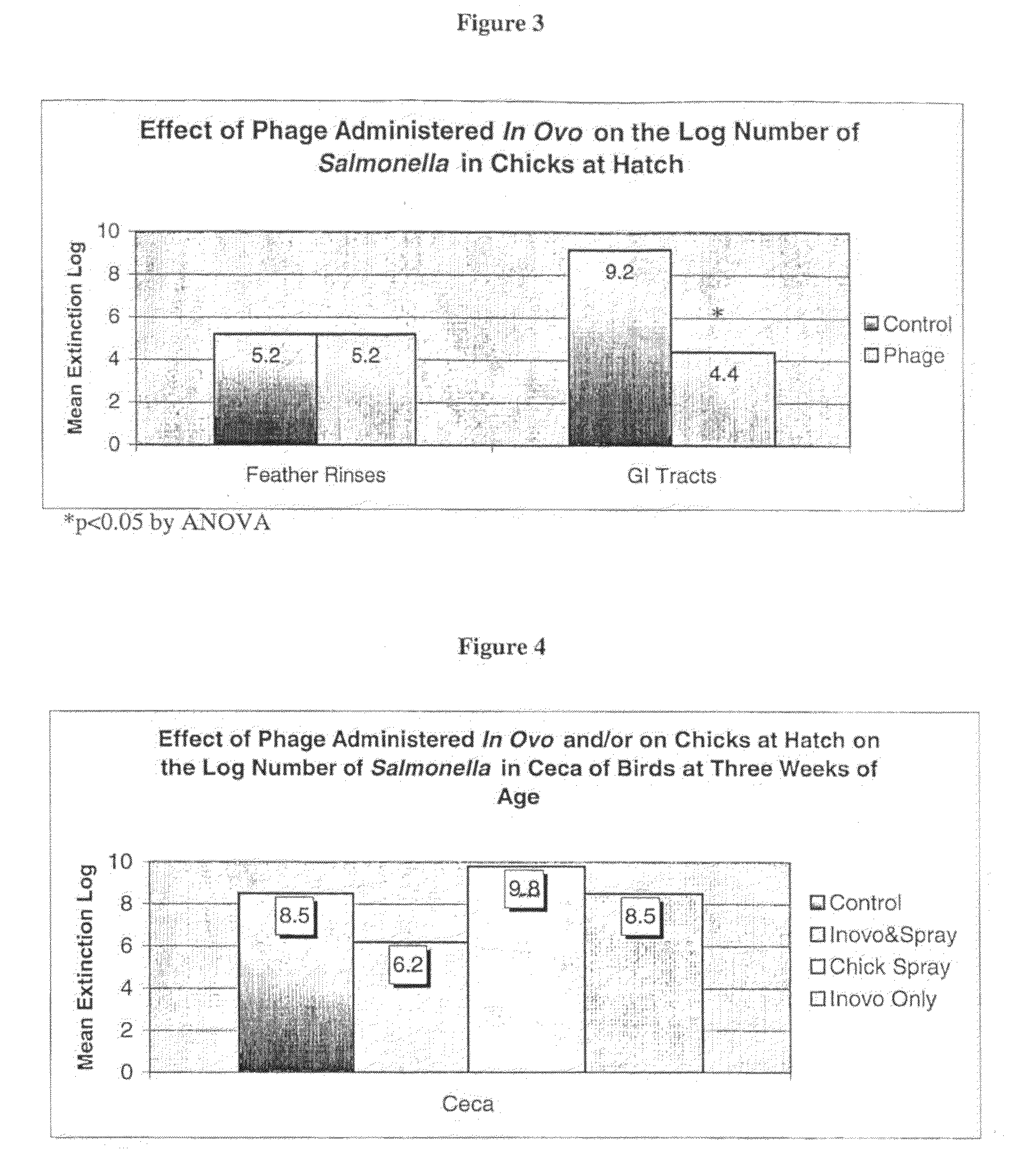
Method for vaccination of poultry by bacteriophage lysate bacterin
InactiveUS20090297561A1Bacterial antigen ingredientsViral antigen ingredientsVaccinationInoculation methods
The invention provides methods of generating phage lysate bacterins, as well as phage lysate bacterin compositions. The invention further encompasses methods of vaccination comprising administering phage lysate bacterin to an animal in need thereof. The invention further encompasses methods of reducing infection or colonization of poultry or poultry eggs using phage bacterin lysates.Method of vaccination comprising administering to an animal in need of immunization an amount of phage lysate bacterin to induce an immune response.
Owner:INTRALYTIX
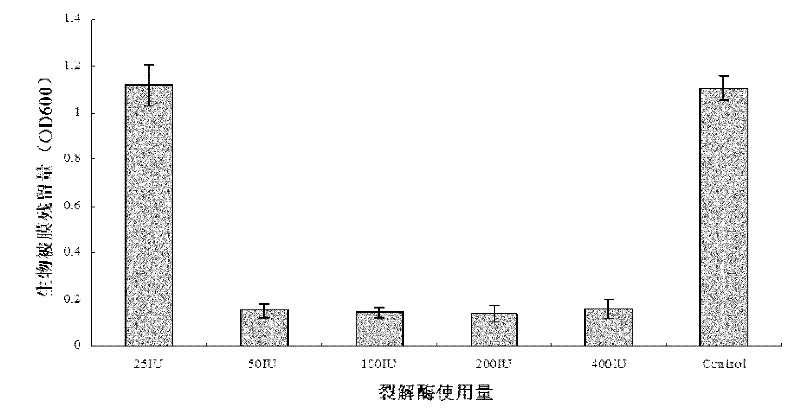
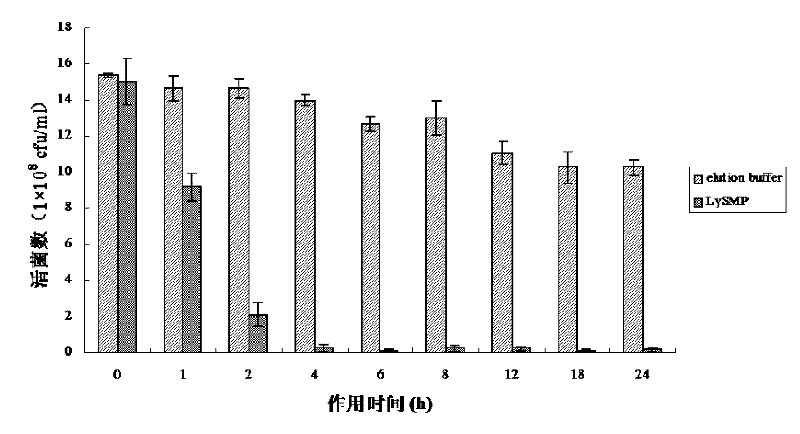
Method for degrading streptococcus suis biofilm by applying phage lyase
ActiveCN102198265AEfficient lysisClean up thoroughlyAntibacterial agentsPeptide/protein ingredientsBiofilmLyase
The invention provides a method for degrading a streptococcus suis biofilm by applying phage lyase, belonging to the field of biotechnology. The method comprises the following steps of: expressing lyase LySMP of which the molecular weight is 55kDa by adopting expression bacteria BL21-lys, purifying with a Ni column to obtain lyase, and degrading the streptococcus suis biofilm obtained by culture on a cell culture plate by using the lyase in vitro. The method provided by the invention can sterilize streptococcus suis SS2-4 and SS2-H and can simultaneously destroy the structure of the biofilm to achieve the effect on thoroughly clearing the biofilm.
Owner:SHANGHAI JIAO TONG UNIV
Bacteriophage lytic enzymes as alternative antimicrobials
Owner:US SEC AGRI
Bacillus cereus phage lyase, preparation method and application thereof
ActiveCN108531469AReduced lytic activityEfficient cracking activityAntibacterial agentsBacteriaFood borneNucleotide
The invention relates to the bioengineering field, in particular to application of a phage lyase PlyHSE3 of food-borne pathogen bacillus cereus. The lyase involved in the invention has a nucleotide sequence shown as SEQ NO 1 and an amino acid sequence shown as SEQ NO 2. The phage lyase has high temperature tolerance and pH tolerance, can be used for prevention and control of bacillus cereus florabacteria, and the lyase can prevent and control pathogenic bacillus cereus under the condition of 4DEG C to 45DEG C. In addition, the lyase can also split pseudomonas aeruginosa. The invention provides a new enzyme preparation source for prevention and control of food-borne pathogen bacillus cereus, other bacillus cereus flora bacteria and pseudomonas aeruginosa at present.
Owner:HAINAN NORMAL UNIV +1
Staphylococcus aureus bacteriophage lyase as well as preparation method and application thereof
InactiveCN111808837ABroad-spectrum antibacterialGood antibacterial effectAntibacterial agentsBacteriaOrganomercurial lyaseNucleotide
The invention discloses a staphylococcus aureus bacteriophage lyase as well as a preparation method and application thereof, and relates to the technical field of bioengineering. The invention provides a staphylococcus aureus bacteriophage lyase LysSA2, and the amino acid sequence of the LysSA2 is Seq ID NO.1; and the invention provides an encoding gene of the staphylococcus aureus bacteriophage lyase LysSA2, and the nucleotide sequence of the encoding gene is Seq ID NO.2. The invention also provides a preparation method of the staphylococcus aureus bacteriophage lyase LysSA2. The method clones staphylococcus aureus bacteriophage SA2 to obtain the bacteriophage lyase LysSA2, and carries out induced expression on soluble lyase by adopting a prokaryotic expression mode. The bacteriophage lyase LysSA2 not only has a relatively strong bacteriostatic effect on staphylococcus, but also has a broad-spectrum bacteriostatic effect on staphylococcus from different sources, and lays a foundationfor treating and inhibiting staphylococcus diseases.
Owner:QINGDAO PHAGEPHARM BIO TECH CO LTD
Genetic toxicant detection vector and detection method
InactiveCN106636164ANo risk of diseaseEasy to operateBacteriaMicrobiological testing/measurementLysisToxicant
The invention discloses a genetic toxicant detection vector and a detection method. The vector is a prokaryotic expression vector connected with a genetic toxicity response promoter, a bacteriophage lytic gene and an escherichia coli terminator in sequence from the end 5' to the end 3'. The detection method comprises the following steps: introducing the genetic toxicant response vector into escherichia coli to obtain recombinant bacteria, and then incubating the recombinant bacteria and a genetic toxicant to realize escherichia coli cell lysis, wherein the escherichia coli recombinant bacteria carry the genetic toxicant response vector; when the recombinant bacteria are in contact with the chemical genetic toxicant, cell lysis occurs in the recombinant bacteria, so that the method for quantitatively detecting the genetic toxicant according to the lysis efficiency is realized. The method is low in time consumption, high in detection sensitivity, easy and convenient for detection, feasible, low in cost and easy to popularize.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of bacteriophage lyase capable of lysing escherichia coli and salmonella
InactiveCN106854247ANon-deterministicHigh activityAntibacterial agentsPeptide/protein ingredientsPolymyxin BPhage lysis
Belonging to the field of animal biomedical engineering, the invention specifically discloses a preparation method of a bacteriophage lyase capable of lysing escherichia coli and salmonella. Through primer design, a lyase gene of bacteriophage ECGD1 is successfully amplified by PCR, and is transferred into an expression system to obtain a recombinant strain. IPTG induction is carried out on the recombinant strain to obtain expression, then the strain is lysed to obtain a soluble recombinant lyase of bacteriophage ECGD1, the recombinant lyase can lyse a plurality of escherichia coli and salmonella, i.e. having a wide lysis spectrum. In addition, the synergistic effect result of the recombinant lyase and polymyxin B shows that the existence of the recombinant lyase lowers the minimum inhibitory concentration of polymyxin B, i.e. the two have a synergistic bacteriostatic effect. Identification on the lysis activity of the recombinant lyase and identification on the wide lysis spectrum thereof reveal that the recombinant lyase of the bacteriophage ECGD1 has potential application value in prevention and control of escherichia coli and salmonella infection.
Owner:SOUTH CHINA AGRI UNIV
Lyase of bacteriophage and sterilization application
InactiveCN105543256AGood water solubilityBroad antibacterial spectrumAntibacterial agentsCosmetic preparationsBacteroidesSide effect
The invention relates to lyase of bacteriophage and sterilization application. The amino acid sequence of the lyase is Seq ID NO.1, the nucleotide sequence of the lyase is Seq ID NO. 2, and hand sanitizer containing the lyase is also provided. The bacteriophage lysine (Endolysin) has the characteristics of high efficiency, a wide spectrum and the like and can be applied in medical treatment and public health, food processing and sanitary products as antibacterial active components. The enzyme preparation can be independently used or used in a compounding mode, bacteria can be specifically inactivated, and a safe enzyme preparation source which is free of toxic and side effects is provided for current bacterial infection prevention and food-derived bacterial contamination prevention.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Bacteriophage lysin with improved antibacterial effect
The invention discloses a bacteriophage lysin with animproved antibacterial effect. The 99th leucine and the 102nd methionine of lyase Bp7e amino acid are respectively mutated to obtain alanine and glutamic acid, by using a prokaryotic expression technique, mutant protein can be obtained, and the mutant protein is identified by using Western-blot and is named Bp7c mutant protein. Bp7e and Bp7e mutant protein are purified, the concentration of Bp7e and Bp7e mutant protein is detected, and the in-vitro pyrolysis experiment and the pyrolysis spectrum detection show that purified Bp7e and Bp7e mutant protein have a broad-spectrum antibacterial function and have pyrolysis effects on micrococcus lysodeikticus, staphylococcus aureus, salmonellae and multiple serotype escherichia colis, and the pyrolysis effect of the Bp7e mutant protein is wholly superior to that of natural lyase Bp7e.
Owner:QINGDAO AGRI UNIV
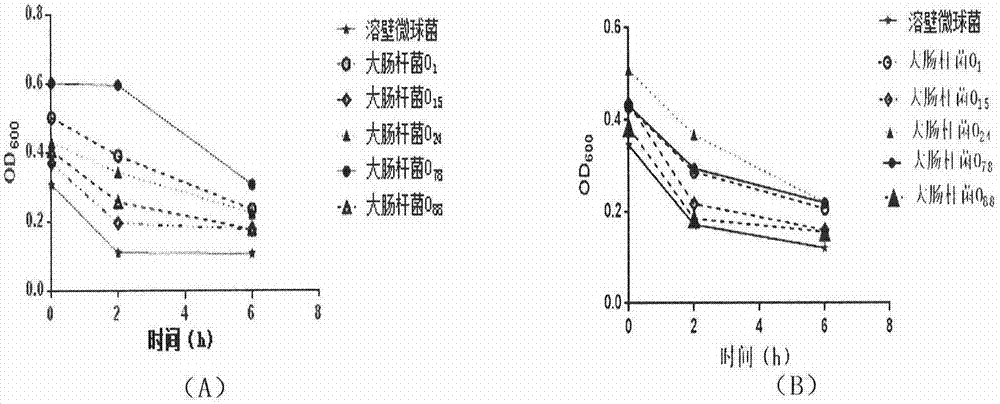
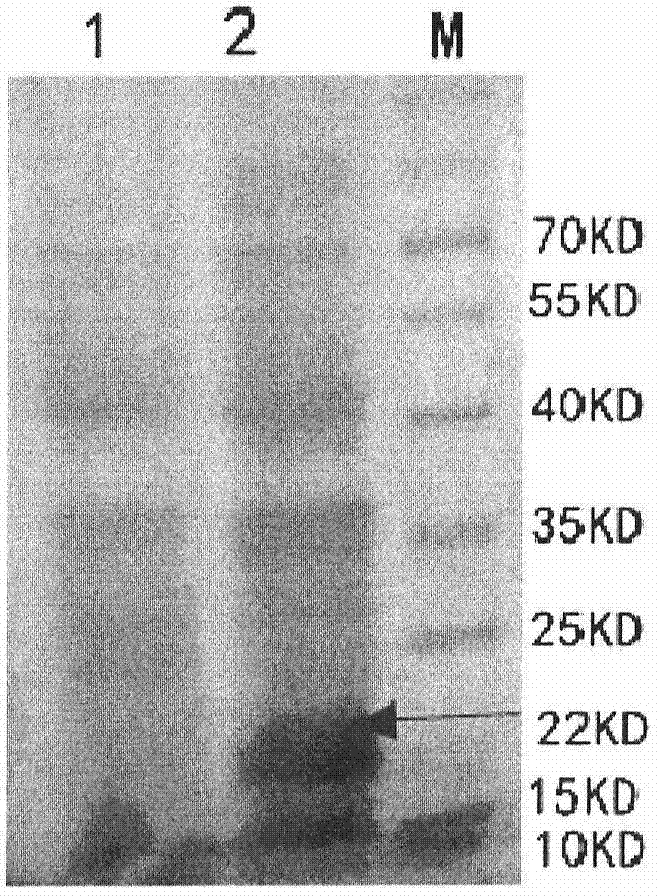
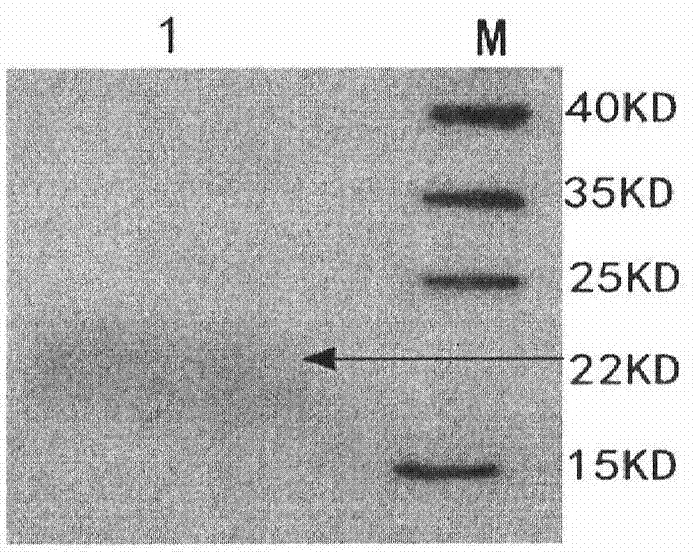
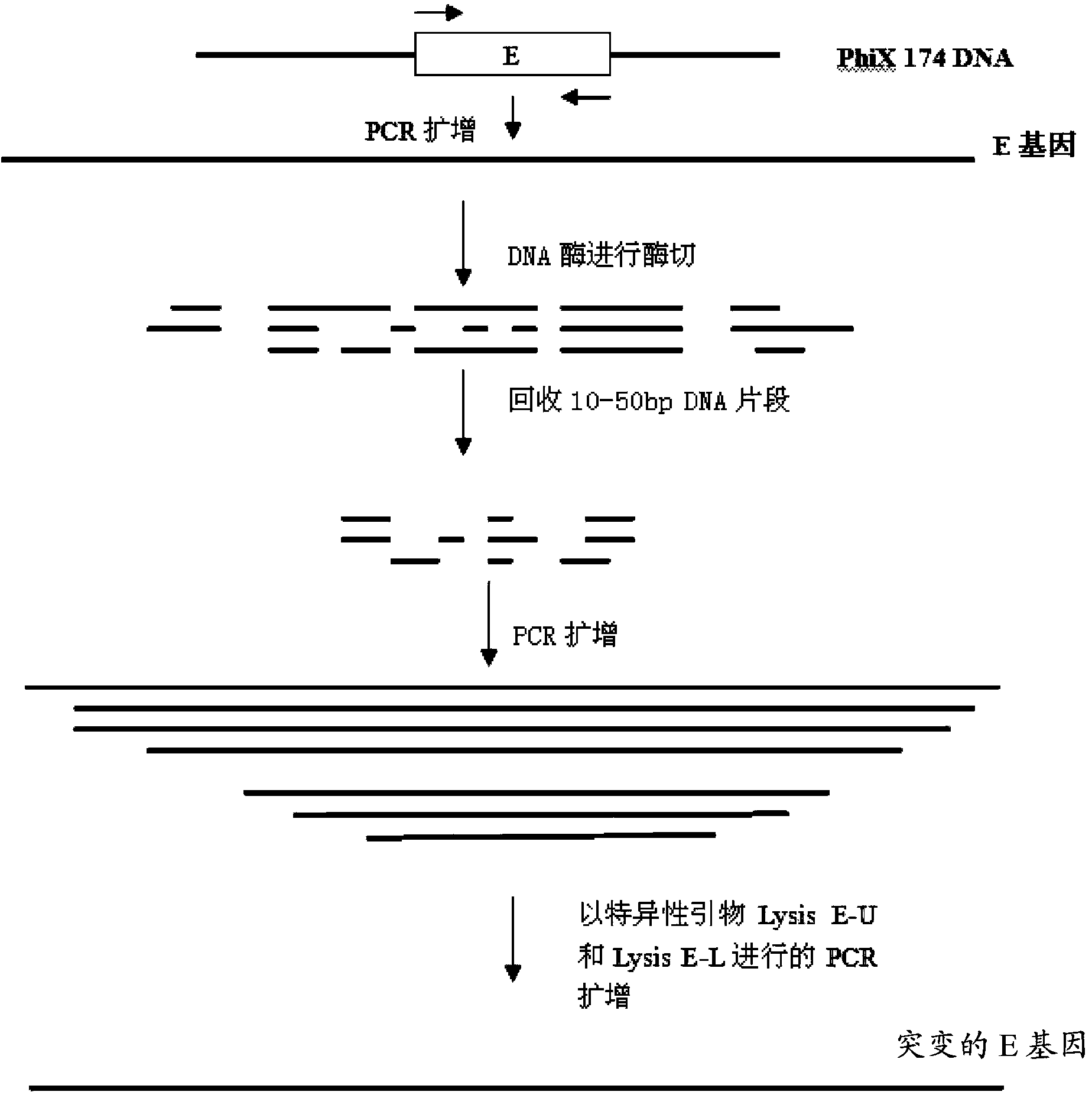
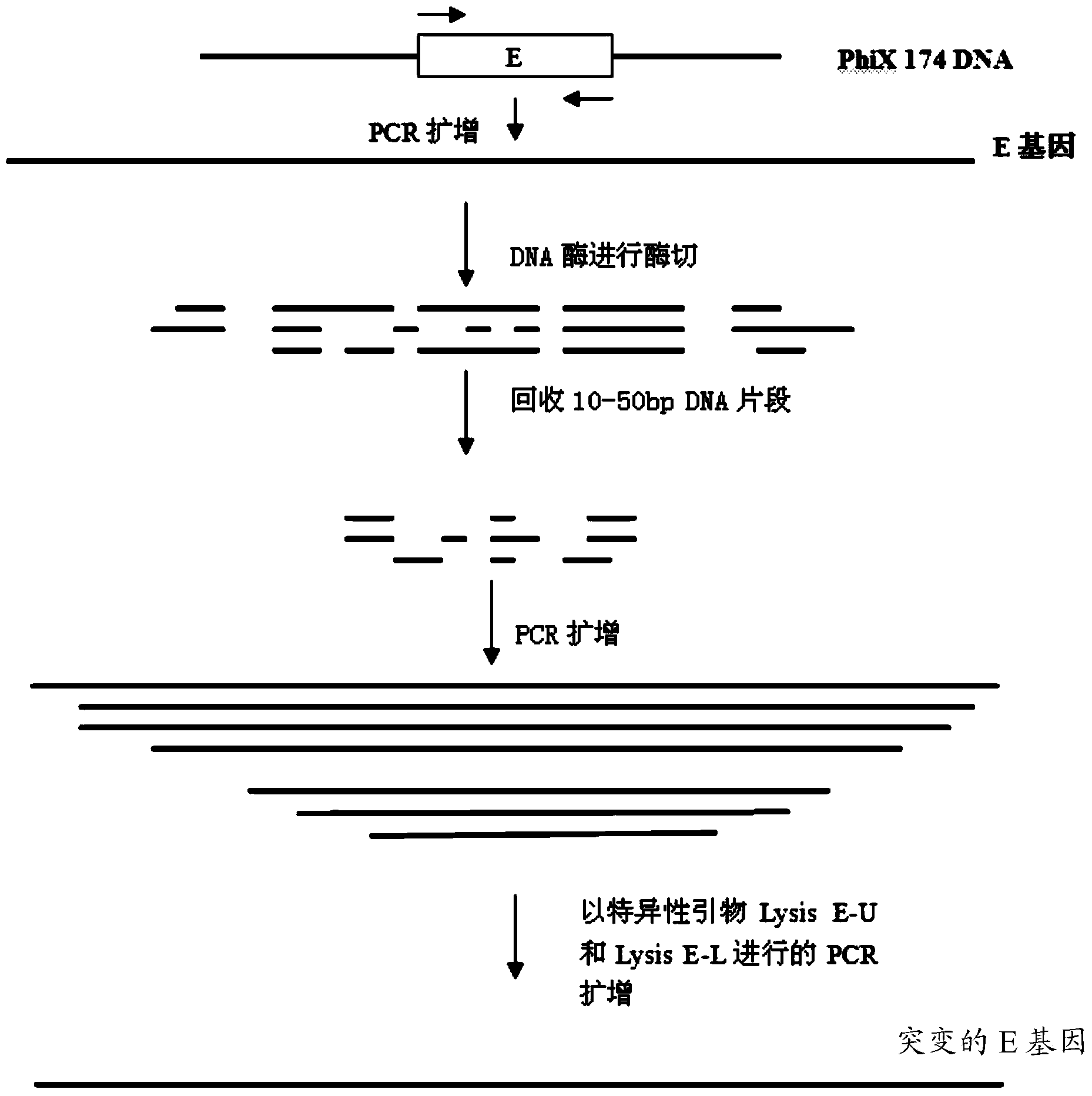
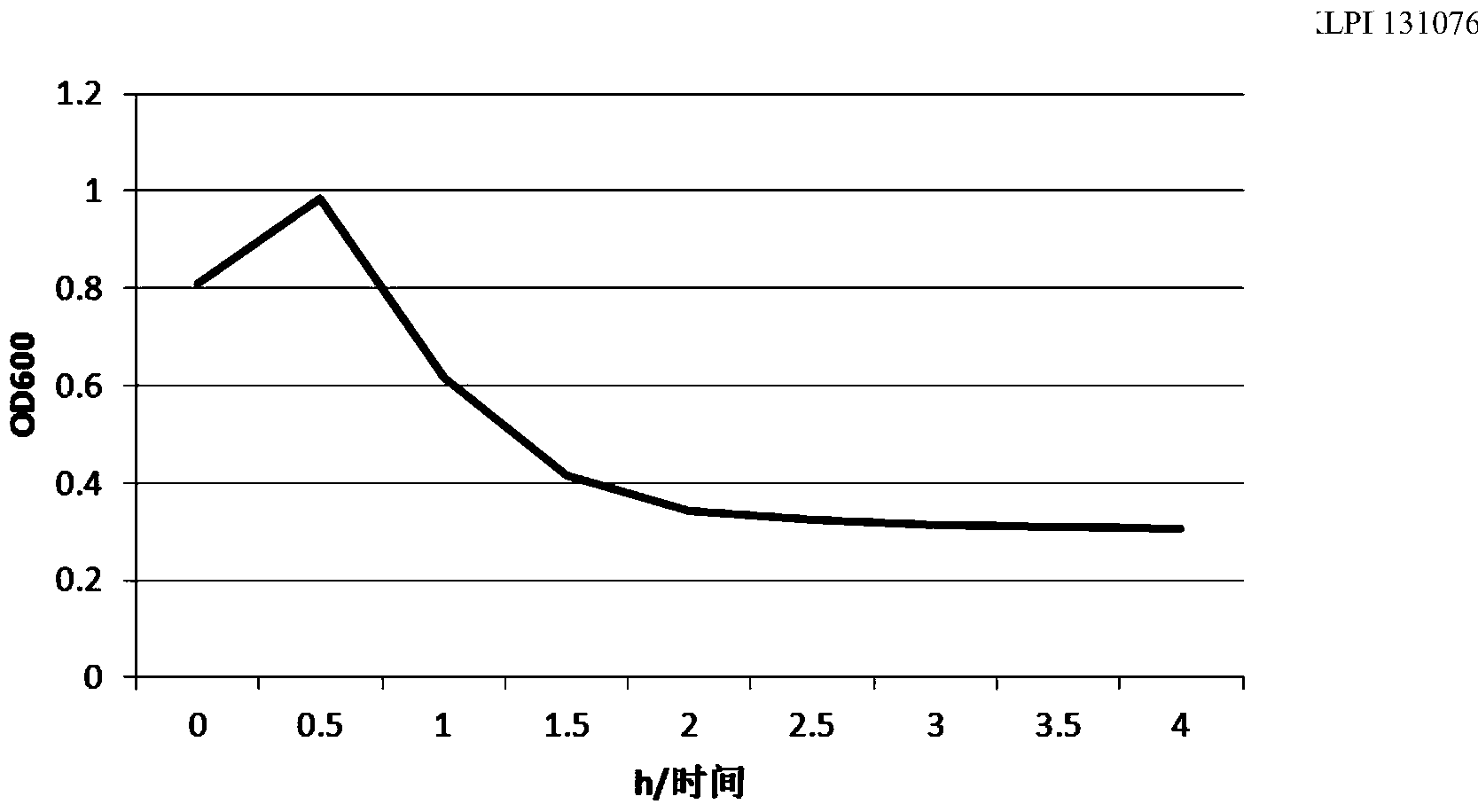
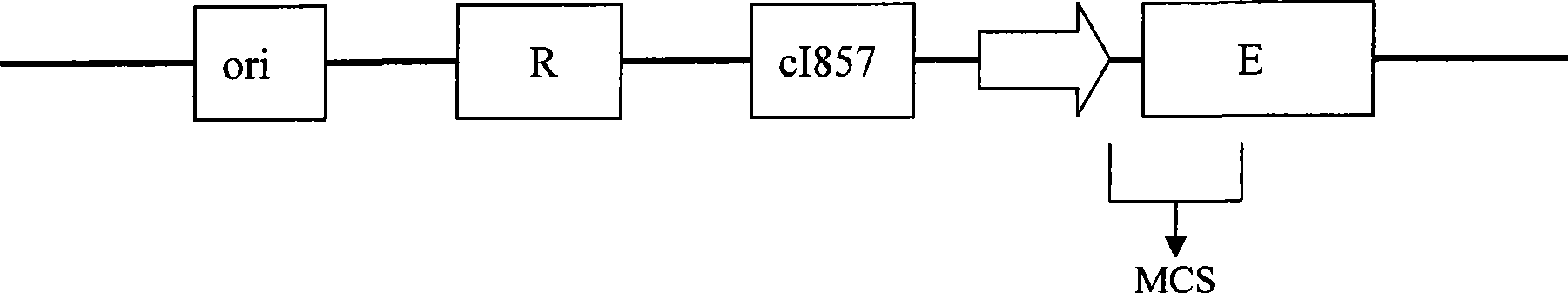
Mutant phage lysis gene E, lysis plasmid vector containing lysis gene and application in preparation of bacterial ghost vaccines
InactiveCN103451195ARepress transcriptionImprove cracking efficiencyAntibacterial agentsBacterial antigen ingredientsCulture bacteriaOrganism
The invention discloses a mutant phage lysis gene E, a lysis plasmid vector containing the lysis gene and an application in preparation of bacterial ghost vaccines. The mutant phage lysis gene E (Eprom) disclosed by the invention is obtained after mutation of a promoter region of the lysis gene E of a phage phiX174, the E gene after mutation can turn the temperature of culture bacteria from the existing 28 DEG C to 37 DEG C, the mutant phage lysis gene E further has higher lysis efficiency, higher starting induction concentration and large-scale production capacity, and the culture lysis efficiency of a fermentation tank is as high as 99.99997%. After the Eprom is connected with pBV220, the high-efficient lysis plasmid vector pBV-Eprom can be obtained. The pBV-Eprom is transformed into actinobacillus pleuropneumoniae to induce the gene expression of the Eprom so as to get a bacterial ghost of the actinobacillus pleuropneumoniae. Porcine transmissible pleuropneumonia bacterial ghost vaccines disclosed by the invention has good safety and immune protection efficacy and can stimulate organisms to produce high-titer antibodies and further provide good cross immunoprotection against attacks of different serotypes of actinobacillus pleuropneumoniae virulent strains.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Co-expression system and construction method of polyvalent bacteriophage lyase genes, live vaccine of carrying system and preparation and application of live vaccine
InactiveCN104673821AEnables cheap scalingNo autolysisAntibacterial agentsBacterial antigen ingredientsMycobacterium smegmatisLatent tuberculosis
The invention discloses a co-expression system and construction method of polyvalent bacteriophage lyase genes, a live vaccine of a carrying system and preparation and application of the live vaccine. Eukaryotic expression plasmids are used as an expression vector, and LysinA, LysinB and Holin gene segments are directionally inserted into the plasmids to simultaneously express the co-expression system of the bacteriophage lyase genes of three kinds of targeted mycobacterium tuberculosis. Mycobacterium smegmatis with good targeting property of macrophages or genetically-modified recombinant BCG is used as a live vector, the co-expression system simultaneously carrying three kinds of genes is electrically transformed into the mycobacterium smegmatis or the genetically-modified recombinant BCG, and then the recombinant therapeutic tuberculosis live vaccine is obtained through expansion in vitro. The live vaccine has a good effect on the field of curing active tuberculosis or latent tuberculosis infection caused by proliferative mycobacterium tuberculosis, dormant mycobacterium tuberculosis and drug-resistant mycobacterium tuberculosis.
Owner:伊正君
DNA loaded Brucella ghost composite vaccine
InactiveCN108690823ALittle side effectsImprove securityAntibacterial agentsBacterial antigen ingredientsSide effectA-DNA
The invention discloses a DNA loaded Brucella ghost composite vaccine. The preparation method comprises following steps: introducing a suicide plasmid that contains a nucleic acid molecule encoding atemperature sensitive regulatory protein cI857, a nucleic acid molecule encoding a bacteriophage splitting protein E, and a nucleic acid molecule encoding a bacterial nuclease protein A into Brucella;utilizing a homologous recombination technology to obtain recombinant Brucella; culturing the recombinant Brucella to obtain a bacterial solution, processing the bacterial solution at a high temperature, collecting bacterial cells, and adding target DNA to obtain the DNA loaded Brucella ghost composite vaccine. The composite vaccine has following advantages: (1) the vaccine has the characteristics of bacterial ghost, compared with a conventional killed vaccine or an attenuated live vaccine, the side effect of the composite vaccine is small, the safety is high, and the protection effect is good; and (2) the bacterial ghost is a safe and effective carrier for delivering DNA vaccines, can introduce nucleic acid vaccines into antigen presenting cells, and performs high efficient expression. The composite vaccine has an important meaning for controlling the epidemic spreading of brucellosis and has a wide application range.
Owner:INNER MONGOLIA HUAXI BIOTECH
Peptidoglycan hydrolase antimicrobials for eradicating lactobacilli that contaminate and reduce ethanol yields in biofuel fermentation
ActiveUS9068204B2Unwanted contaminationIncrease lytic activityBacteriaBiofuelsBiotechnologyBacteroides
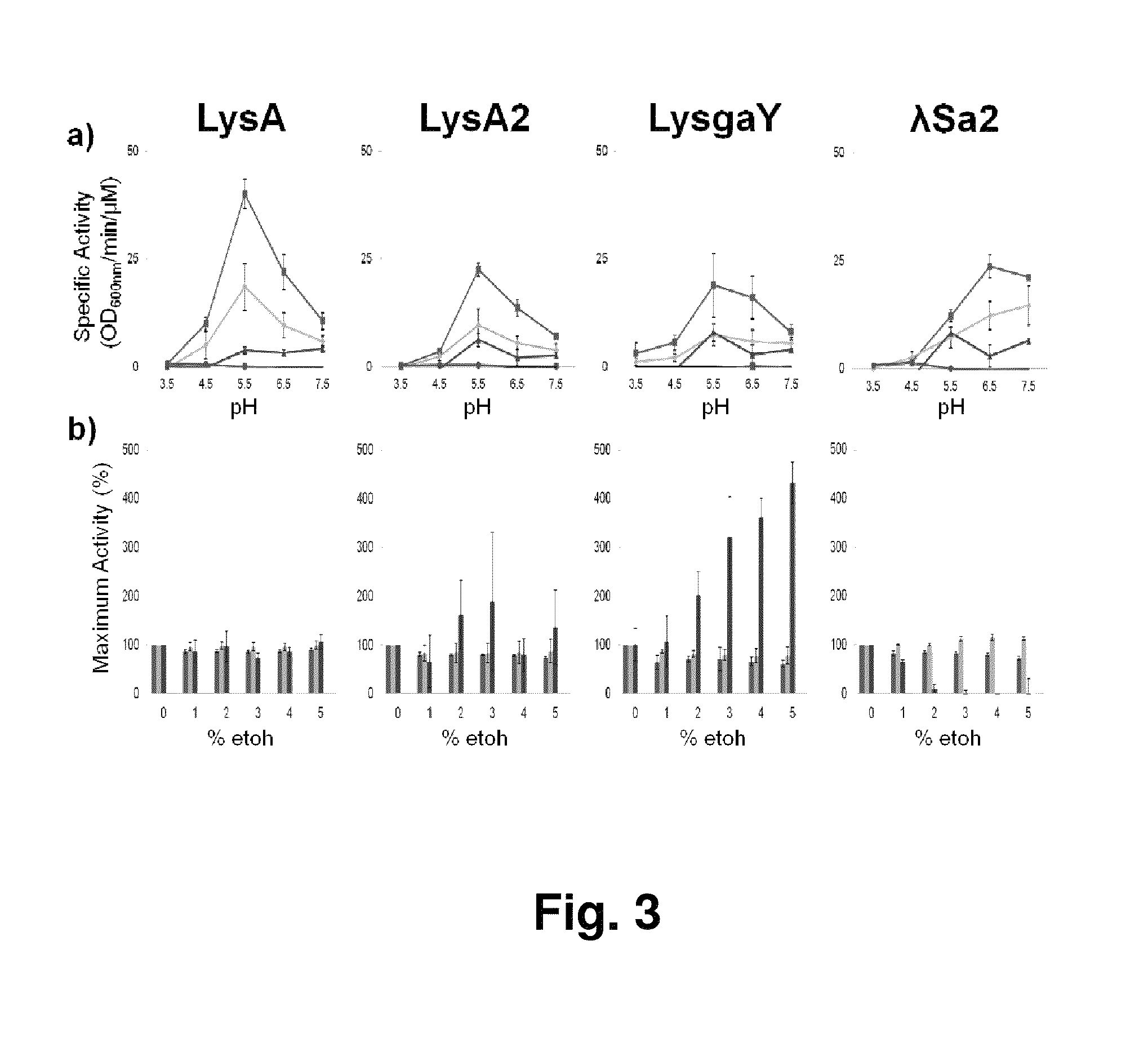
Ethanol losses due to bacterial contamination in fermentation cultures weakens the economics of biofuel production. Lactobacillus species are the predominant contaminant. Bacteriophage lytic enzymes are peptidoglycan hydrolases which degrade Gram positive cell walls when exposed externally and are a novel source of antimicrobials. The streptococcal phage λSA2 endolysin construct demonstrated strong lytic activity towards 17 of 22 strains of lactobacilli, staphylococci or streptococci maintaining optimal specific activity under fermentation conditions toward L. fermentum substrates. Lactobacillus bacteriophage endolysin constructs LysA, LysA2 and LysgaY showed exolytic activity towards ˜60% of the lactobacilli tested including four L. fermentum isolates from fuel ethanol fermentations. Presence of ethanol (≦5%) did not affect lytic activity. Lysins were able to reduce both L. fermentum and L. reuteri contaminants in mock fermentations of corn fiber hydrolysates. Recombinant LysA and λSa2 expressed in the yeast Saccharomyces cerevisiae are functional; LysA was shown to reduce lactobacilli in experimentally infected fermentations.
Owner:UNITED STATES OF AMERICA
Genetically engineered lysin with function of killing staphylococcus, as well as preparation method and application thereof
InactiveCN110684760AEfficient killingBroad cracking spectrumAntibacterial agentsPeptide/protein ingredientsStaphylococcus cohniiOrganomercurial lyase
The invention discloses a genetically engineered lysin with a function of killing staphylococcus and a preparation method. According to the preparation method, a lysin LysGH15 is subjected to genetically engineered modification to obtain a novel bacteriophage lysin LysGH15(+). The bacteriophage lysin LysGH15(+) shows stronger bactericidal activity and wider host spectrum, in comparison with a lysin before modification, can kill staphylococcus aureus, staphylococcus epidermidis, staphylococcus haemolyticus, staphylococcus hominis, staphylococcus saprophyticus, staphylococcus cohnii, fermented staphylococcus xylosus and the like, provides a potential novel medicine to prevention and treatment of diseases caused by staphylococcal infection, and has a good application value.
Owner:JILIN UNIV
Application of bacteriophage endolysins Lysep3 in preparation of broad-spectrum antibacterial drug
ActiveCN111420037ARealize large-scale productionPerfect protein purification systemAntibacterial agentsFungiOrganomercurial lyaseEnzyme Gene
The invention provides an application of bacteriophage endolysins Lysep3 in preparation of a broad-spectrum antibacterial drug. The invention finds for the first time that the Lysep3 has a better inhibitory effect on gram-positive bacteria, and has an obvious inhibitory effect on a plurality of bacteria under the combined action of an outer membrane penetrant, and has lower hemolytic activity on mouse red blood cells and cytotoxicity to murine derived macrophages; and in addition, the natural bacteriophage endolysins Lysep3 is used as a template, a specific expression vector is constructed byoptimizing an endolysins Lysep3 gene sequence, so that the expression of the bacteriophage endolysins Lysep3 in an eukaryotic expression system (pichia pastoris) is realized, a protein purification system is established and perfected, the large-scale production of the bacteriophage endolysins Lysep3 can be realized, and the bacteriophage endolysins Lysep3 is used in the fields of antibacterial drugs and feed additives, and has important application value and broad market prospects.
Owner:FEED RESEARCH INSTITUTE CHINESE ACADEMY OF AGRICULTURAL SCIENCES
Composition, preparation method and application of composition in treatment of bacterial diseases
PendingCN113368221AClear in timeRestoring a sterile environmentAntibacterial agentsPeptide/protein ingredientsDiseaseSterile environment
The invention discloses a composition. The composition comprises lactoferrin and bacteriophage, wherein the bacteriophage is used for cracking infected bacteria, timely removing harmful bacteria in a body and quickly and efficiently recovering a sterile environment in the body; the lactoferrin can better play a role in improving the immunity in the body without the harmful bacteria, so that discomfort such as inflammation, ulcer, redness and swelling, abscess and the like caused by bacterial infection can be quickly and efficiently eliminated, and the body is recovered to be healthy. The bacteriophage and the lactoferrin are compounded, the bacteriophage can be attached to lactoferrin molecules, and the bacteriophage is prevented from being inactivated by resistance factors by means of 'shielding' of the lactoferrin and successfully reaches the focus of infection. Besides, the lactoferrin is used as a bactericide, can be subjected to non-specific binding with surface molecules of the bacteria and can quickly and accurately locate the harmful bacteria, so that accurate infection of the bacteriophage on the harmful bacteria is helped.
Owner:南京北极光生物科技有限公司
Clostridium perfringensphage lyase and application thereof
ActiveCN112852794AEfficient killingNo side effectsBacteriaPeptide/protein ingredientsBiotechnologyLyase
The invention provides clostridium perfringensphage lyase and an application thereof serving as an antibacterial substance in prevention of poultry necrotic enteritis and food pollution control, and belongs to the field of bioengineering. The amino acid sequence of the clostridium perfringensphage lyase disclosed by the invention is SeqIDNO.2. The enzyme preparation capable of efficiently killing the clostridium perfringens is developed by utilizing a bioengineering technology, the preparation can be independently used or used in a compounded manner, the clostridium perfringens can be specifically inactivated, and a safe enzyme preparation source without toxic and side effects is provided for preventing and treating poultry necrotic enteritis and controlling clostridium perfringens pollution in food at present.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Lyase capable of killing streptococcus equi subspecies. Equi and medical application of lyase
PendingCN112501189AEfficient killingBroad cracking spectrumAntibacterial agentsBacteriaLyaseStreptococcus equi subsp equi
The invention provides lyase capable of killing streptococcus equi subspecies. Equi and medical application, and provides a new bacteriophage lyase which can be independently used or compounded with other substances for use, has strong lytic activity and a wide lytic spectrum, can effectively kill streptococcus equi subsp. Equi and streptococcus suis. The invention provides a novel medicine for preventing and treating diseases caused by streptococcus equi subsp. Equi and streptococcus suis infection.
Owner:JILIN UNIV
Method of breaking wall of bacteriophage expressing lytic gene under control of magnesium ion
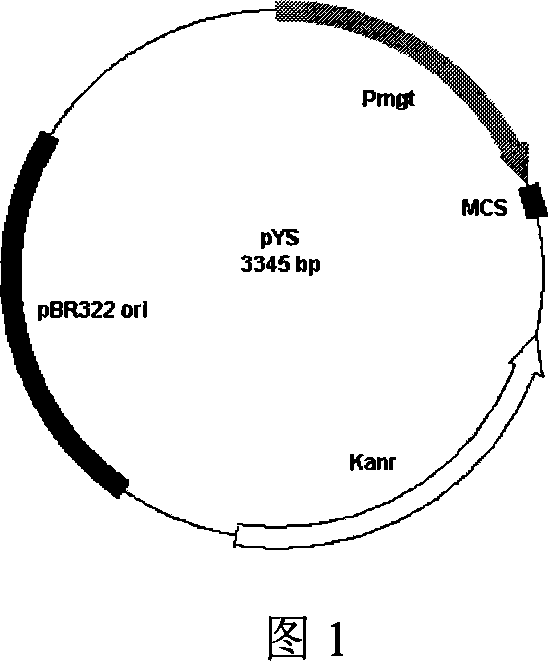
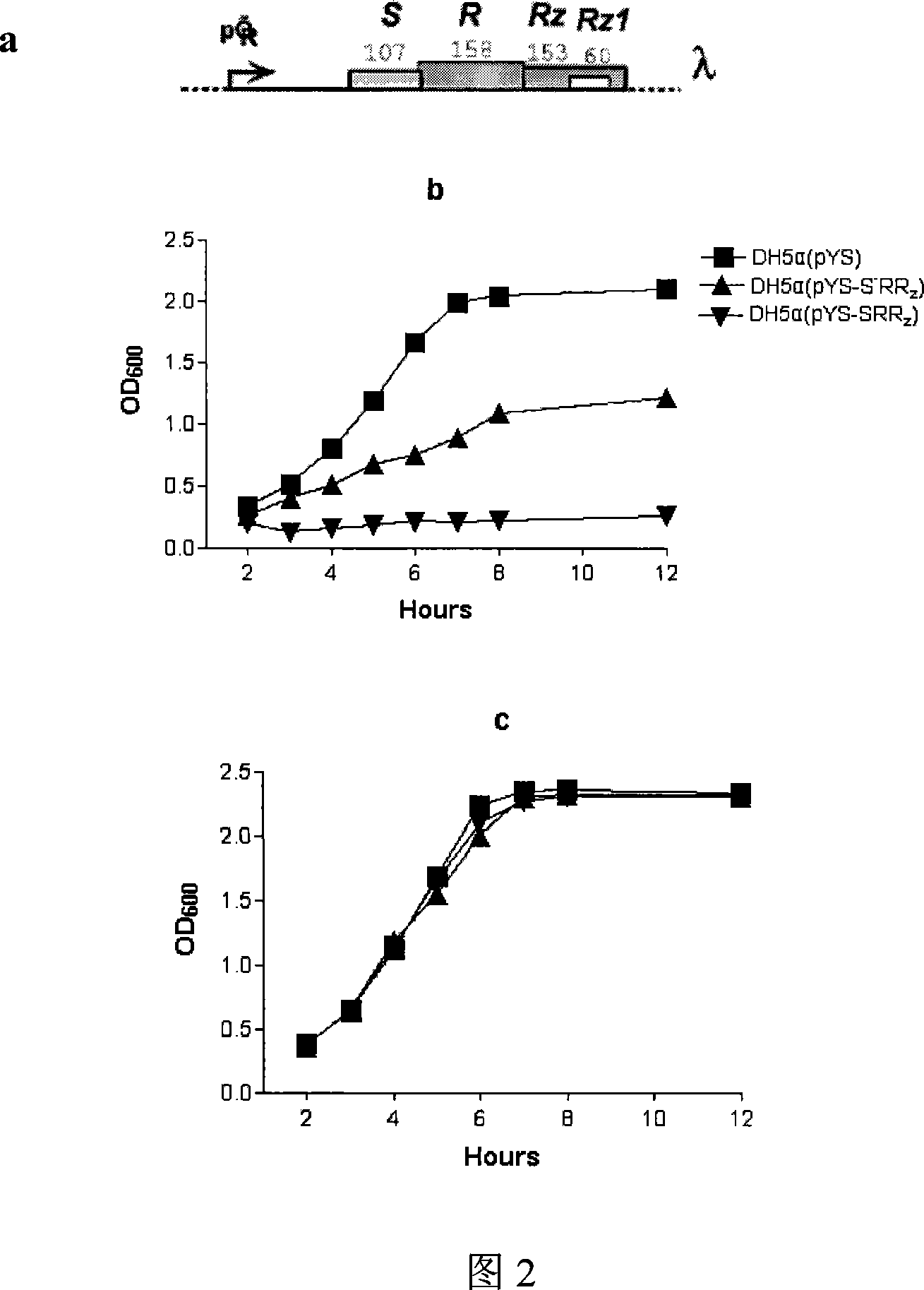
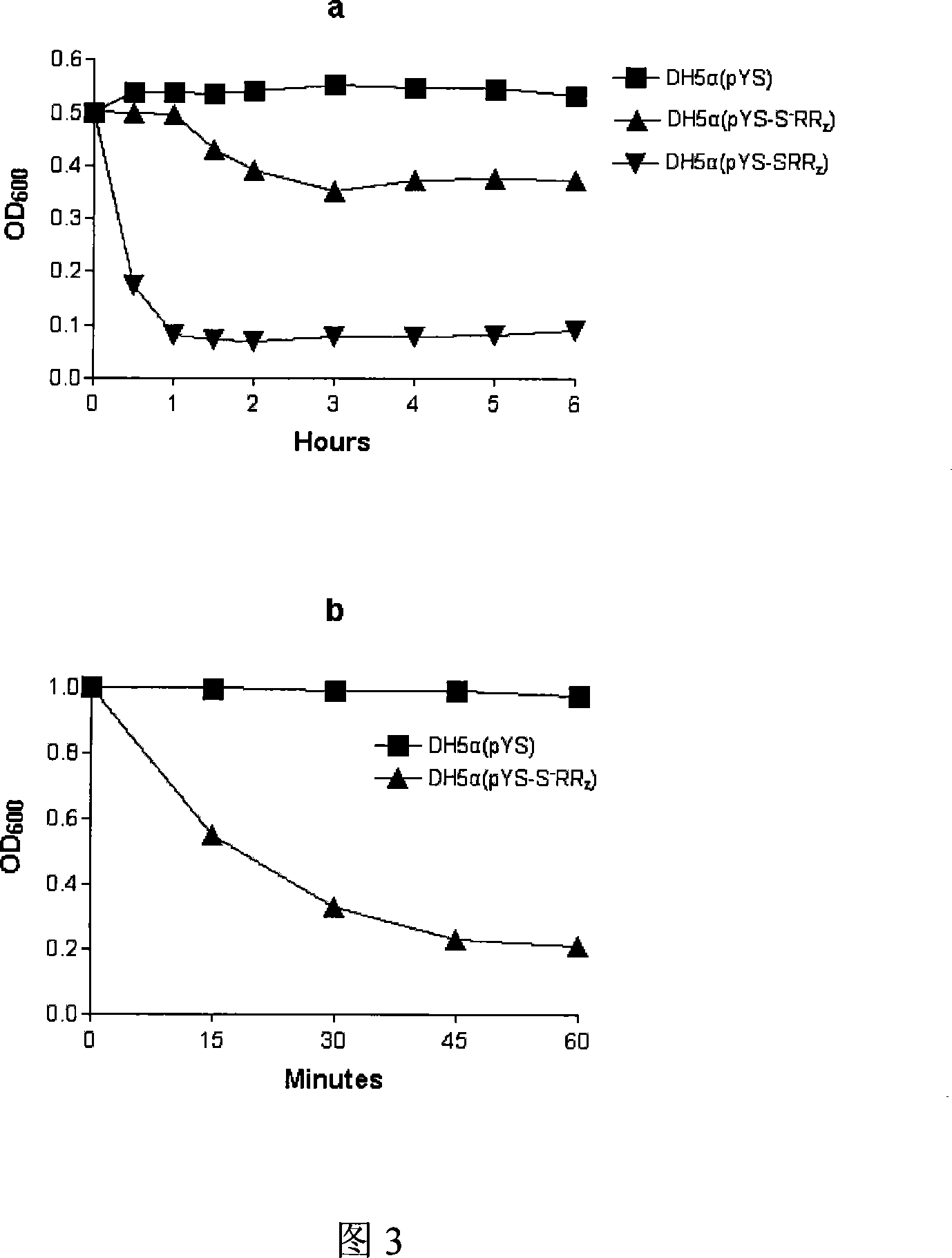
The present invention relates to gene engineering, and is especially method of expressing bacteriophage lytic gene under the control of magnesium ion. The method features that bacteriophage lytic gene is first cloned to the expression vector pYS controlled by magnesium ion and then transformed into the host bacteria, and the magnesium ion concentration is then controlled for the lytic gene to be expressed in the host bacteria. The present invention makes it possible to express lytic gene in colibacills for the host bacteria to self crack effectively under mild condition.
Owner:YANGZHOU UNIV
Light green balloon bacteriophage AVP and use thereof
A light green balloon bacteriophage AVP is brought forward, deposited in China typical culture preservation center with that deposit number of CCTCC NO:M 2018155, deposited on 25 March 2018. The new light green balloon bacteriophage AVP can lyse the bacteriophage AVP lysate of Aeromonas aeruginosa, the bacteriophage has a wide lysis spectrum, not only can it lyse the pale green balloon bacteria, It can also lyse Staphylococcus aureus and Staphylococcus epidermidis. It can lyse standard strains or clinical strains isolated from environment or diseased animal feces. It can be used alone or in combination with other substances to provide a safe and non-toxic bacteriophage sterilization product for disinfection and purification of environment.
Owner:JILIN UNIV
Bacteriophages expressing antimicrobial peptides and uses thereof
ActiveUS10676721B2Reduce deliveryReduced viabilityPeptide/protein ingredientsHydrolasesOrganomercurial lyaseAntimicrobial peptides
The present invention is generally related to engineered bacteriophages expressing antimicrobial peptides or lytic enzymes or fragments thereof for targeting a broad spectrum of bacterial hosts, and for the long-term suppression of bacterial phage resistance for reducing bacterial infections. In some embodiments, bacteriophages express antimicrobial peptides or antimicrobial polypeptides (e.g. phage lytic enzymes) which are secreted from the host bacteria, or alternatively released upon lysis of the bacterial host cell. Aspects of the present invention also relate to the use of the engineered bacteriophages for the reduction of bacterial infections, both in a subject or for bioremediation purposes, in clinical settings and wound healing.
Owner:TRUSTEES OF BOSTON UNIV +1
Method for preparing bacterial ghost vaccine of haemophilus parasuis as well as product and application thereof
InactiveCN103446581ARepress transcriptionImprove cracking efficiencyAntibacterial agentsMicroorganism based processesEPROMHaemophilus
The invention discloses a method for preparing a bacterial ghost vaccine of haemophilus parasuis as well as a product and application thereof. The method comprises the following steps of connecting a mutational bacteriophage splitting gene E Eprom (as shown in SEQ ID No:1) with pBV220 to obtain an efficient splitting plasmid vector pBV-Eprom; converting the pBV-Eprom into haemophilus parasuis, propagating at 37 DEG C, and inducing the Eprom gene to express at 42 DEG C, and collecting the product which is unexpressed finally to obtain haemophilus parasuis bacterial ghost, wherein the Eprom is obtained by carrying out mutation on a promoter region of the bacteriophage splitting gene E, and the temperature for culturing bacteria is changed to 37 DEG C from the existing 28 DEG C by the Eprom; moreover, the splitting efficiency is high, the initial induced concentration and the large-scale production capacity are high, and the culture-splitting efficiency of a fermentation tank is as high as 99.99995%. The bacterial ghost vaccine of the haemophilus parasuis disclosed by the invention has good safety and immune protective efficacy, can be used for stimulating a body to generate a high-titration antibody, and also can be used for providing good cross immune protection for attack of a virulent strain of the haemophilus parasuis with different serotypes.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Detecting method for accurately and rapidly detecting target superbacteria
PendingCN109750085AAccurate trackingHigh detection specificityMicrobiological testing/measurementSurvival StatusQuantitative determination
The invention belongs to the technical field of medical treatment sanitation, and particularly relates to a detecting method for accurately and rapidly detecting target superbacteria. According to themethod, floating bacteria in the environment are adopted as a sample collecting object; the method comprises the specific steps of sampling, enriching, screening culture, qualitative or quantitativemeasuring evaluation and the like. By means of the characteristic that phage can split ATP released by bacteria in the survival status so that luciferase catalyzes fluorescein to emit light, the typeor content of 'superbateria' can be visually qualitatively or quantitatively determined; due to the specificity of phage, no isolated culture is needed even in a sample containing mixed bacteria, rapid and accurate detection and determination can be achieved, and therefore the good application value is achieved.
Owner:希拓生物科技有限公司
Method for expressing anthrax bacteria gamma phage lyase and its special gene
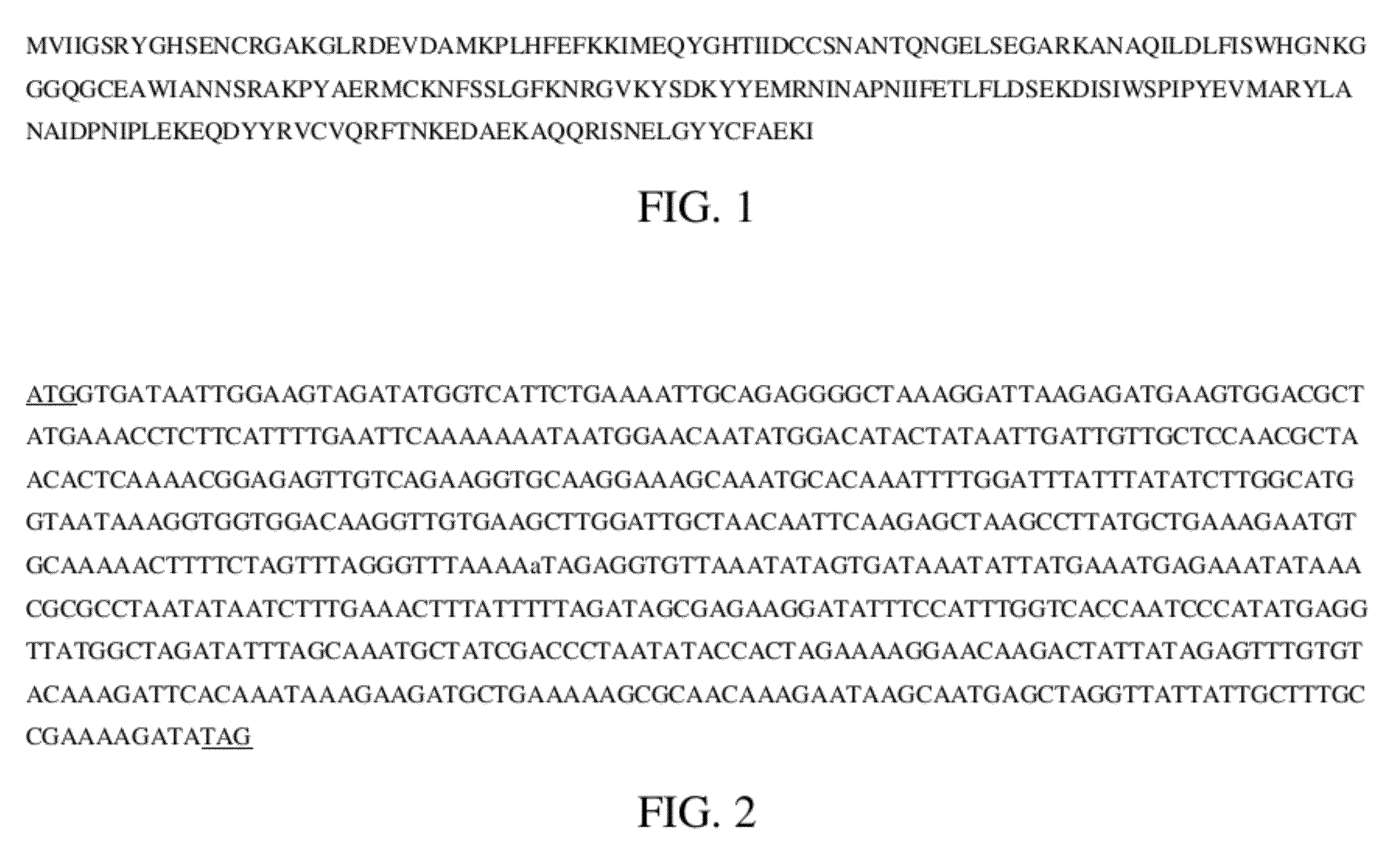
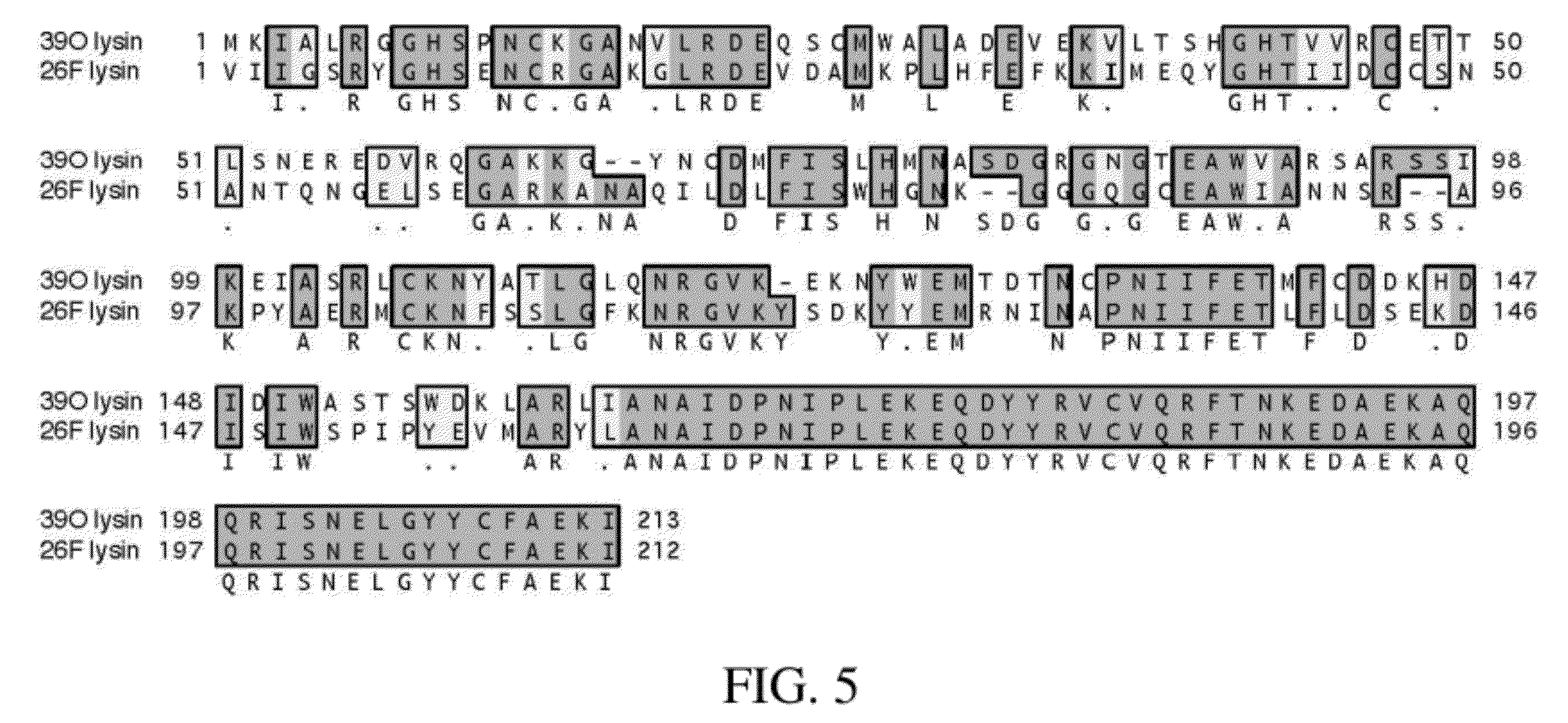
InactiveCN1821413AStrong cracking abilityStrong specificityBacteriaEnzymesEscherichia coliNucleotide
The present invention discloses one method of expressing anthrax bacteria gamma phage lyase and its special gene. The gene has one of the following nucleotide sequence: 1) SEQ ID No. 2 in the sequence list; 2. polynucleotide sequence coding the protein sequence of SEQ ID No. 3 in the sequence list; and 3. the nucleotide sequence capable of hybridizing with the DNA sequence limited by SEQ ID No. 2 in the sequence list under high strict condition. The optimized anthrax bacteria gamma phage lyase gene of the present invention is expressed in colibacillus system in the high expression level with expressed anthrax bacteria gamma phage lyase as high as 75 % of the all bacillus protein, has powerful anthrax bacteria schizolyzing capacity and very high specificity, and may be used in the diagnosis and prevention of anthrax and the theoretical research of anthrax treatment.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Design and construction method of high efficiency temperature controlled positive screening cloning carrier
InactiveCN101182533AQuality improvementAddressing Negative Background IssuesVector-based foreign material introductionCDNA libraryNormal growth
The present invention provides methods for designing and constructing novel molecular cloning vectors for cloning DNA fragments. The invention utilizes the mechanism that the expression product of the bacteriolytic gene E of the bacteriophage ΦX174 can lead to the death of the host Escherichia coli cell to design and construct the cloning vector. When the target fragment is inserted into the cloning site on the vector, it will cause the expression of the E gene to be silent, so the positive transformant containing the recombinant vector grows normally into a colony clone when it is cultivated at the induction temperature (42 ° C), and the vast majority (99.9 %) negative transformants will die due to E gene expression when cultured at this temperature, and cannot grow into colony clones. The invention can be used for designing and constructing common cloning vectors and T vectors. The cloning vector of the present invention has the technical feature of inhibiting the generation of negative clones, so it is especially suitable for constructing genome libraries and cDNA libraries in addition to being used for single gene cloning.
Owner:SOUTHWEST UNIVERSITY
Novel method for extracting phage genome DNA
The invention provides a novel method for extracting phage genome DNA. The novel method comprises the following steps: after amplifying phage lysate, removing cell debris and nucleic acid substances of host bacteria, adding PEG6000 to settle phage, after resuspending by TM buffer liquid, avoiding chloroform extraction, directly adding nuclease, removing the genome of the remaining host bacteria, then replacing protease K by using urea, after degeneration of coat protein, separating the protein from the genome DNA by agarose gel electrophoresis, and then recycling the genome DNA of the phage byusing a freeze thawing and recycling method. The method is simple and convenient to operate, is particularly suitable for phage sensitive to chloroform, and is wide in application range, experiment costs are greatly reduced, and extracting time is shortened.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
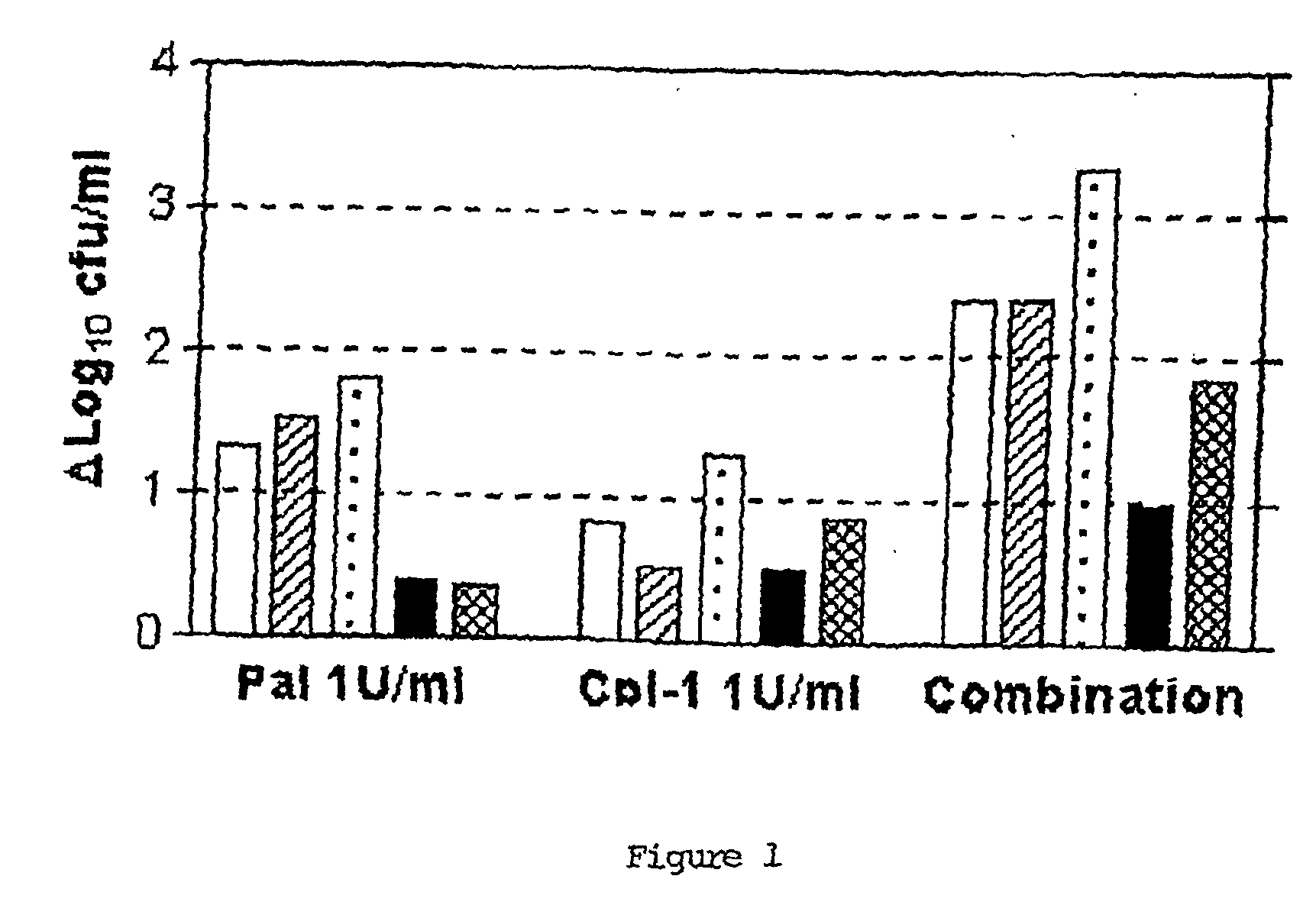
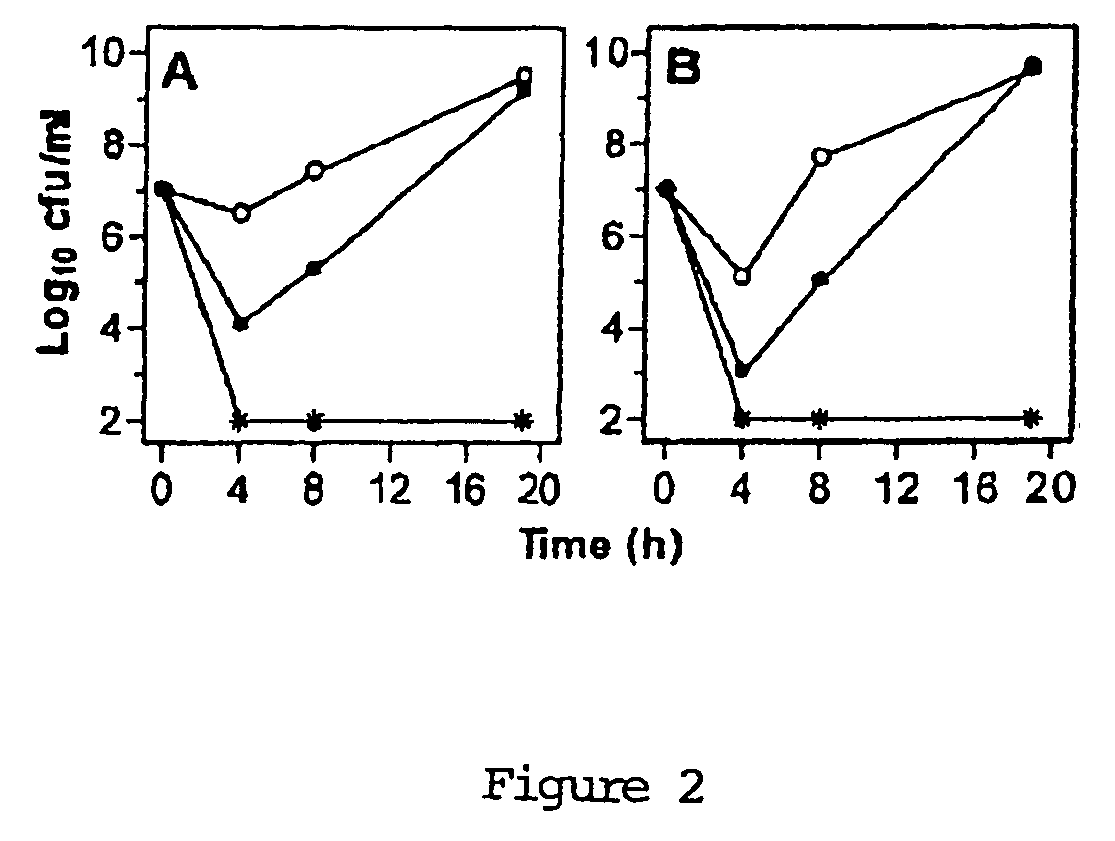
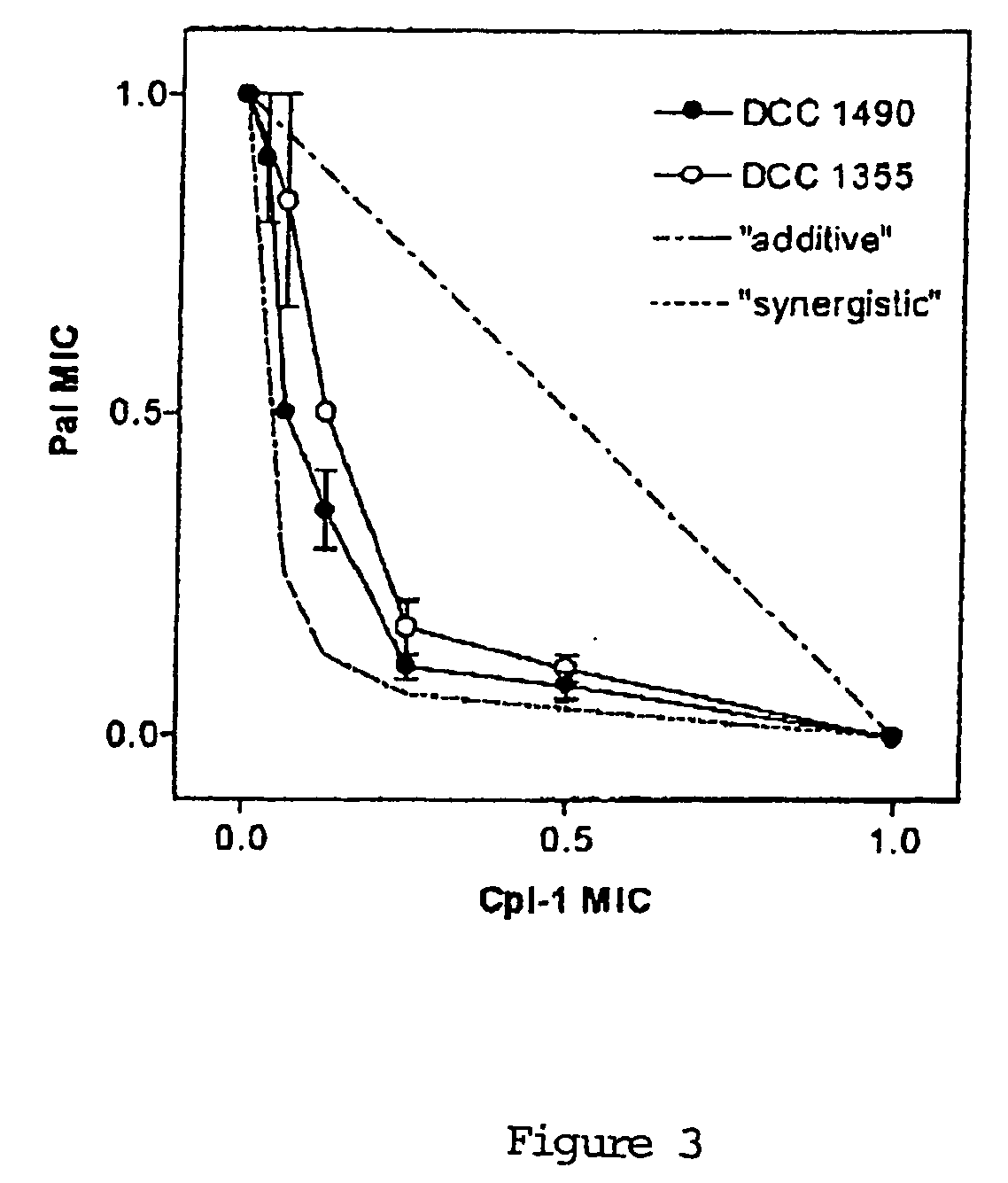
Uses of sysnergistic bacteriophage lytic enzymes for prevention and treatment of bacterial infections
InactiveUS20060159671A1Reduce the amount requiredPotential damageAntibacterial agentsBiocideMicrobial agentLyase
Methods for treating and preventing bacterial infections are described using at least two lytic enzymes obtained from a bacteriophage isolated from Streptococci. Two of these enzymes, Pal and Cpl-1, showed synergy when tested for cleavage of peptidoglycan in the cell walls of Streptococcus pneumoniae. Moreover, the combination of these two enzymes resulted in killing of both penicillin sensitive as well as penicillin resistant strains of S. pneumoniae. The synergy displayed by the combined use of these two enzymes may establish a means for identification of agents that mimic or enhance this activity and may lead to the identification of new antimicrobial agents and pharmaceutical compositions useful for treating and preventing a variety of bacterial infections in mammals. Further compositions are described for disinfecting or sanitizing porous and non-porous surfaces suspected of harboring infectious organisms.
Owner:THE ROCKEFELLER UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com