Application of bacteriophage endolysins Lysep3 in preparation of broad-spectrum antibacterial drug
A technology of phage lysing enzymes and antibacterial drugs, which is applied in the fields of biomedicine and genetic engineering, can solve the problems of high production cost of natural lysing enzymes, unclear bactericidal effect and mechanism, and achieve important application value and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
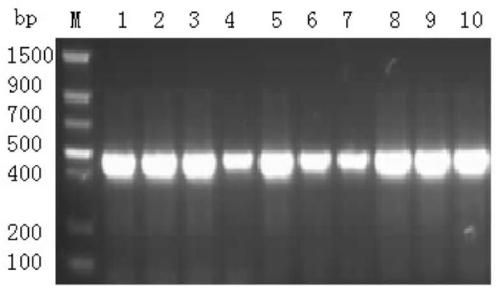
[0064] The acquisition of embodiment 1 lyase Lysep3 gene fragment
[0065] Optimization and design of lyase Lysep3 gene expression sequence:
[0066] The gene encoding the lyase Lysep3 was optimized according to the yeast codon preference (SEQ ID NO:2), and 8 CAT histidine tags and 2 TAA terminator sequences were added to the 3' end of the optimized gene sequence (SEQ ID NO:2). ID NO:3), and add XhoI restriction site and Kex2 restriction site at the 5' end of the optimized gene sequence by the method of specific primer PCR amplification, and add XbaI restriction site at the 3' end, that is Complete the construction of the gene expression cassette. The above sequence was completed by Shanghai Sangon Bioengineering Co., Ltd.
Embodiment 2
[0067] Example 2 Construction of Yeast Recombinant Expression Vector
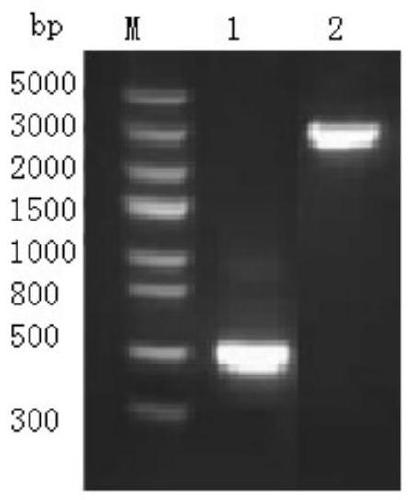
[0068] 1. Amplify the lyase Lysep3 gene fragment obtained in Example 1 with specific primers containing the XhoI and XbaI restriction site sequences, and perform double digestion to recover the purified fragment. At the same time, the pPICZαA vector (purchased from Invitrogen) was digested with XhoI and XbaI, and the product was verified by Tricine-SDS-PAGE electrophoresis ( figure 1 ).
[0069] The double enzyme digestion system is as follows:
[0070]
[0071] After adding the sample to the above enzyme digestion system, place it in a PCR instrument at 37°C for 4 hours, and detect it by electrophoresis on a 2% agarose gel. Electrophoresis conditions: 120V, 30min. The digested products were recovered with a DNA product recovery kit. After the lyase Lysep3 gene and the pPICZαA vector were digested with XbaI and XhoI, the lyase Lysep3 gene was ligated with the linearized pPICZαA vector with T4 DNA liga...
Embodiment 3
[0088] Example 3 Construction of recombinant yeast strain containing lyase Lysep3 gene
[0089] 1. Linearization of recombinant vector pPICZαA-Lysep3
[0090] Use PmeI to digest the constitutive recombinant expression vector pPICZαA-Lysep3. The enzyme digestion system and reaction conditions are as follows:
[0091]
[0092] After adding the sample to the above enzyme digestion system, place it in a PCR instrument at 37°C for 4 hours, and detect it by electrophoresis on a 2% agarose gel. Electrophoresis conditions: 120V, 30min. Electrophoresis results showed that the pPICZαA-Lysep3 recombinant vector was completely linearized.
[0093] 2. Preparation of competent Pichia pastoris X-33
[0094] 1) Pick a single colony of X-33 on the YPD plate, inoculate it into 10 mL of YPD liquid medium, and cultivate overnight at 29°C and 250 rpm;
[0095] 2) Take Pichia pastoris X-33 overnight culture solution and inoculate it into 100mL YPD liquid medium with 1% inoculum amount, cultiv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com