Streptococcus zooepidemicus protective antigen Sec_205 and preparation method thereof
A protective antigen, Streptococcus equi technology, applied in the direction of bacterial antigen components, chemical instruments and methods, antibacterial drugs, etc., can solve the problem that it is difficult to develop subunit vaccine infection, and achieve a good immune response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
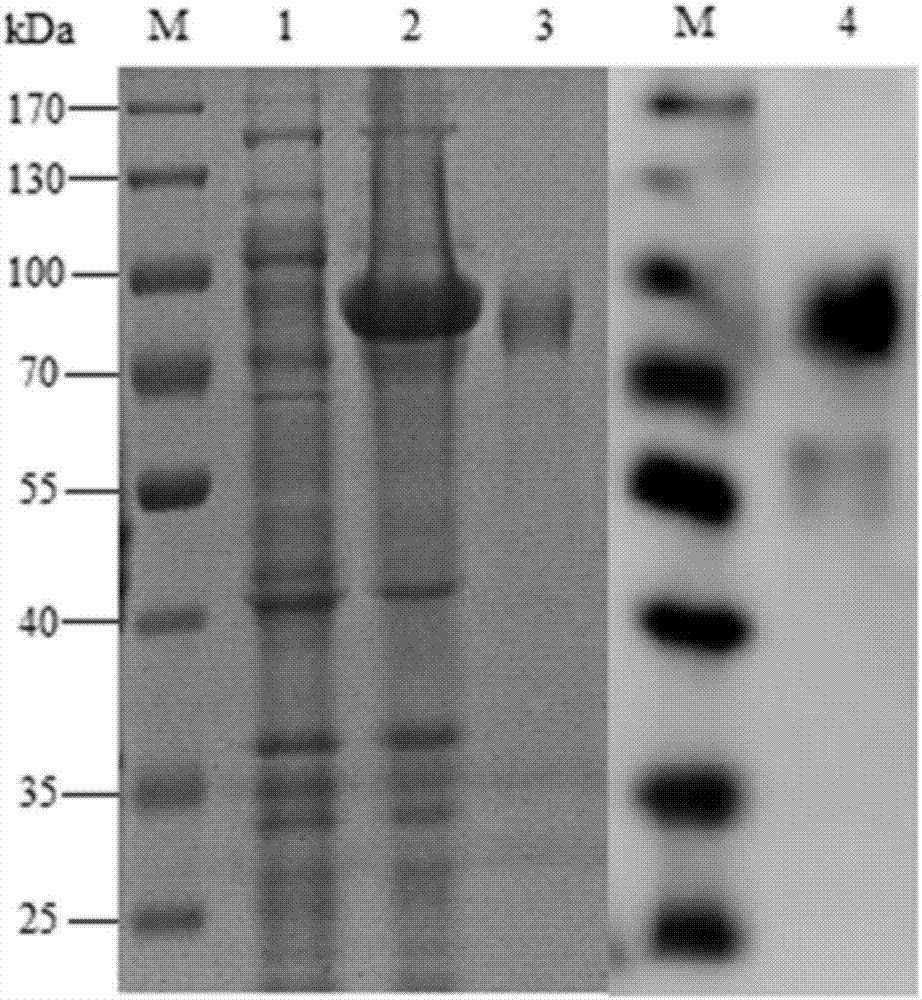
[0036] This embodiment provides a protective antigen Sec_205 of Streptococcus equi subspecies zooepidemicus, which is a recombinant protein of SEZ Sec_205, encoded by the sesec_205 gene (SEQ ID NO.1), consisting of 650 amino acid residues, with a molecular weight of 70.9 kDa, amino acid The sequence is shown in SEQ ID NO.2.
[0037] The forward primer of the sesec_205 gene has a BamH I restriction site, and the reverse primer has a Hind III restriction site. The forward and reverse primers were designed from the genome sequence of the NCBI database (Genbank CP001129.1).
[0038] Further, this embodiment also provides a method for preparing the above-mentioned Streptococcus equi subspecies zooepidemic protective antigen Sec_205, the specific steps comprising:
[0039] PCR amplification: use the genome of C55138 as a template, and use the following primers to perform PCR amplification and clone the sesec_205 gene under conventional conditions;
[0040] Forward primer (SEQ ID N...
Embodiment 2
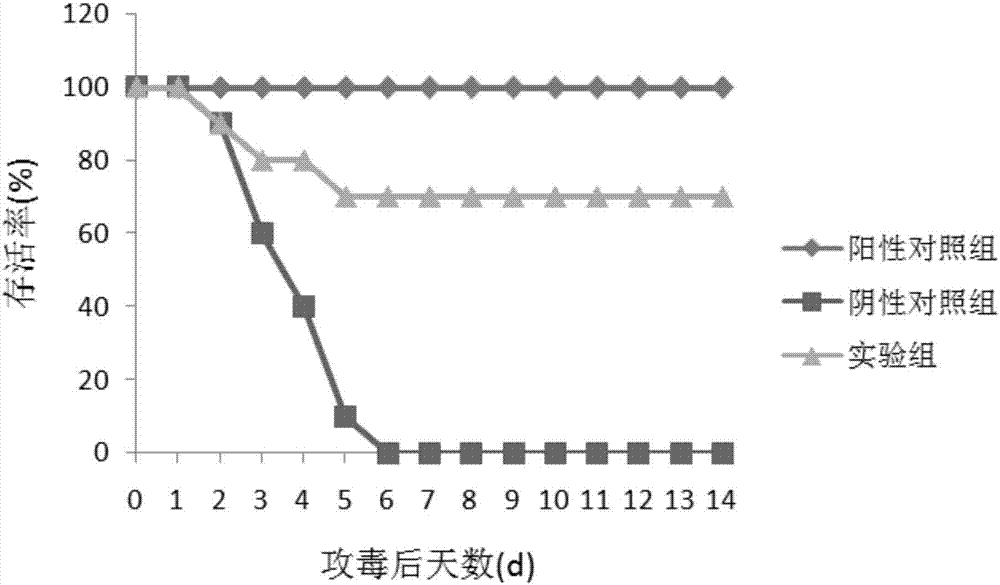
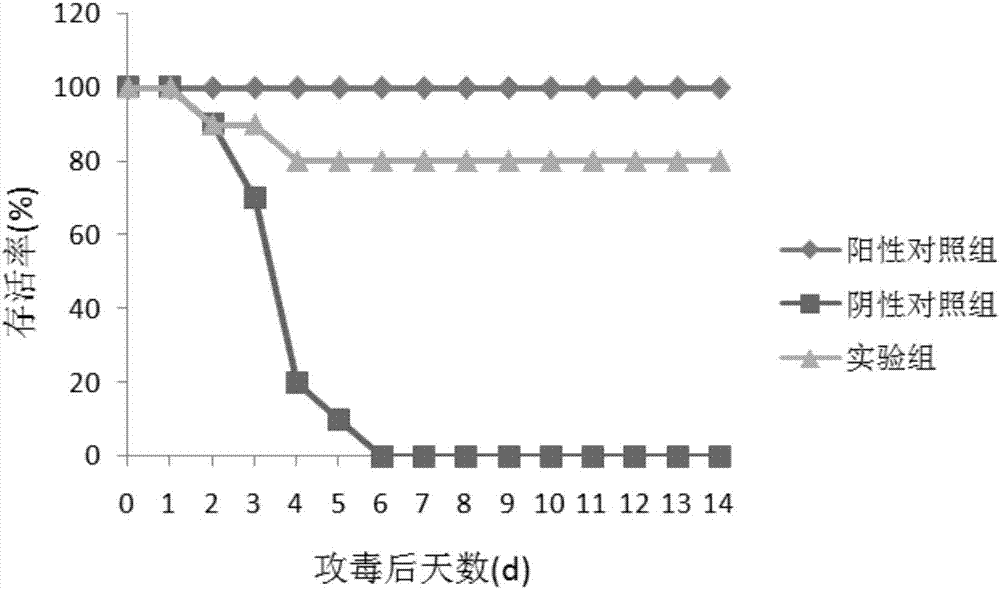
[0051] Embodiment 2 Mice immunization and virus challenge test
[0052] In this example, the SEZ Sec_205 recombinant protein obtained in Example 1 was tested for mouse immunization and challenge, and the test steps included:
[0053] 1. Thirty 6-week-old KM female mice were randomly divided into 3 groups, 10 in each group;
[0054] 2. Experimental group: After 50 μg of purified rSec_205 was emulsified with Montanide Gel 01PR (SEPPIC, France) adjuvant, the first group of mice was immunized by intraperitoneal injection, and 14 days later, the mice were immunized for the second time in the same way;
[0055] 3. Positive control group: C55138 was inactivated and emulsified with Montanide Gel 01 PR (SEPPIC, France) adjuvant, and mixed with 1×10 9 The mice were immunized with CFU / mL by intraperitoneal injection, and after an interval of 14 days, the second immunization was carried out in the same way;
[0056] 4. Negative control group: the mice in the second group were intraperit...
Embodiment 3
[0062] Embodiment three, passive protection test analysis
[0063] In order to confirm that the protection is due to Sec_205-specific stimulation of the immune response, mice were passively immunized with anti-rSec_205 mouse serum, and then challenged.
[0064] 1. Thirty 6-week-old KM female mice were randomly divided into 3 groups, 10 in each group;
[0065] 2. Experimental group: immunize the first group of mice with 100 μL anti-rSec_205 second-immune mouse serum intravenously;
[0066] 3. Positive control group: Inject the second group of mice intravenously with SEZ inactivated vaccine second-immune mouse serum;
[0067] 4. Negative control group: Inject the third group of mice intravenously with the serum of normal mice;
[0068] 5. After 24 hours of immunization, all mice were challenged with SEZ C55138 (2×10 5 CFU / mL);
[0069] 6. Observe and record the growth of all mice.
[0070] Experimental results: 1) All mice in the positive control group survived the challeng...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com