Lyase of bacteriophage and sterilization application
A bacteriophage lyase and bacteriophage technology, applied in the field of bioengineering, can solve the problems of pulmonary edema, irritant toxicity, eye and respiratory tract irritation, etc., and achieve the effects of short action time, broad antibacterial spectrum and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, the extraction of phage genome
[0036] (1) Crude phage particles
[0037] (1) Preparation of host bacteria: pick Pseudomonas aeruginosa (Pseudomonasaeruginosa) from solid medium [1] A single colony was inoculated in 5mL LB liquid medium, and cultured with shaking at 37°C for 6-8h.
[0038] (2) Preparation of pure phage culture solution: Pick a single phage plaque, inoculate it in 5 mL logarithmic phase host bacterial culture solution, culture it with shaking at 37°C for 4-6 hours, then centrifuge the lysate at 10000×g for 10 minutes, and the supernatant The solution is the pure culture solution of phage.
[0039] (3) Preparation of crude phage particles: Transfer overnight culture to 100mL liquid LB medium, inoculum size is 1%, amplify and cultivate to logarithmic phase (OD 600About 0.4), add 5 mL of phage pure culture solution, shake culture at 37°C for 6-8 hours to obtain phage lysate. Add DNaseI and RNaseA to the lysate to a final concentration of ...
Embodiment 2
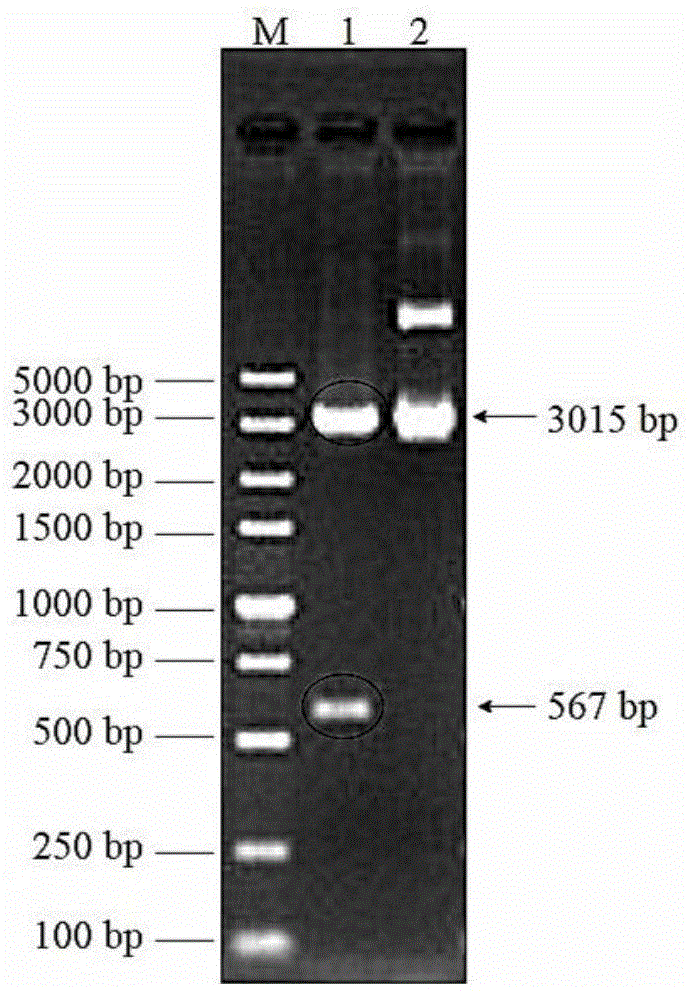
[0049] Embodiment 2, construction of recombinant plasmid
[0050] 1. Obtaining the target fragment
[0051] (1) Primer design
[0052] According to the gene sequence of Pseudomonas aeruginosa bacteriophage K8 lyase, design primers:
[0053] LkesF: 5′-CGCGGATCCATGGCTCTAACTGAGCAAG-3′
[0054] LkesR: 5′-CGCGGATCCTTACTTGAAGGATTGATAGG-3′
[0055] (2) 50μL reaction system:
[0056]
[0057] (3) PCR reaction conditions:
[0058]
[0059] 2. Chemical transformation experiment (construction of pGEM-Teasy recombinant vector)
[0060] (1) Ligate the gp57 gene PCR product with the pGEM-Teasy vector, the connection system is:
[0061] 10μL reaction system:
[0062]
[0063] Mix the reaction system evenly, and connect overnight at 16°C.
[0064] (2) Escherichia coli DH5a chemically competent cell preparation:
[0065] a. Bacterial culture: Pick a single colony of Escherichia coli DH5a in 5 mL of liquid LB medium and culture overnight at 37°C. Transfer the overnight cultur...
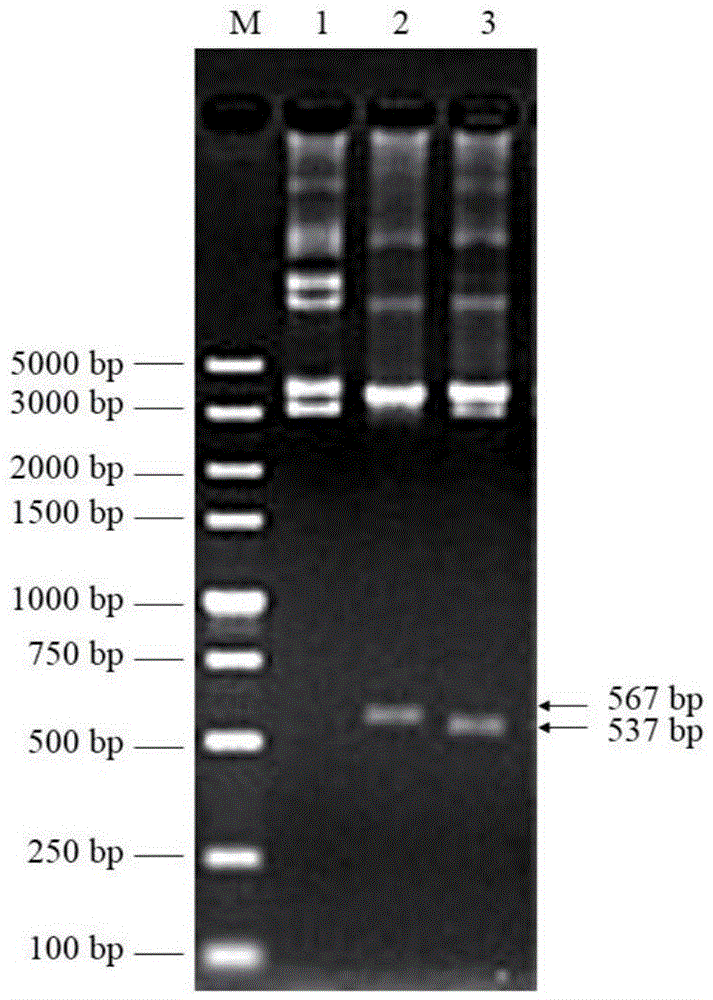
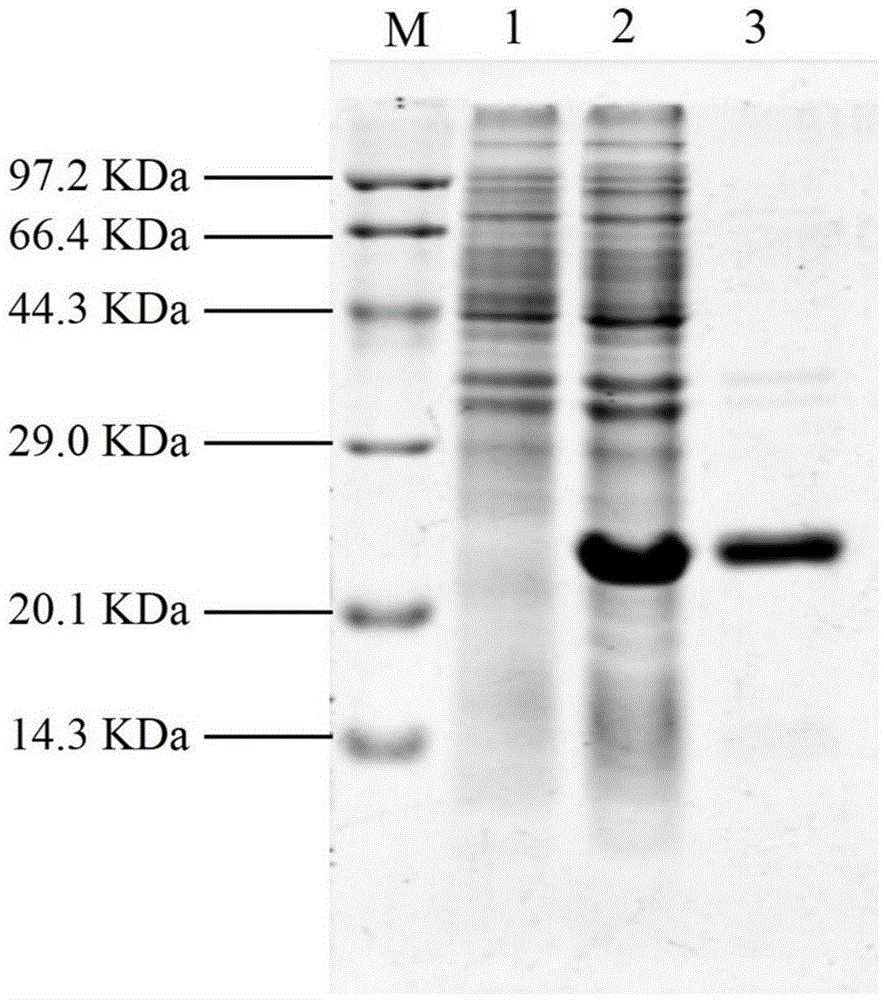
Embodiment 3
[0090] Embodiment 3, high expression of lyase in Escherichia coli
[0091] Inoculate the frozen engineered bacteria JK1501 containing positive recombinant plasmids into 20 mL of LB liquid medium (containing ampicillin 100 μg / mL, kanamycin 25 μg / mL), 37 ° C, 220 r / min, shaking culture overnight, the next day, According to the inoculation amount of 1%, the bacterial solution was added to fresh 1 LLB liquid medium (containing ampicillin 100 μg / mL, kanamycin 25 μg / mL), and cultivated to OD at 37 ° C and 180 rpm 600=0.6, add inducer IPTG (to a final concentration of 1 mmol / L). After 4 hours of induction culture, centrifuge at 4000 r / min for 15 minutes to collect the bacteria. Resuspend each 1g of bacteria (wet weight) in 3mL of bacteriostasis buffer (Tris-HCl20mmol / L, NaCl250mmol / L, PMSF1mmol / L, pH7.4), mix well, break the cells by ultrasonic, and centrifuge at 10000× g, centrifuge at 4°C for 15 minutes to remove insoluble cell debris, and filter the supernatant with a 0.22 μm st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com