Streptococcus equi subsp. Equi HLJ2018D-LX strain and application thereof in preparation of streptococcus equi subsp. Equi inactivated vaccine
A HLJ2018D-LX, Streptococcus equi technology, applied in vaccines, veterinary vaccines, bacteria and other directions, can solve the threats to the sustainable development of the donkey industry, hinder the development and spread of the donkey industry and other problems, achieve good immunogenicity and reduce morbidity. , good effect of cultivating characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Isolation and culture identification of Streptococcus equi equi subspecies donkey-derived strain HLJ2018D-LX strain
[0026] According to the new biological product declaration requirement, in conjunction with a large amount of test data that the present invention obtains, with reference to " Chinese Veterinary Pharmacopoeia " (2005 edition), the poisonous species used for making seedlings has been identified, and the poisoned species standard of the trial procedure (draft) is now carried out as follows illustrate.
[0027] 1 Source of Poison Seeds
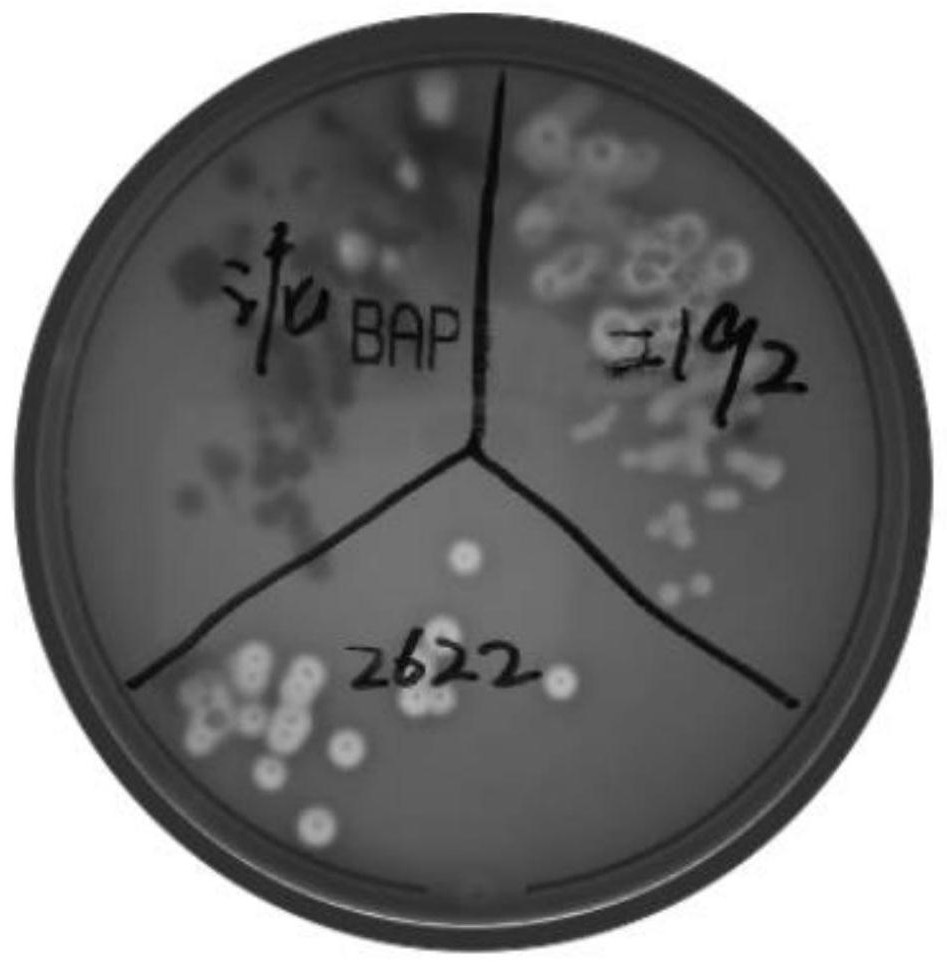
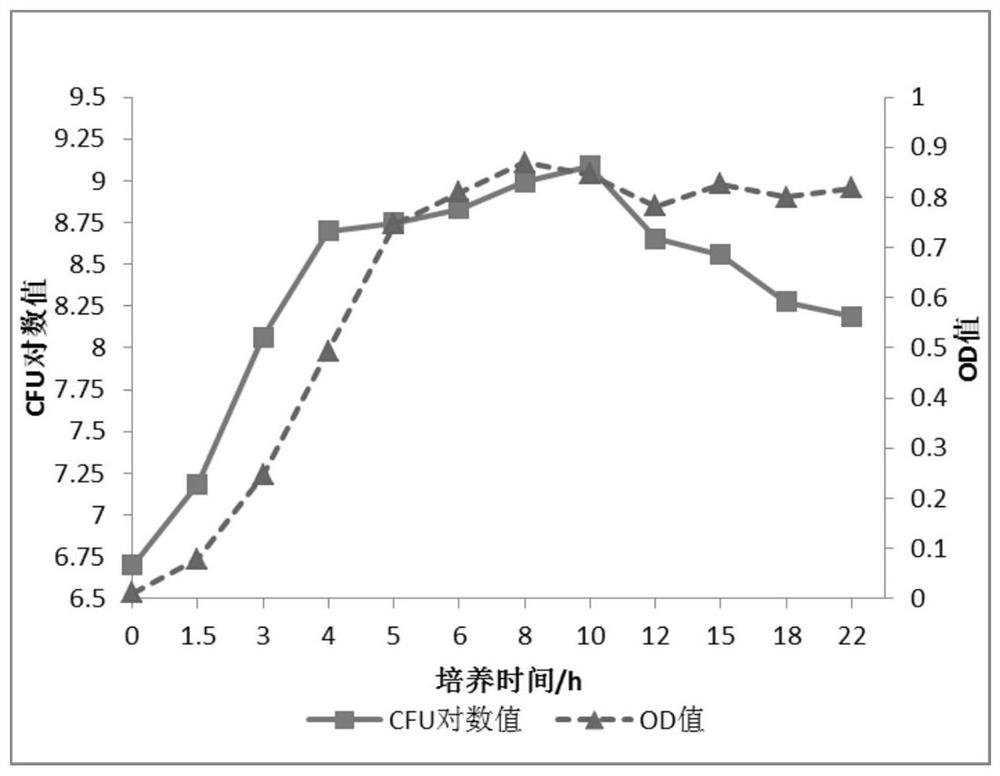
[0028] The HLJ2018D-LX strain of the present invention is obtained from the viscous purulent nasal fluid discharged from young foals caused by typical Streptococcus equi subsp. equi infection. Inoculate the nose swab of donkey foal infected with adenovirus by streaking on Columbia blood plate culture medium where typical β hemolysis occurs, such as figure 1 shown.
[0029] Inoculate the isolated and purified β-...
Embodiment 2
[0044] The preparation of embodiment 2 inactivated vaccines
[0045] 1 inactivation process
[0046] Formaldehyde solutions with final concentrations of 0.1% (v / v), 0.15% (v / v), and 0.2% (v / v) were selected for inactivation at 4, 28°C, and 37°C, and the results showed that 0.2% (v / v) Formaldehyde is inactivated at 28°C for 48 hours, and the inactivation effect is complete. Therefore, it is finally determined that 0.2% formaldehyde is inactivated at 28°C for 48 hours, coated with Columbia blood plate, cultured at 37°C for 3 days, and sterile, the inactivation is qualified, and then the inactivation is continued for 12 hours. Wash 2 times with PBS to remove formaldehyde.
[0047] 2 emulsification process
[0048] Use the commercialized MONTANIDE PET GEL A adjuvant from Sepic Company of France to mix and emulsify to make oil-in-water inactivated vaccine. Before emulsification, mix the same batch of inactivated Streptococcus equi subsp. Mixed at a ratio of 5%. Make up to volu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com