Staphylococcus aureus bacteriophage lyase as well as preparation method and application thereof
A technology of phage lyase and Staphylococcus, which is applied in the field of Staphylococcus aureus phage lyase and its preparation, and can solve problems such as the lack of separation and purification of Staphylococcus aureus phage lyase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 Staphylococcus aureus phage lyase gene cloning and vector construction
[0046] 1.1 Strains and vectors
[0047] Staphylococcus aureus phage SA2 was deposited in the General Microbiology Center of the China Committee for the Collection of Microbial Cultures, with the preservation date being July 27, 2017, and the preservation code is CGMCC No.14331.
[0048] Escherichia coli DH5α competent cells and Escherichia coli BL21 competent cells were purchased from Treasure Bioengineering (Dalian) Co., Ltd.
[0049] The expression vector pCold TF was purchased from Bao Biological Engineering (Dalian) Co., Ltd.
[0050] 1.2 Primer design
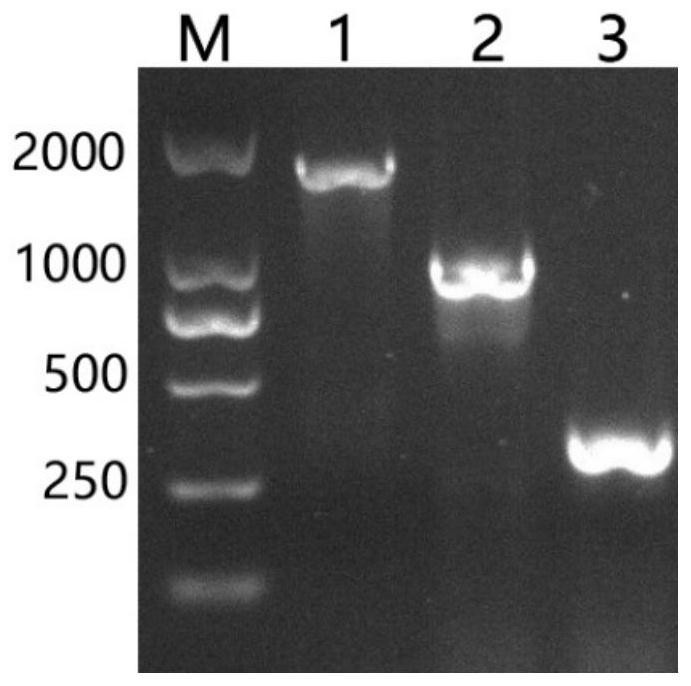
[0051] According to the whole genome sequence of phage SA2, determine the lysing enzyme LysSA2 gene sequence of phage SA2, and according to the structural domain characteristic of lysing enzyme LysSA2, clone the coding sequence of lysing enzyme LysSA2, lysing enzyme LysSA2-1 and lysing enzyme LysSA2-2 respectively; Wherein The c...
Embodiment 2
[0069] Example 2 Induced Expression and Purification of Recombinant Expression Plasmid Bacteria
[0070] 2.1 Transfer of recombinant expression plasmids into BL21 competent cells
[0071] Take 1 μL of recombinant plasmid and add 100 μL of E. coli BL21 competent cells, mix well, and ice-bath for 30 minutes; heat shock in 42°C water bath for 90 seconds, quickly place on ice for 3 minutes, add 900 μL of LB broth, and incubate at 37°C for 90 minutes; take 100 μL of bacteria The solution was spread on the nutrient agar plate containing Amp and incubated overnight at 37°C.
[0072] 2.2 Induced expression of recombinant expression plasmid bacteria
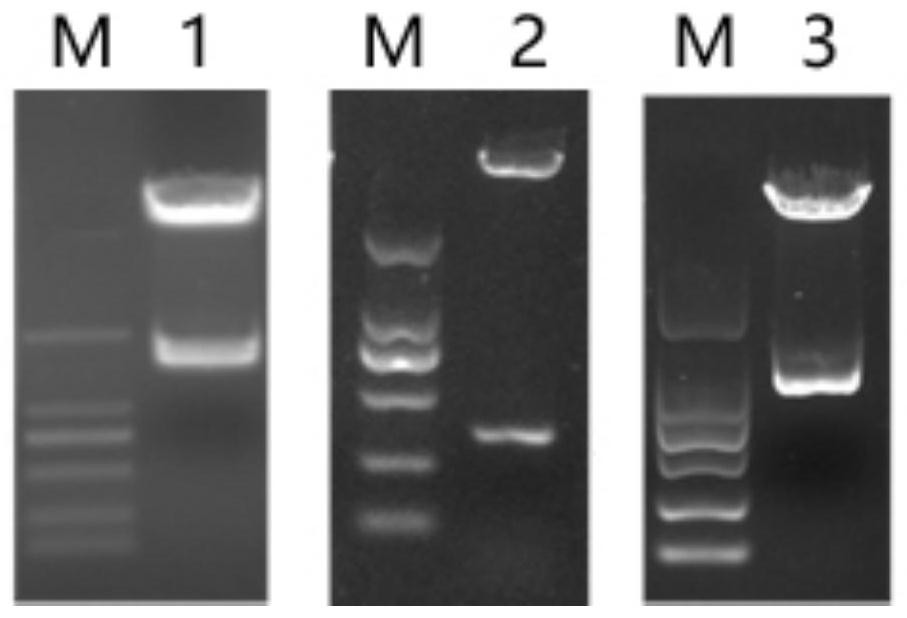
[0073] Randomly pick a single colony transferred into BL21 competent cells, inoculate it into LB liquid broth containing Amp, culture it with shaking at 37°C until the OD600 is about 0.6-0.8, add IPTG with a final concentration of 0.1mM, and shake overnight at 16°C to induce expression. The recombinant proteins were named lyases LysSA2,...
Embodiment 3
[0077] Embodiment 3 lyase LysSA2, LysSA2-1 and LysSA2-2 in vitro antibacterial activity assay and antibacterial spectrum detection
[0078] 3.1 Determination of antibacterial activity of lyases LysSA2, LysSA2-1 and LysSA2-2 in vitro
[0079] Staphylococcus aureus was cultured to the logarithmic phase in LB liquid medium. After the bacterial liquid was centrifuged, the pellet was washed twice with PBS, and then the bacterial cells were resuspended with PBS and the absorbance OD600 was adjusted to about 0.65. In a 96-well plate, mix 100 μL of the resuspended bacteria with 100 μL of recombinant proteins LysSA2, LysSA2-1 and LysSA2-2 at a concentration of 500 μg / mL, and use the induced expression of the empty plasmid pCold TF protein as a control, 3 in each group In parallel, culture in a 37°C incubator. The OD600 value was measured at intervals of 30 min after the culture started, and the curve of OD600 changing with time was drawn.
[0080]The results showed that the OD600 val...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com