Phage lytic enzymes as well as gene, gene recombinant expression vector and application thereof
A technology of bacteriophage lyase and expression vector, applied in the field of bacteriophage lyase and its gene, gene recombinant expression vector and application field, can solve the problem of lack of effective prevention and treatment of drug-resistant bacterial infection, etc., and achieve good lysing bacterial activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Cloning of Phage Lyase P9ly Gene
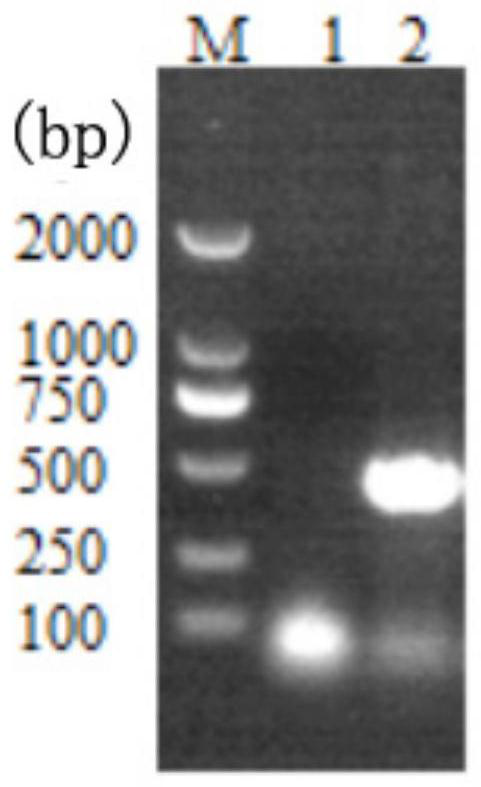
[0037] 1. Amplification of the lyase gene (using the lytic phage PDS9 genomic DNA corresponding to Shigella BDS9 as a template).
[0038](1) The primer sequences used for the amplification of phage PDS9 lyase gene P9ly include SEQ ID NO: 3 and SEQ ID NO: 4, wherein, SEQ ID NO: 3 is a forward primer: 5'-CATG CCATGG CAATGGATATTTTTTGATATGTTACG-3'; SEQ ID NO: 4 is reverse primer: 5'-CG GAATTC TCTATAAGCAGCCCATGTGC-3'. Wherein, the underlined part of the forward primer and the reverse primer represent NcoI and EcoRI restriction sites, respectively.
[0039] (2) The amplification reaction system is as follows:
[0040] Table 1 Components of PCR amplification reaction system
[0041]
[0042]
[0043] (3) The amplification conditions are as follows:
[0044] Mix the reaction system evenly, pre-denature at 94°C for 10min, then denature at 94°C for 45s, anneal at 58°C for 45s, extend at 72°C for 90s, and after 30 cycles, extend at 7...
Embodiment 2
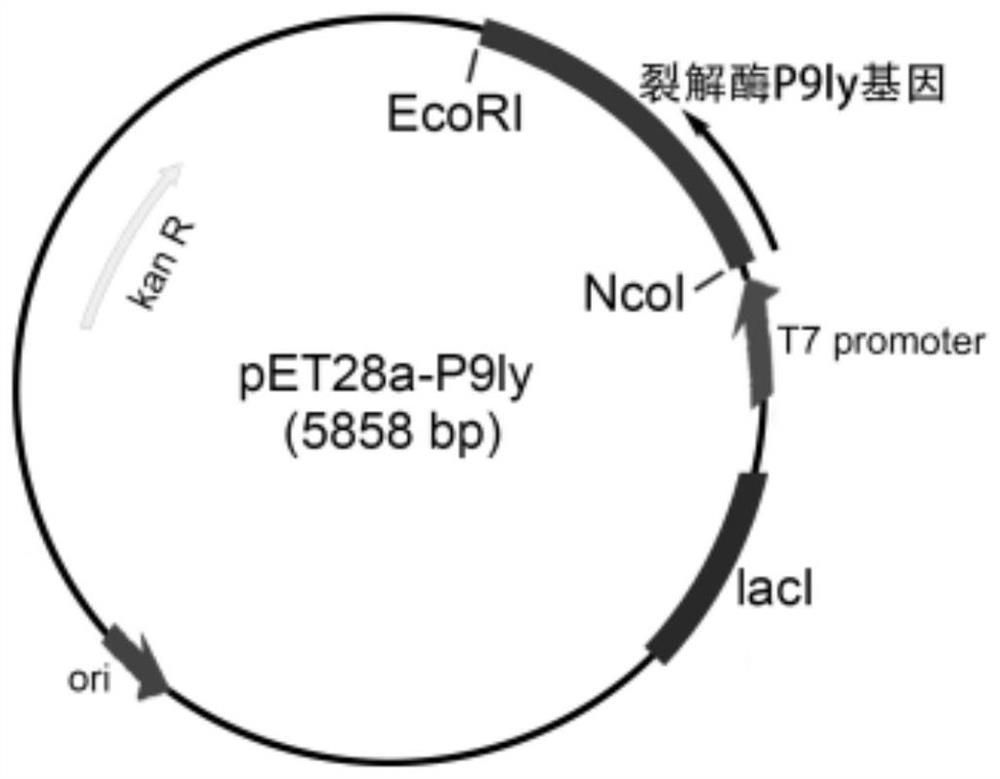
[0051] Construction of recombinant expression vector pET28a-P9ly
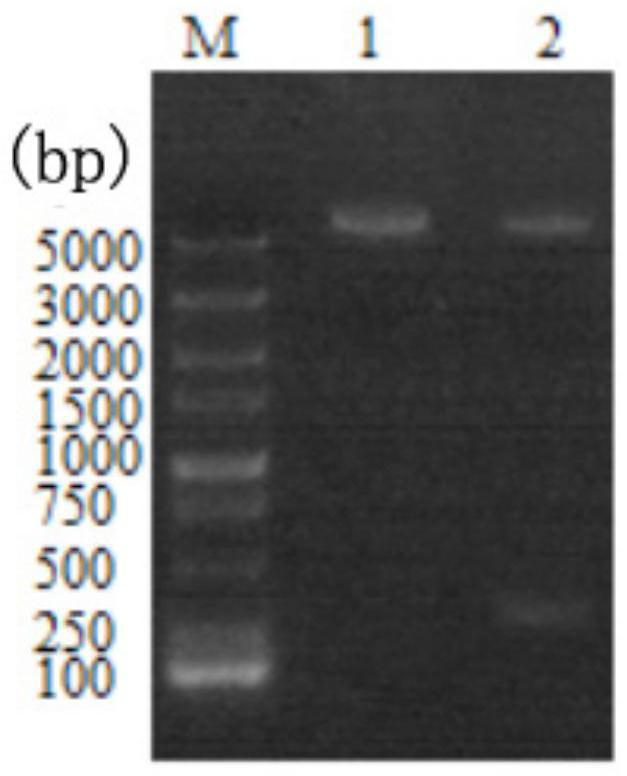
[0052] In order to connect the target gene fragment to the expression vector pET28a, it is necessary to make the target fragment have a fragment with cohesive ends, that is, a restriction site.
[0053] 1. Preparation of linear vector pET28a with cohesive ends
[0054] In order to connect the target gene fragment to the expression vector pET28a, it is necessary to make the target fragment have a fragment with cohesive ends, that is, a restriction site. Similarly, in order to allow the target fragment to be inserted into the vector, it is also necessary to make the vector have cohesive ends and make their restriction sites the same.
[0055] (1) Plasmid extraction: Use the plasmid extraction kit (Bitec), the operation steps are as follows:
[0056] ① Strain activation: Dip the sterile inoculation loop into the strain preservation solution frozen at -80°C, and inoculate it on the Kan + LB plate, cultivate over...
Embodiment 3
[0074] Induced Expression and Enzyme Activity Determination of Lyase P9ly Recombinant Protein in Escherichia coli BL21(DE3)
[0075] The constructed recombinant expression vector pET28a-P9ly was transformed into Escherichia coli BL21(DE3), the strain containing the recombinant plasmid was cultured overnight, and the bacterial solution was inoculated into Kan + (final concentration 50 μg / mL) of LB liquid medium, 37 ° C shaker culture to its OD value of 0.6-0.8; take out 4 mL of bacterial liquid as a control experiment; add lactose (final concentration of 1 mg / mL) to the remaining bacterial liquid ), placed at 37° C., 150 rpm shaker for induction culture for 10 hours, and sampled 5 mL.
[0076] 1. Lyase P9ly protein SDS-PAGE detection
[0077] Centrifuge the 5mL bacterial liquid taken out at 9000rpm for 10min, discard the supernatant, add a final concentration of 30mM imidazole solution to suspend the bacterial cells, ultrasonically disrupt the bacterial cells (power 36%, beat ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com