DNA loaded Brucella ghost composite vaccine
A technology of Brucella and Brucella nucleic acid, applied in the field of Brucella slough compound vaccine, can solve the problem that the protective immune response is not high enough, the gene silence is not expressed, and the protective antigen immunogenicity is not good, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
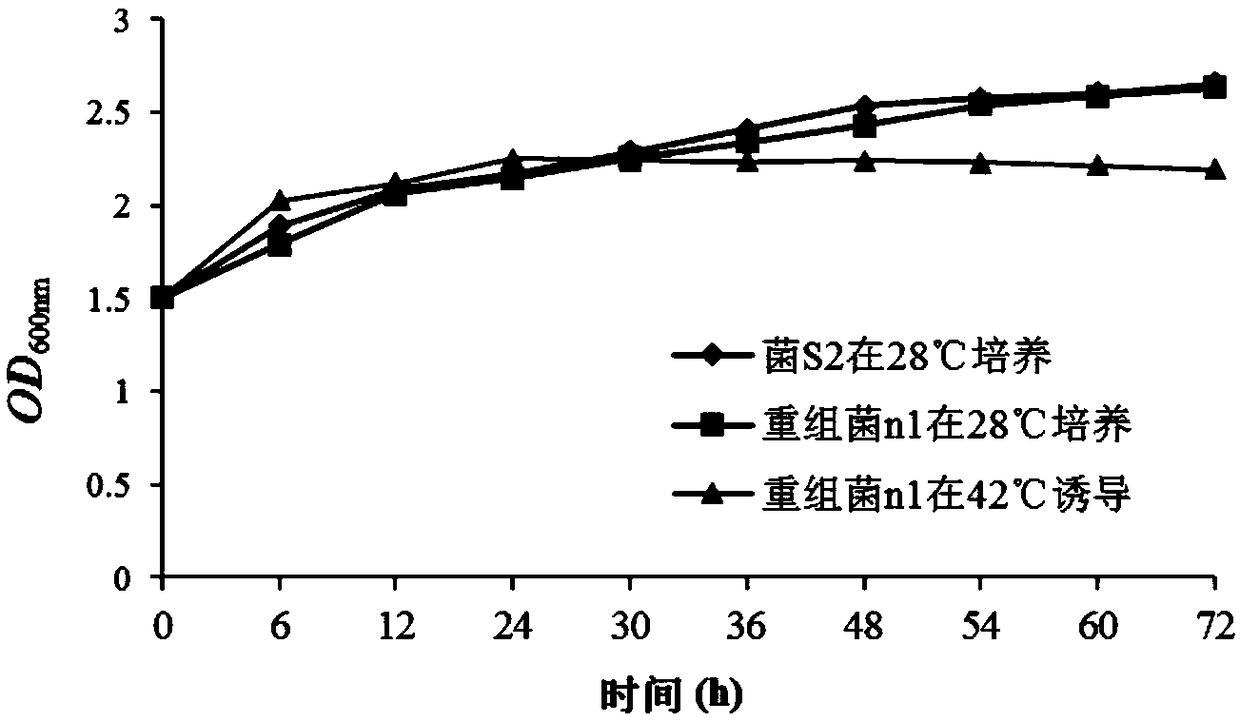
[0073] Embodiment 1, the preparation of the brucella slough composite vaccine loaded with DNA
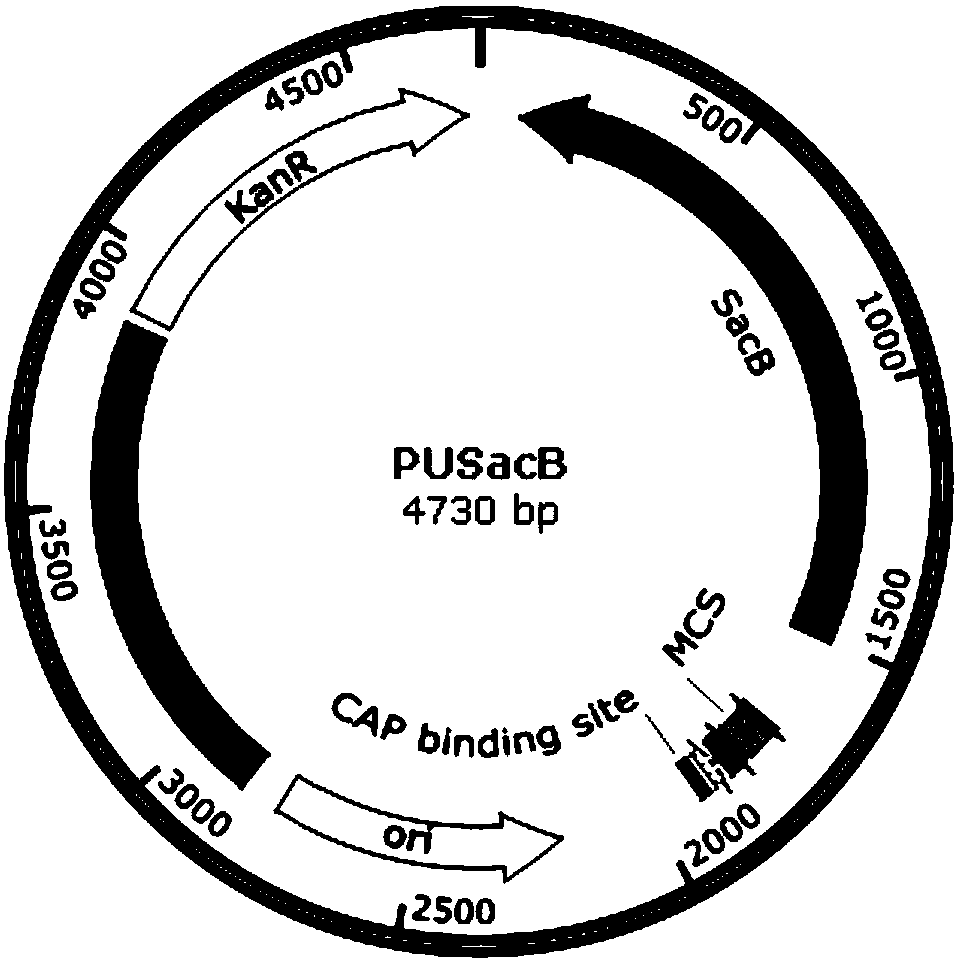
[0074] 1. Construction of temperature-controlled cleavage type suicide plasmid PUSacB-ΔwboA-TLC
[0075] 1. Synthesis of primers
[0076] According to the upstream and downstream homology arm nucleotide sequences of the wboA gene (genebank number: HQ845203.1) of the whole genome of Brucella disclosed by GenBank, primers wboA-N-F, primers wboA-N-R, primers wboA-C-F and Primers wboA-C-R.
[0077] The nucleotide sequences of each primer are as follows:
[0078] Primer wboA-N-F: 5'-CGC GAGCTC CTGGCGTCAGCAATCAGAG-3' (the underline is the recognition site of restriction endonuclease SacⅠ);
[0079] Primer wboA-N-R: 5'-CGC GGATCC GTGCAACGACCTCAACTTCC-3' (the underline is the recognition site of restriction endonuclease BamHI);
[0080] Primer wboA-C-F: 5'-CGC GTC GAC ACGCCATCGAACCTTTATCTG-3' (the underline is the recognition site of restriction endonuclease SalI);
[0081] Prime...
Embodiment 2
[0163] The application of the brucella slough composite vaccine loaded with DNA prepared by embodiment 2, embodiment 1
[0164] 1. Safety test
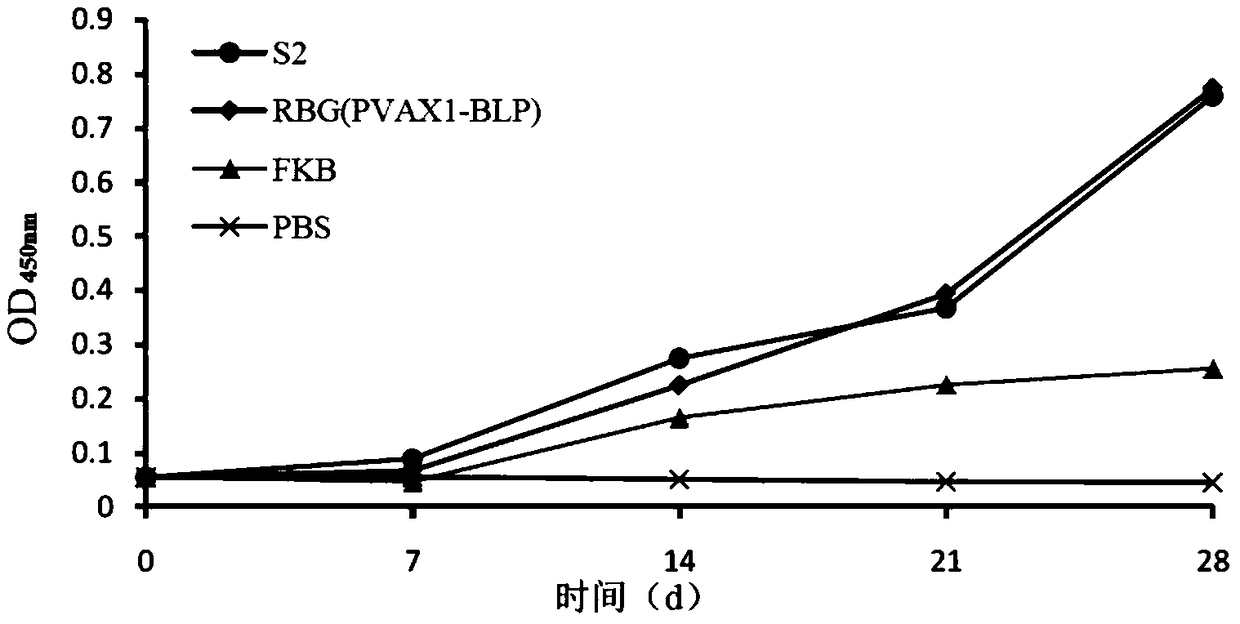
[0165] 1. Take 40 healthy female BALB / C mice aged 6-8 weeks and weighing 18-20 g, and randomly divide them into 4 groups, 10 mice in each group.
[0166] 2. After completing step 1, the four groups of mice were treated as follows:
[0167] Group 1 (S2 group): each mouse was inoculated with Brucella live vaccine S2 by intraperitoneal injection; the inoculation dose was 5×10 8 CFU / piece;
[0168] The 2nd group (RBG (PVAX1-BLP) group): every mouse is prepared by intraperitoneal injection the brucella crassa slough complex vaccine of loading DNA prepared in embodiment 1; Inoculation dose is 5 * 10 9 CFU / piece;
[0169] The 3rd group (FKB group): each mouse is inoculated with Brucella formaldehyde inactivated vaccine by intraperitoneal injection; Inoculation dose is 5 * 10 9 CFU / piece;
[0170] Group 4 (negative control group): each ...
Embodiment 3
[0193] Embodiment 3, the preparation of the smooth type brucella slough compound vaccine loaded with DNA
[0194] 1. Construction of temperature-controlled cleavage suicide plasmid PUSacB-Δbp26-TLC
[0195] 1. Synthesis of primers
[0196] According to the nucleotide sequence of the upstream and downstream homology arms of the bp26 gene (genebank number: AY166769) published by GenBank, primers bp26-N-F, primer bp26-N-R, primer bp26-C-F and primer bp26 were designed and synthesized -C-R. The nucleotide sequences of each primer are as follows:
[0197] Primer bp26-N-F: 5'-CGC GAGCTC CGTGTTGTGCGCCTGAAGCGCAAT-3' (the underline is the recognition site of restriction endonuclease SacⅠ);
[0198] Primer bp26-N-R: 5'-CGC GGATCC GACGAGCATGATTGTGGAAAATGA-3' (the underline is the recognition site of restriction endonuclease BamHI);
[0199] Primer bp26-C-F: 5'-CGC GTC GAC CTTGGCCGTGTGGTGGAAATCAG-3' (the underline is the recognition site of the restriction endonuclease SalI);
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com