Through the application of techniques set forth herein, those of skill in the art will be enabled to obtain .Iadd.a recombinant .Iaddend.DNA .[.segments encoding all or a portion of the HA synthase
gene.]. .Iadd.segment identified in FIG. 5.Iaddend.. Through isolation of.[.the HA
gene.]. .Iadd.this DNA, .Iaddend.from whatever source is chosen, one will have the ability to realize significant advantages such as an ability to manipulate the host that is chosen to express the .[.HA synthase
gene.]. .Iadd.
recombinant DNA segment identified in FIG. 5, .Iaddend.the
fermentation environment chosen for HA production, as well as genetic manipulation of the underlying gene. As those of skill in the art will recognize in light of the present disclosure, this will provide additional significant advantages both in the ability to control the expression of the gene and in the nature of the
gene product that is realized.
In accordance with the present invention, .[.the HA synthase gene.]. .Iadd.recombinant DNA segment identified in FIG. 5, .Iaddend.when from a prokaryotic source such as a Streptococcal source, is obtained by the following general steps. First,
genomic DNA is obtained from Streptococcal cells which are capable of producing the enzyme, such as S. equisimilis strain D181. This DNA is then partially digested with a selected
restriction enzyme, such as
EcoRI, to provide fragments having an average length that is compatible with the insert length allowed by the
cloning vector that is subsequently employed. Due to the size of the .[.HA synthase enzyme.]. .Iadd.recombinant DNA segment identified in FIG. 5, .Iaddend.one will desire to obtain DNA that has an average length of up to 15 kb, and preferably about 5-10 kb. The use of the partial
digestion technique is a significant factor in the practice of the invention in that it avoids the potential problem of an incomplete gene that will be realized if the selected
enzyme gene has a
restriction enzyme .[.site within the HA synthase
coding region.]. .
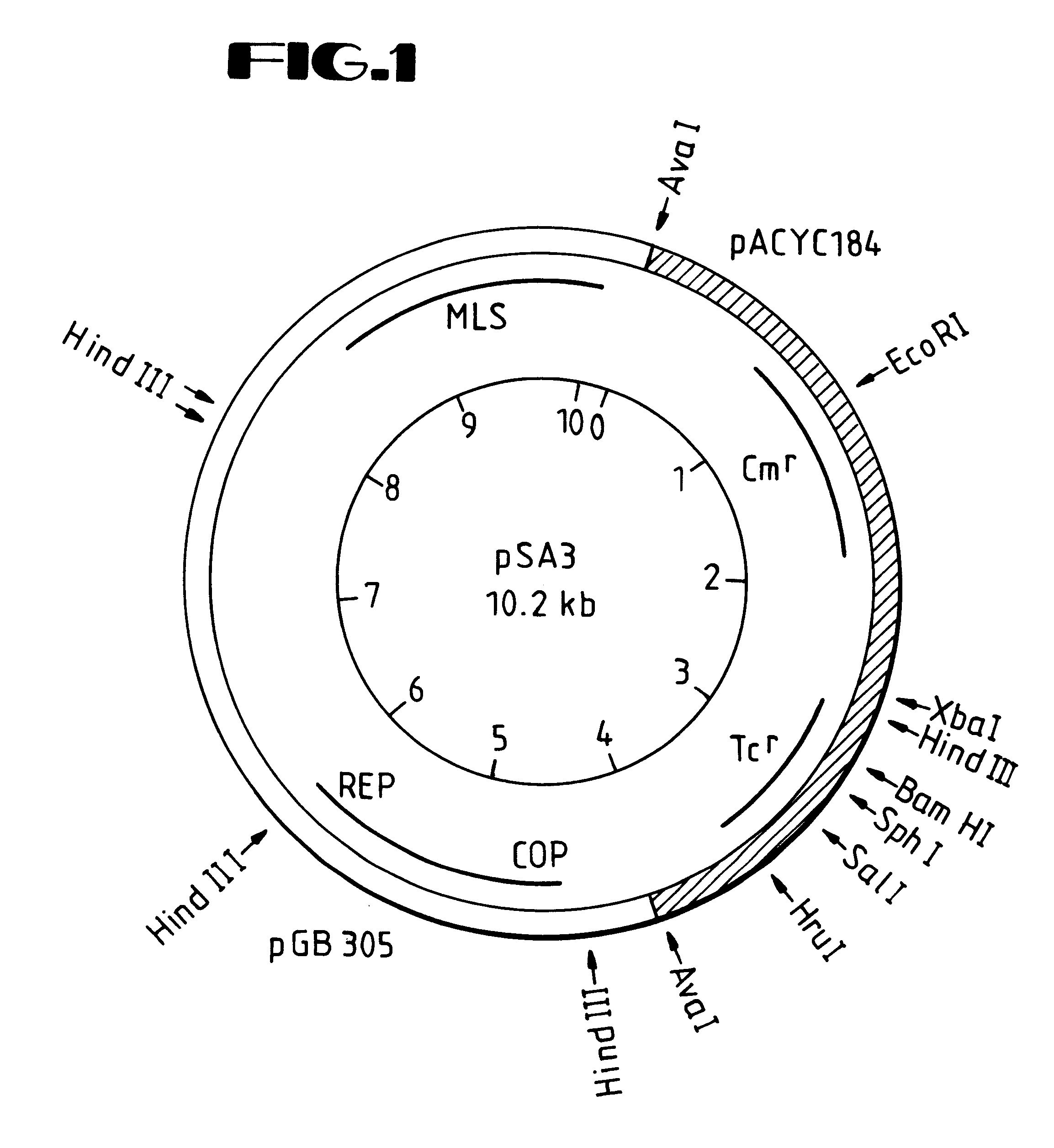
Vectors such as these, exemplified by the pSA3 vector of Dao and Ferretti (4), allow one to perform clonal colony selection in an easily manipulated host such as E. coli, followed by subsequent transfer back into a
Streptococcal strain for production of HA. This is advantageous in that one can augment the
Streptococcal strain's inherent ability to synthesize HA through gene dosaging (i.e., providing extra copies of the .[.HA synthase gene.]. .Iadd.recombinant DNA segment identified in FIG. 5 .Iaddend.by amplification). The inherent ability of the streptococci to synthesize HA can also be augmented through the formation of extra copies, or amplification, of the
plasmid that carries the .[.HA synthase gene.]. .Iadd.recombinant DNA segment identified in FIG. 5.Iaddend.. This amplification can account for up to a 10-fold increase in
plasmid copy number and, therefore, the .[.HA synthase gene.]. .Iadd.recombinant DNA segment identified in FIG. 5 .Iaddend.copy number.
Once a positive clone is identified, and the presence of .[.putative HA synthase.]. .Iadd.the recombinant DNA segment identified in FIG. 5 .Iaddend.is confirmed, it will be desirable to subject the cloned insert to
restriction enzyme mapping and DNA
sequence analysis. This will both identify the
amino acid sequence of the underlying .[.enzyme.]. .Iadd.DNA product.Iaddend., and provide the ability to further engineer the .[.HA synthase DNA.]. .Iadd.recombinant DNA segment identified in FIG. 5.Iaddend.. It is recognized that .[.HA synthase DNA.]. .Iadd.the recombinant DNA segment identified in FIG. 5 .Iaddend.may be engineered in a variety of manners and for many purposes. For example, it is possible to tailor the sequence by deletion of a transmembrane spanning domain that would normally insert the .[.enzyme.]. .Iadd.segment product .Iaddend.into the bacterial membrane. Removal of this region or regions from, and possibly addition of a leader
signal sequence to .[.the HA synthase DNA.]. .Iadd.recombinant DNA segment identified in FIG. 5 .Iaddend.would then direct the completed enzyme to be secreted from the
cell into the medium. Furthermore, manipulation and / or alteration of the underlying
nucleotide sequences can be achieved to provide for a
recombinant enzyme having improved kinetic attributes. Thus, in light of the present disclosure, once the mapping and
sequence analysis is complete, those of skill in the art will have the ability to reengineer the gene into desired expression constructs, including positioning the gene to bring it under the control of whatever
promoter system that is desired.
A preferred technique for isolation of HA is described in U.S. Pat. No. 4,517,295 in which the organic
carboxylic acid,
trichloroacetic acid, is added to the bacterial suspension at the end of the
fermentation. The
trichloroacetic acid causes the bacterial
cell to clump and die and facilitates the ease of separating these cells and associated debris from HA, the desired product. The clarified supernatant is concentrated and dialyzed to remove low molecular weight contaminants including the
organic acid. The aforementioned procedure utilizes Millipore TM
filtration through filter cassettes containing 0.22 mm pore size filters.
Diafiltration is continued until the
conductivity of the solution decreases to approximately 0.5
mega-ohms.
 Login to View More
Login to View More