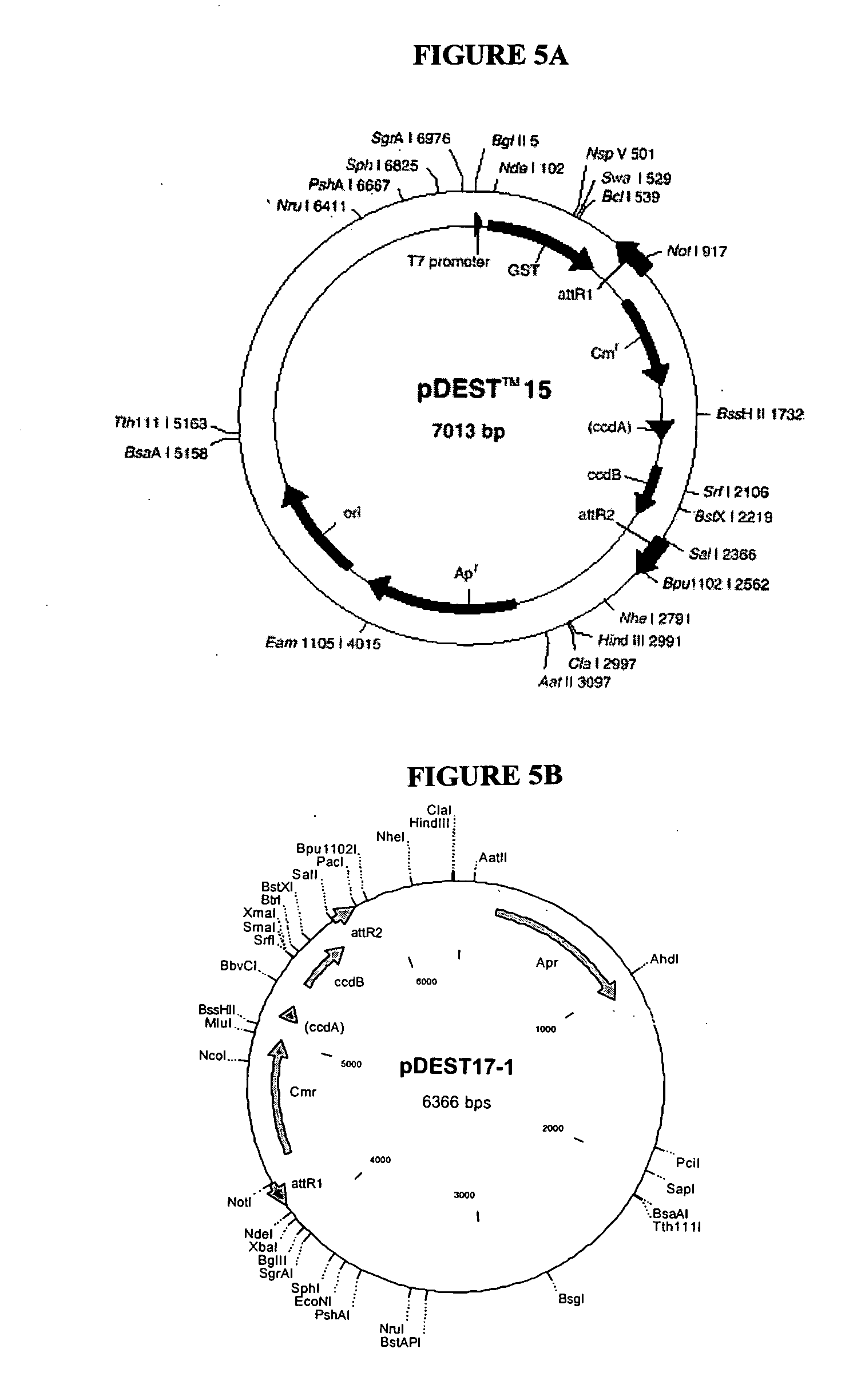
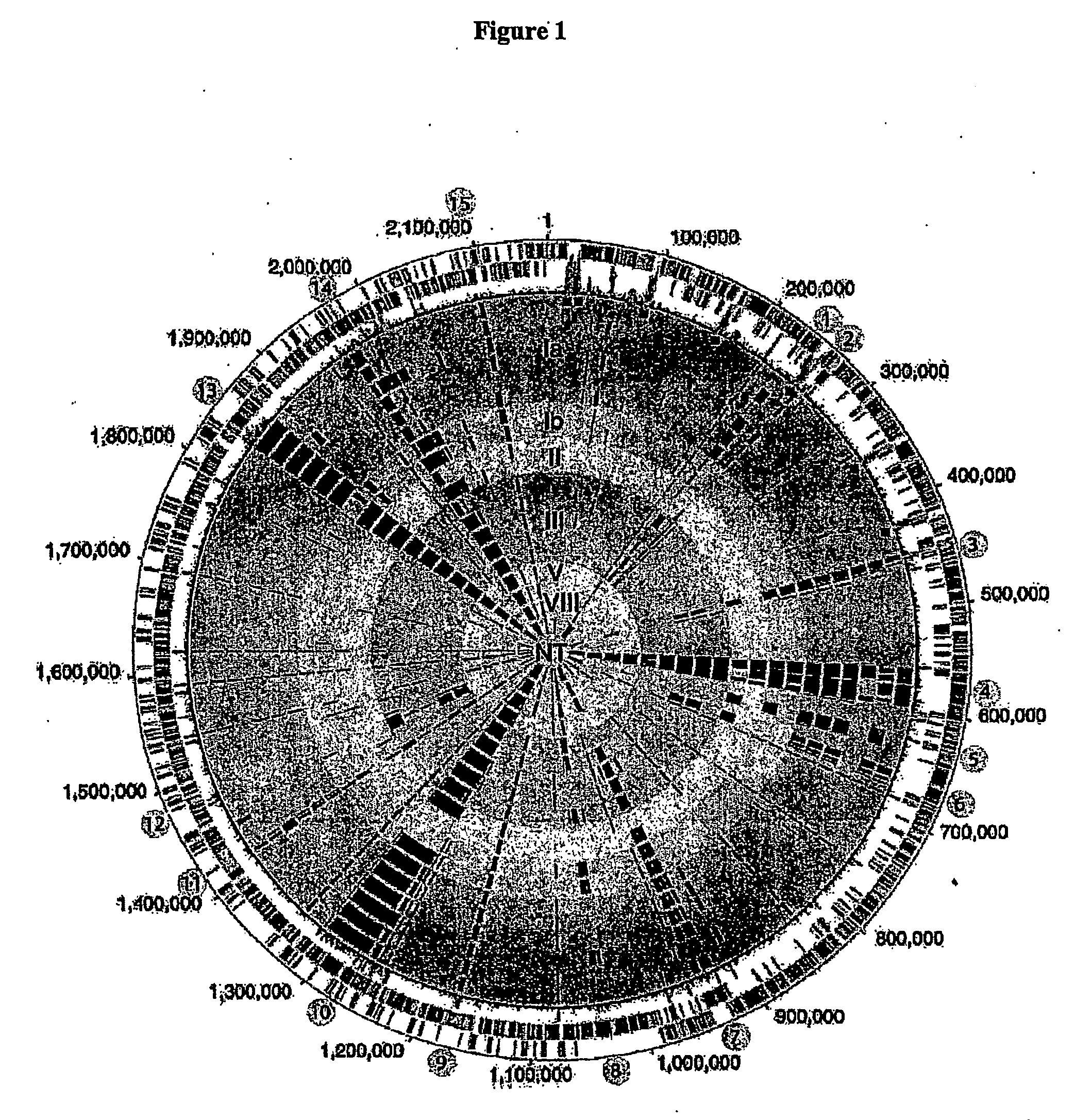
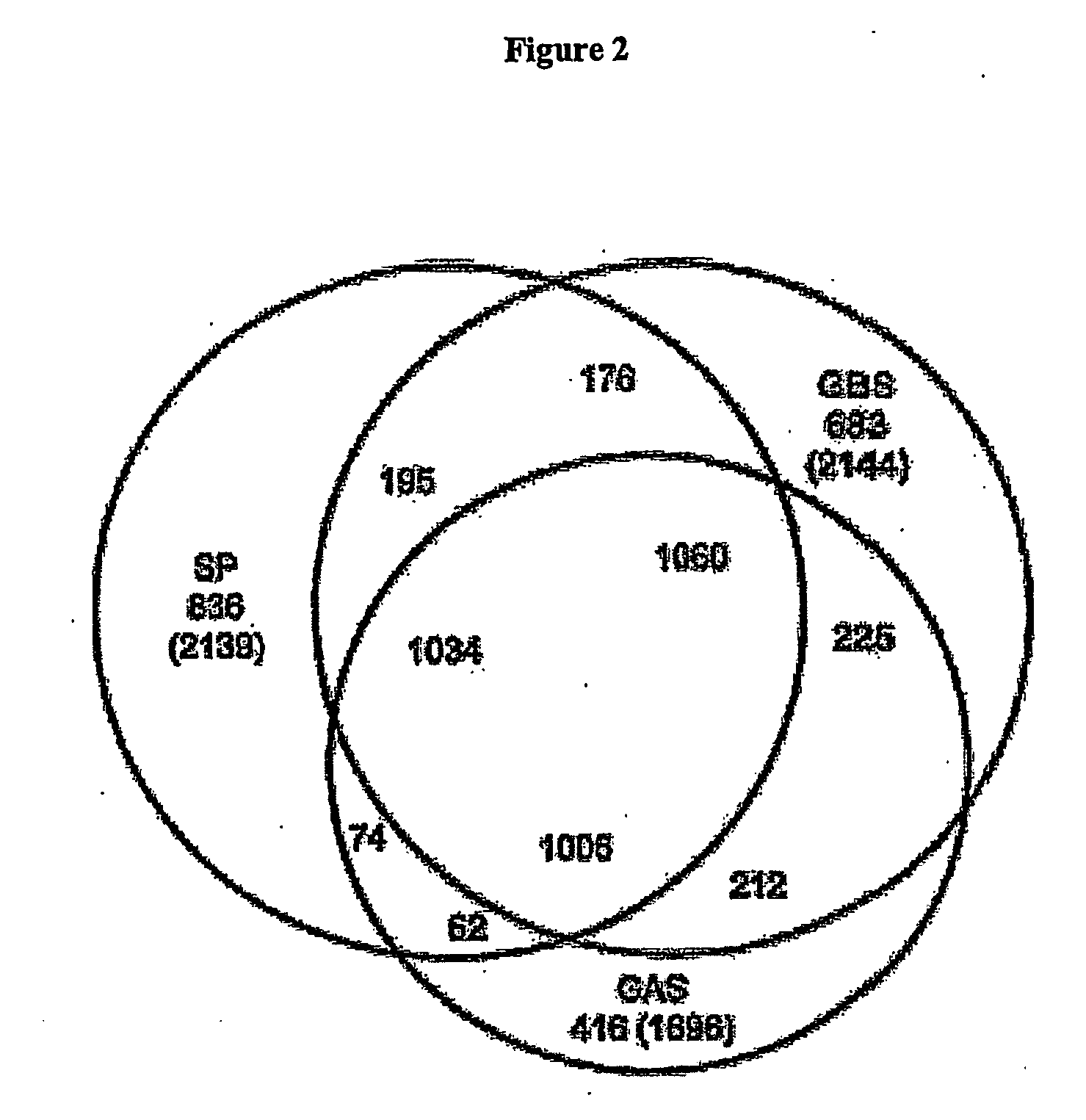
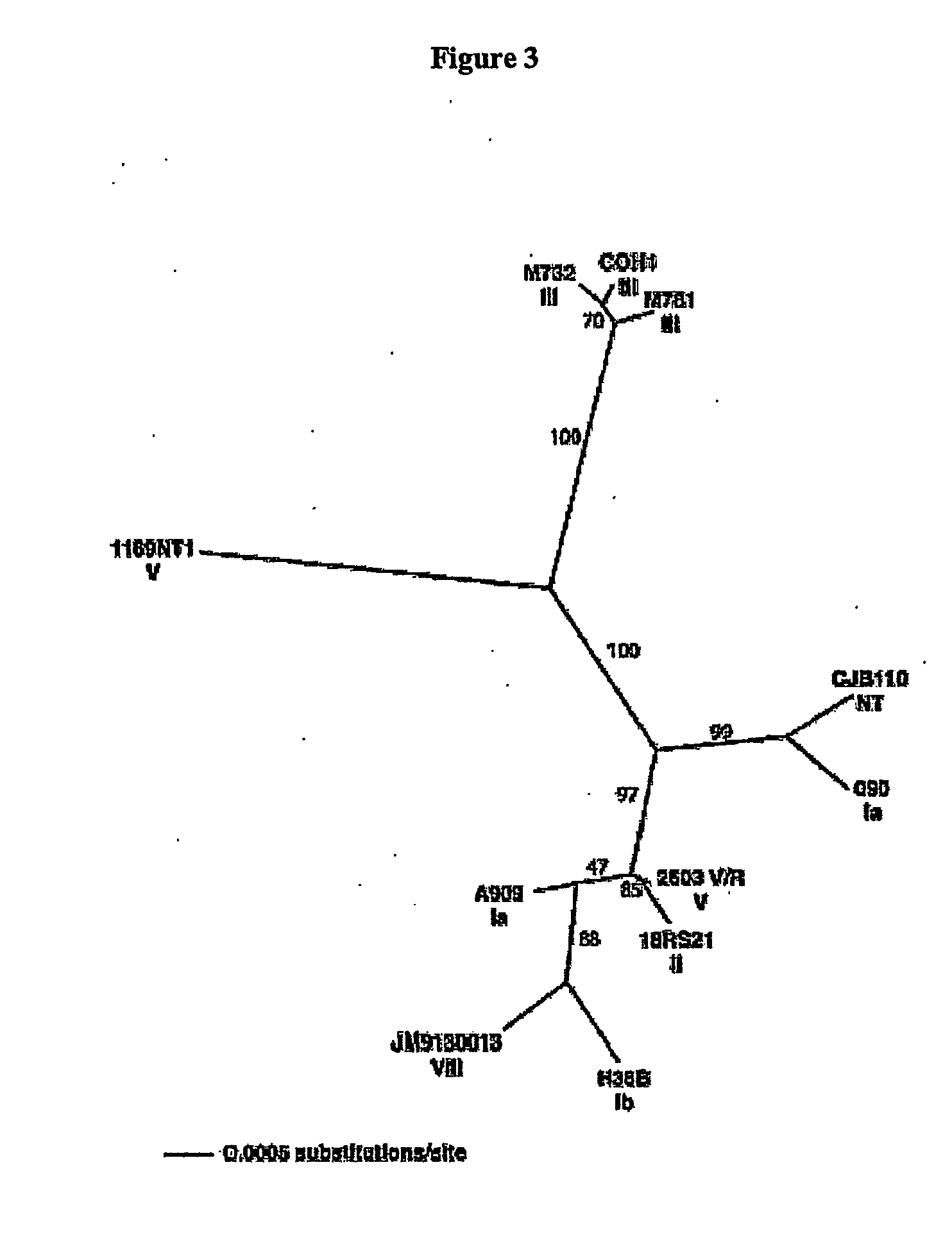
Patents
Literature
62 results about "STREPTOCOCCUS ANTIGEN" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
It is an infrequent, but usually pathogenic, part of the skin microbiota. It is the predominant species harboring the Lancefield group A antigen, and is often called group A streptococcus (GAS). However, both Streptococcus dysgalactiae and the Streptococcus anginosus group can possess group A antigen.
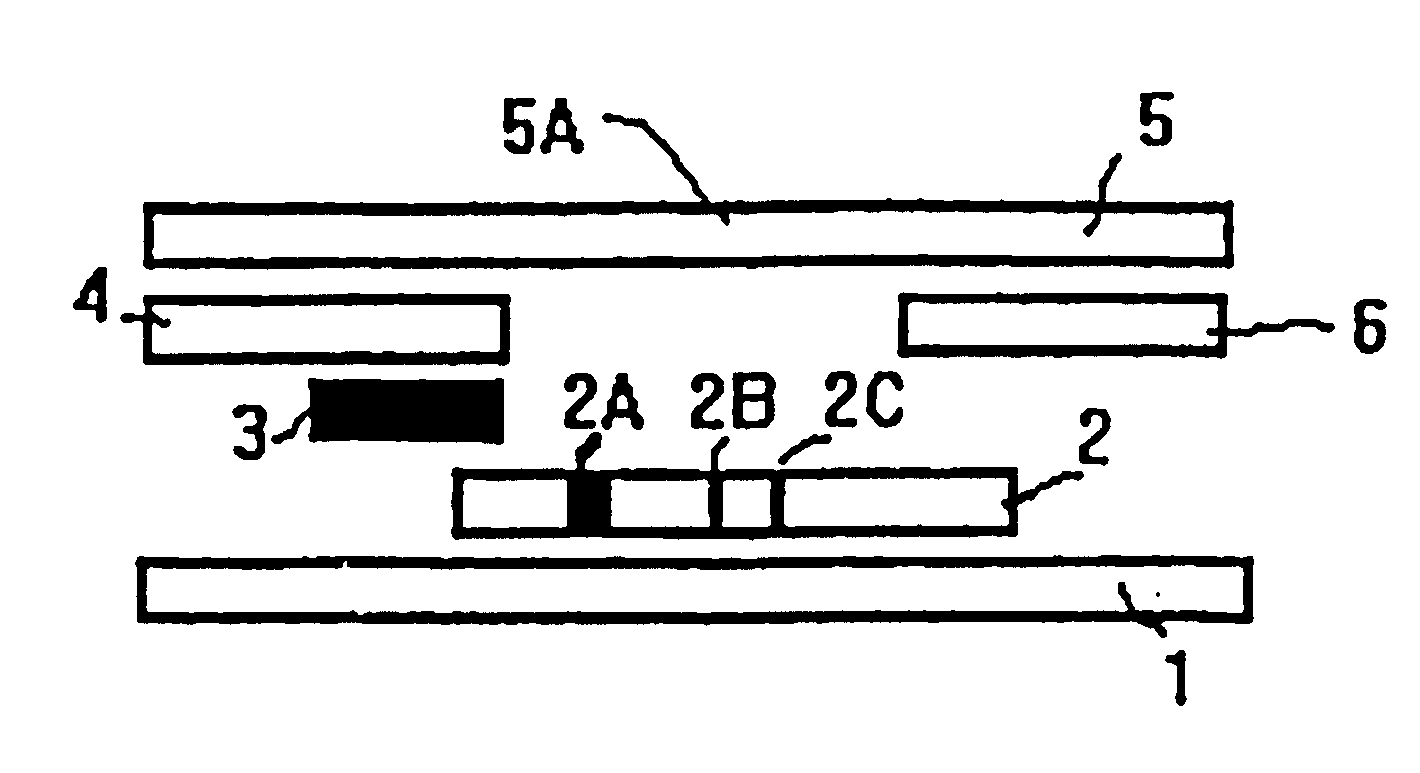
Methods of use of one step immunochromatographic device for Streptococcus A antigen
InactiveUS6979576B1Reduce the numberLess manipulationBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenReagent
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:SEKISUI DIAGNOSTICS
Nucleic acids and proteins from streptococcus groups A & B
InactiveUS20060210582A1Antibacterial agentsSenses disorderStreptococcus pyogenesStreptococcus agalactiae
The invention provides proteins from group B streptococcus (Streptococcus agalactiae) and group A streptococcus (Streptococcus pyogenes), including amino acid sequences and the corresponding nucleotide sequences. Data are given to show that the proteins are useful antigens for vaccines, immunogenic compositions, and / or diagnostics. The proteins are also targets for antibiotics.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Conserved and specific streptococcal genomes
InactiveUS20070053924A1Antibacterial agentsBacterial antigen ingredientsStreptococcus pyogenesCoccidia
The invention relates to polynucleotides which are conserved or specific to one or more species of Streptococcus, Streptococcus species serotypes, and / or serotype isolates. In particular, the invention relates to polynucleotides from Streptococcus which are conserved or specific to one or more of the species of S. pneumoniae (“pneumococcus” or “S. pn.”), S. pyogenes (“group A streptococcus” or “GAS”), and S. agalactiae (“group B streptococcus” or “GBS”). The invention further relates to polynucleotides which are conserved or specific to one or more Streptococcal species serotypes, such as GBS serotypes Ia, Ib, II, III, IV, V, VI, VII, and VIII. The invention still further relates to polynucleotides which are conserved or specific to one or more clinical isolates of a Streptococcus species.
Owner:TETTELIN HERVE +1
Novel streptococcus antigens
Streptococcus proteins and polynucleotides encoding them are disclosed. Said proteins are antigenic and therefore useful vaccine components for the prophylaxis or therapy of streptococcus infection in animals. Also disclosed are recombinant methods of producing the protein antigens as well as diagnostic assays for detecting streptococcus bacterial infection.
Owner:ID BIOMEDICAL CORP LAVAL
Streptococcus Pyogenes Antigens
The present invention relates to antigens, more particularly an antigen of Streptococcus pyogenes (also called group A Streptococcus (GAS)) bacterial pathogen which is useful as vaccine component for therapy and / or prophylaxis.
Owner:ID BIOMEDICAL
Microparticles with adsorbed polypeptide-containing molecules
ActiveUS20050220883A1Without usingEasy to produceSsRNA viruses negative-senseAntibacterial agentsHemagglutininLactide
Microparticles with absorbed polypeptide-containing molecules formed without the use of surfactant, methods of making such microparticle compositions, and uses thereof, are disclosed. The microparticles comprise a polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, and the like. Preferred polymers are poly(D,L-lactide-co-glycolides), more preferable those having a lactide / glycolide molar ratio ranging from 40:60 to 60:40 and having a molecular weight ranging from 20,000 Daltons to 70,000 Daltons. Preferred polypeptide containing molecules are bacterial and viral antigens (including HIV antigens, meningitis B antigens, streptococcus B antigens, and Influenza A hemagglutinin antigens).
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Streptococcus antigens
The present invention relates to polypeptides of Streptococcus pneumoniae which may be used for prophylaxis, diagnostic and / or therapy purposes.
Owner:ID BIOMEDICAL
Immunogenic And Therapeutic Compositions For Streptococcus Pyogenes
ActiveUS20090117113A1Antibacterial agentsWhole-cell/virus/DNA/RNA ingredientsImmunogenicityStreptococcus pyogenes
Owner:GSK VACCINES
Streptococcus antigens
Streptococcus polypeptides and polynucleotides encoding them are disclosed. Said polypeptides may be useful vaccine components for the prophylaxis or therapy of streptococcus infection in animals. Also disclosed are recombinant methods of producing the protein antigens as well as diagnostic assays for detecting streptococcus bacterial infection.
Owner:ID BIOMEDICAL
Streptococcus protective antigen C5a and preparation method thereof
InactiveCN102746388AStrong immune responseSignificant passive immune protectionAntibacterial agentsBacterial antigen ingredientsForward primerProtective antigen
The present invention relates to a streptococcus antigen C5a and a preparation method thereof. The streptococcus protective antigen C5a is an SEZC5a recombinant protein, which consists of 571 amino acids and has a molecular weight of 60.3kDa; a forward primer has one BamHI restriction enzyme cutting site, and a reverse primer has one EcoRI restriction enzyme cutting site. According to the preparation method of the streptococcus protective antigen C5a, the SEZ C5a recombinant protein is treated with cloning, expression and purification; and a series of biological engineering technologies and experiments on mice are applied to conduct system analysis on an rSCPZ. After vaccination, the rSCPZ can provide high protective efficacy; an anti-rSCPZ mice double-immunized serum has significant passive immune protection on mice; and the mice immunized by the rSCPZ show high level of antibody titer in serum. The anti-rSCPZ antibody can induce high level of bactericidal capability; an scpZ gene has a transcription level in SEZ (Streptococcus zooepidemicus) infected mice higher than that of culture in vitro; and the rSCPZ can adhere to hep-2 cells and inhibit cell infection ability of SEZ.
Owner:广东艾佩克科技有限公司
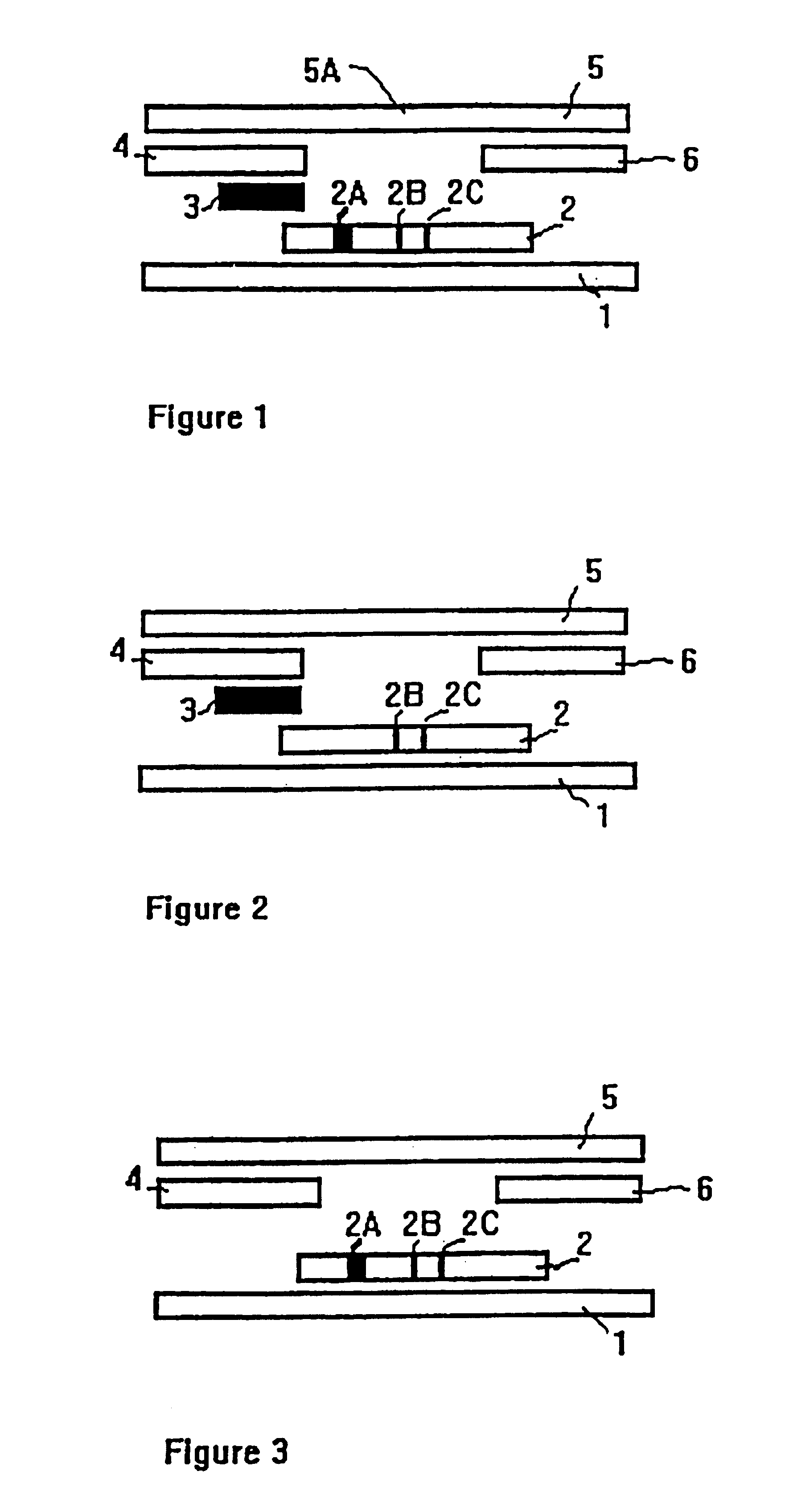
Method of use of one step immunochromatographic device for streptococcus a antigen
InactiveUS20060154315A1Reduce the numberLess manipulationBiological testingImmunoassaysReagentSTREPTOCOCCUS ANTIGEN
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:GENZYME CORP
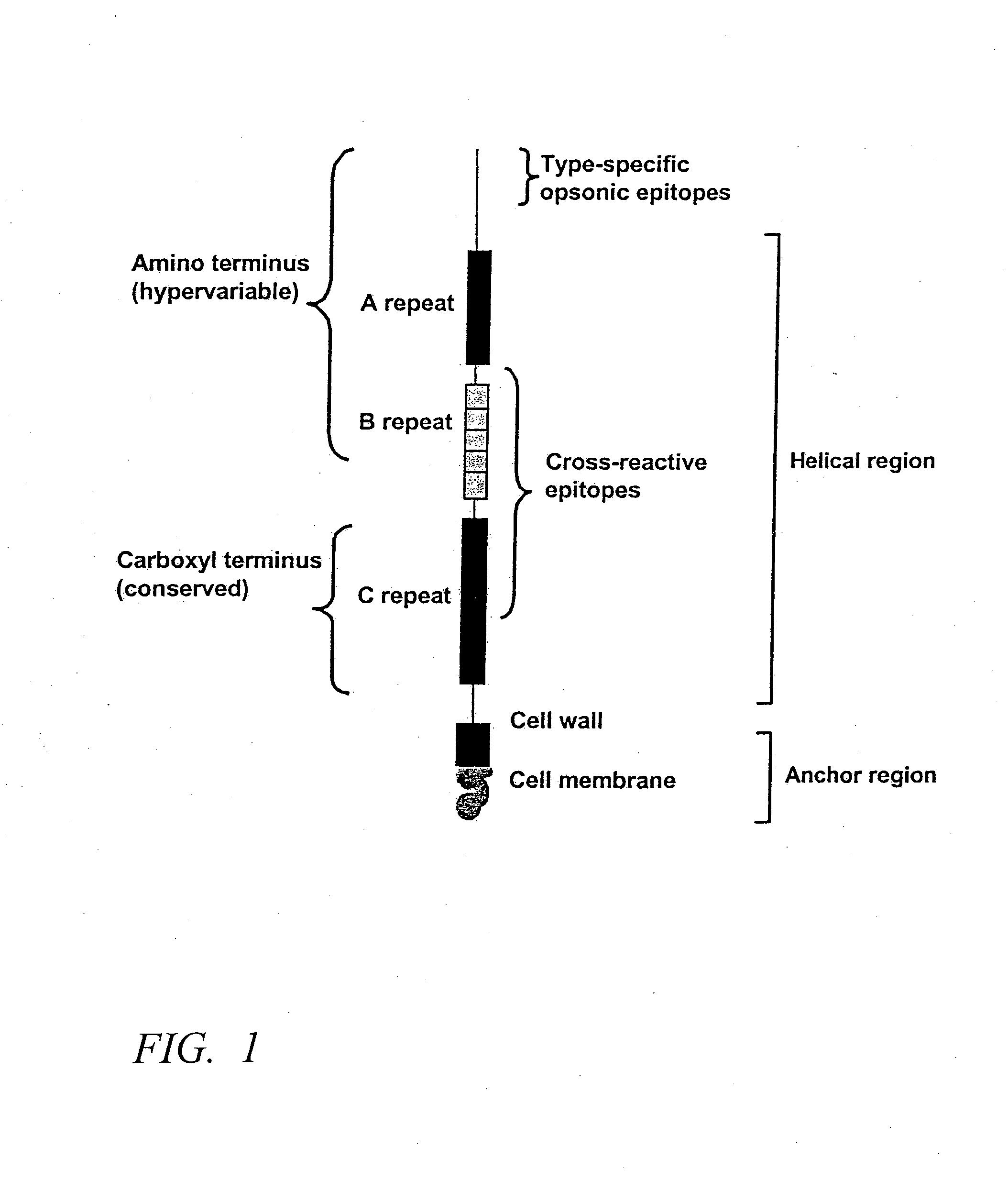
Antigen of hybrid M protein and carrier for group A streptococcal vaccine
Recombinant hybrid streptococcal M protein antigens are provided which elicit protective antibodies against Group A streptococci and prevent rheumatic fever. Recombinant hybrid genes which encode the antigen are provided. Vaccine compositions and methods of administering the compositions are provided to elicit immunity against Group A streptococci.
Owner:UNIV OF TENNESSEE RES FOUND
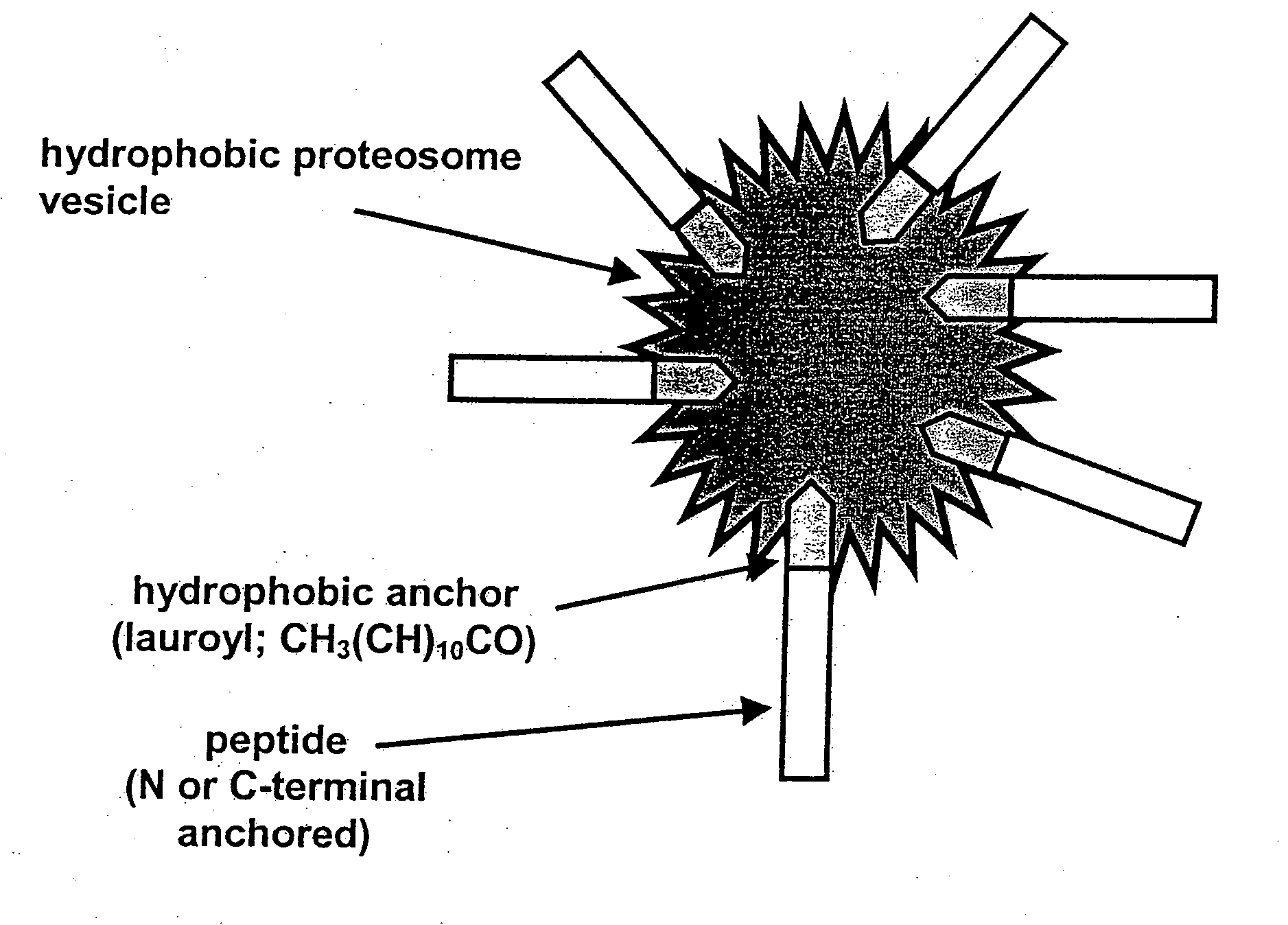
Vaccine
Effective stimulation of immune responses is achieved through the use of a group A streptococcal antigen combined with proteosome adjuvant. The compositions are provided in particular for intranasal administration. The vaccine compositions are provided for use in inducing an immune response in an individual for the treatment or prophylaxis of group A streptococcal infection in an individual, preferably via prevention or reduction of colonisation of the throat following intranasal administration.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES +1
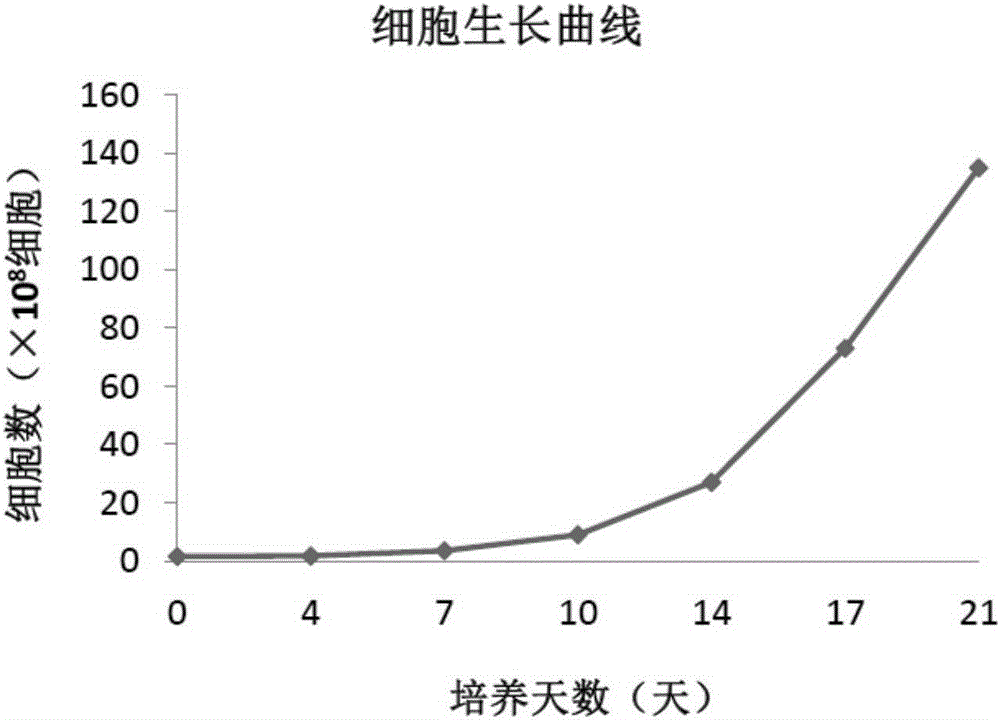
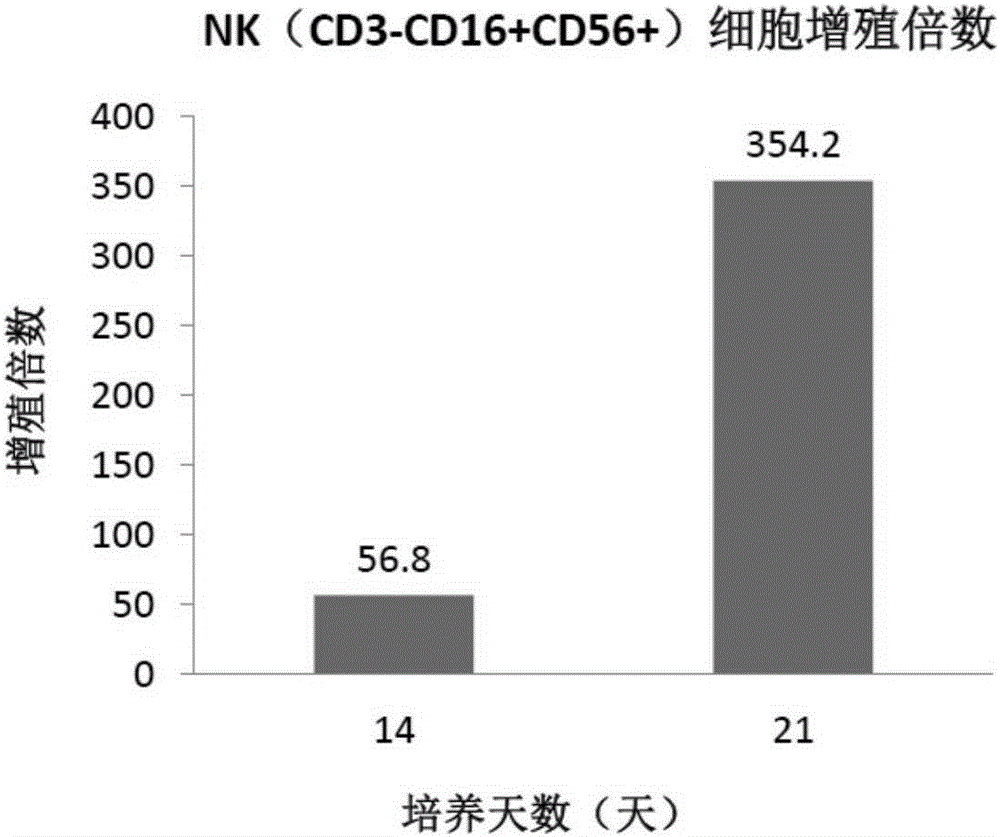
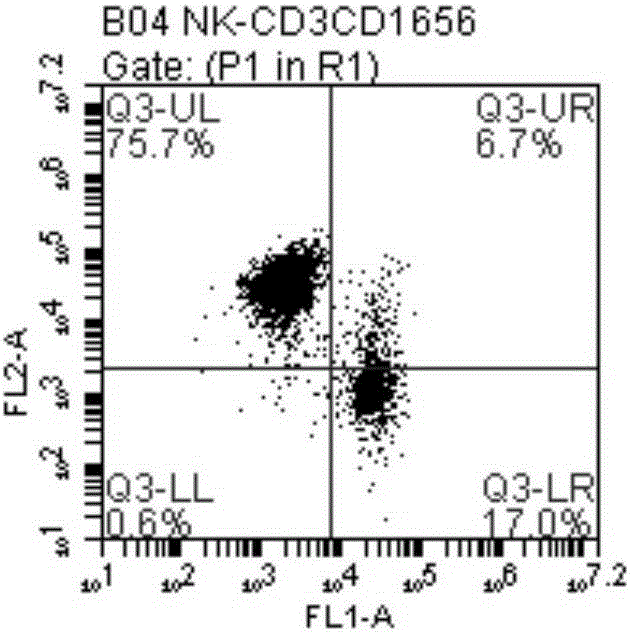
Method for amplifying NK cells through in-vitro cultivation
InactiveCN105176926AIncrease the number ofHigh purityBlood/immune system cellsSerum igeSerum free media
The invention relates to a method for amplifying NK cells through in-vitro cultivation, in particular to a method for greatly amplifying the NK cells from autologous peripheral blood mononuclear cells through in-vitro cultivation. The method includes the steps of activating the NK cells with group-A streptococcus preparations as the irritant and cultivating the NK cells through the combined common stimulation of cell factors IL-2 so that the obtained NK cells can have the advantages of being large in number, high in purity and cytotoxicity and the like and can meet the clinical requirements. Specifically, the autologous peripheral blood mononuclear cells are inoculated into a serum-free medium to be cultivated, and the group-A streptococcus preparations and the cell factors IL-2 are added for inducing activation and proliferation. The method is easy, convenient and rapid to operate; the NK cells can be activated and amplified only through the group-A streptococcus preparations and the cell factors IL-2; the amplification times, flow phenotype and cytotoxicity of the NK cells are ensured, the cultivation cost of the NK cells is greatly reduced, and use and popularization are simple and easy.
Owner:SHANGHAI CLAISON BIOTECH
Therapeutic vaccine for malignant tumors and composition thereof
The invention relates to a therapeutic vaccine for malignant tumors and a composition thereof. The therapeutic vaccine for malignant tumors is a tumor cell line which contains plasmid of antisense nucleic acid of human transforming growth factor beta (TGF-beta2); the therapeutic vaccine composition for malignant tumors comprises the therapeutic vaccine for malignant tumors and an immunopotentiator, and the immunopotentiator is one selected from the group consisting of a Corynebacterium parvum preparation, a non-cell Corynebacterium parvum preparation, a BCG polysaccharide, a nucleic acid preparation, a Nocardia rubra-cell wall skeleton preparation, a group A Streptococcus preparation, a non-cell group A Streptococcus preparation, a Pseudomonas aeruginosa preparation, a non-cell Pseudomonas aeruginosa preparation, a Brucella preparation, a non-cell Brucella preparation, a non-cell Mycobacterium vaccae preparation and a non-cell Mycobacterium smegmatis preparation, preferably the non-cell Corynebacterium parvum preparation; and the malignant tumors include a lung cancer, a liver cancer, a pancreatic cancer, leukemia, lymphoma, an ovarian cancer, a colon cancer, a stomach cancer and a breast cancer.
Owner:熊慧
Methods of use of one step immunochromatographic device for streptococcus a antigen
InactiveUS20050277163A1Avoid lateral flowReduce the numberBiological testingImmunoassaysReagentSTREPTOCOCCUS ANTIGEN
A method to determine the presence or absence of Streptococcus Group A antigen in a sample, comprising the following steps: extracting the antigen from the sample in an assay chamber with two or less extraction reagents, wherein the two reagents may be added to the assay chamber in no particular sequence; introducing a lateral flow immunochromatographic assay device into the extraction reagents containing the extracted antigen without further addition of reagents or manipulation of the sample; forming an antigen-indicator labeling reagent complex; and determining the presence or absence of the antigen in the sample by the presence or absence of a signal formed by the binding of the antigen-indicator labeling reagent complex to an indicator capture reagent specific for said antigen-indicator labeling reagent complex.
Owner:GENZYME CORP
Diagnostic and therapeutic methods for rheumatic heart disease based upon group a streptococcus markers
InactiveUS20120276130A1Enhance cellular immunityIncrease in antigen-specific CTL productionAntibacterial agentsBacterial antigen ingredientsStreptococcus pyogenesRheumatic Heart Diseases
This invention is in the field of identifying patients having rheumatic heart disease (RHD) associated with Streptococcus pyogenes (Group A Streptococcus; GAS) infection and identifying patients at risk of developing RHD associated with GAS infection. The invention also provides methods and compositions for preventing and treating RHD associated with GAS infection.
Owner:MARGARIT & ROS IMMACULADA +4
Group b streptococcus antigens
The present invention relates to polypeptides, epitopes and antibodies directed to these epitopes, more particularly to the Sip polypeptide of Group B streptococcus (GBS), also called Streptococcus Agalactiae which may be used to prevent, diagnose and / or treat streptococcal infection.
Owner:ID BIOMEDICAL
Group A streptococcus oligosaccharide protein conjugate as well as preparation method and application thereof
InactiveCN109045292AImprove applicabilityUnrestricted drug resistance problemAntibacterial agentsBacterial antigen ingredientsAntigenC5a peptidase
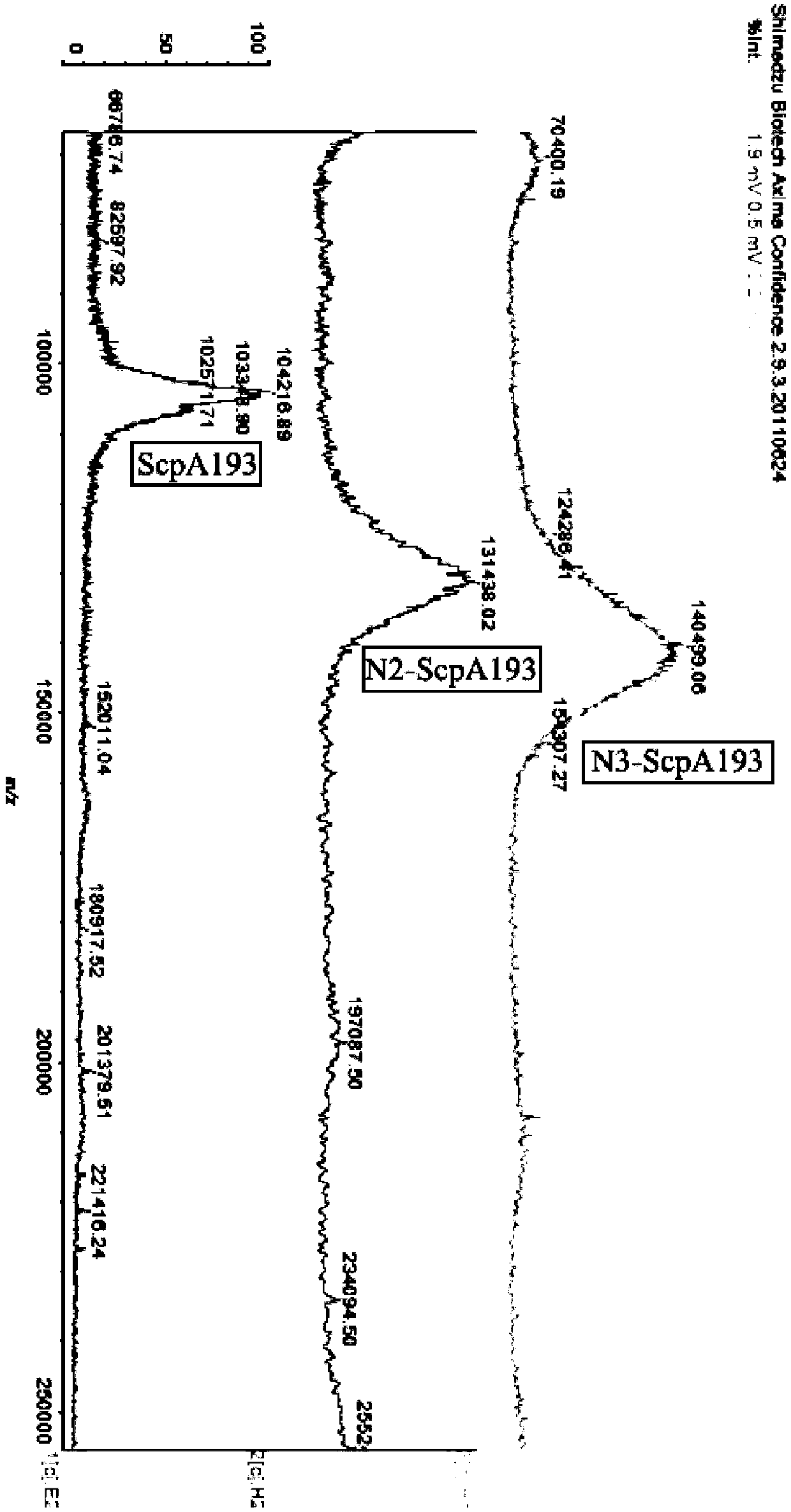
The invention relates to a group A streptococcus oligosaccharide protein conjugate as well as a preparation method and application thereof. In the group A streptococcus oligosaccharide protein conjugate, an oligosaccharide antigen is a clear and single oligomeric hexaose or nonaose derivative which can be synthesized according to a chemical method, a connecting arm is succinimide, and used novel carrier protein ScpA193 is a group A streptococcus virulence factor C5a peptidase deactivated through directional mutation. The invention further relates to the preparation method of the group A streptococcus oligosaccharide protein conjugate and application thereof to preparation of an anti-group A streptococcus vaccine. The group A streptococcus oligosaccharide protein conjugate can be simultaneously induced into two types of antibodies with different targets synergistically resisting group A streptococci, and is a composite vaccine. The vaccine has better applicability, can more effectivelyinhibit the group A streptococci, and is not limited by the bacterial resistance problem caused by large-scale use of antibiotics.
Owner:SHANDONG UNIV
Streptococcus suis vaccines and diagnostic tests
InactiveUS20070111236A1Accurate responseAccurate measurementAntibacterial agentsBacteriaAntigenDiagnostic test
The invention relates to Streptococcus suis infection in pigs, vaccines directed against those infections and tests for diagnosing Streptococcus suis infections. The invention provides an isolated or recombinant nucleic acid encoding a capsular gene cluster of Streptococcus suis or a gene or gene fragment derived thereof. The invention further provides a nucleic acid probe or primer allowing species or serotype-specific detection of Streptococcus suis. The invention also provides a Streptococcus suis antigen and vaccine derived thereof.
Owner:STICHTING WAGENINGEN RES
Group A Streptococcus multivalent vaccine
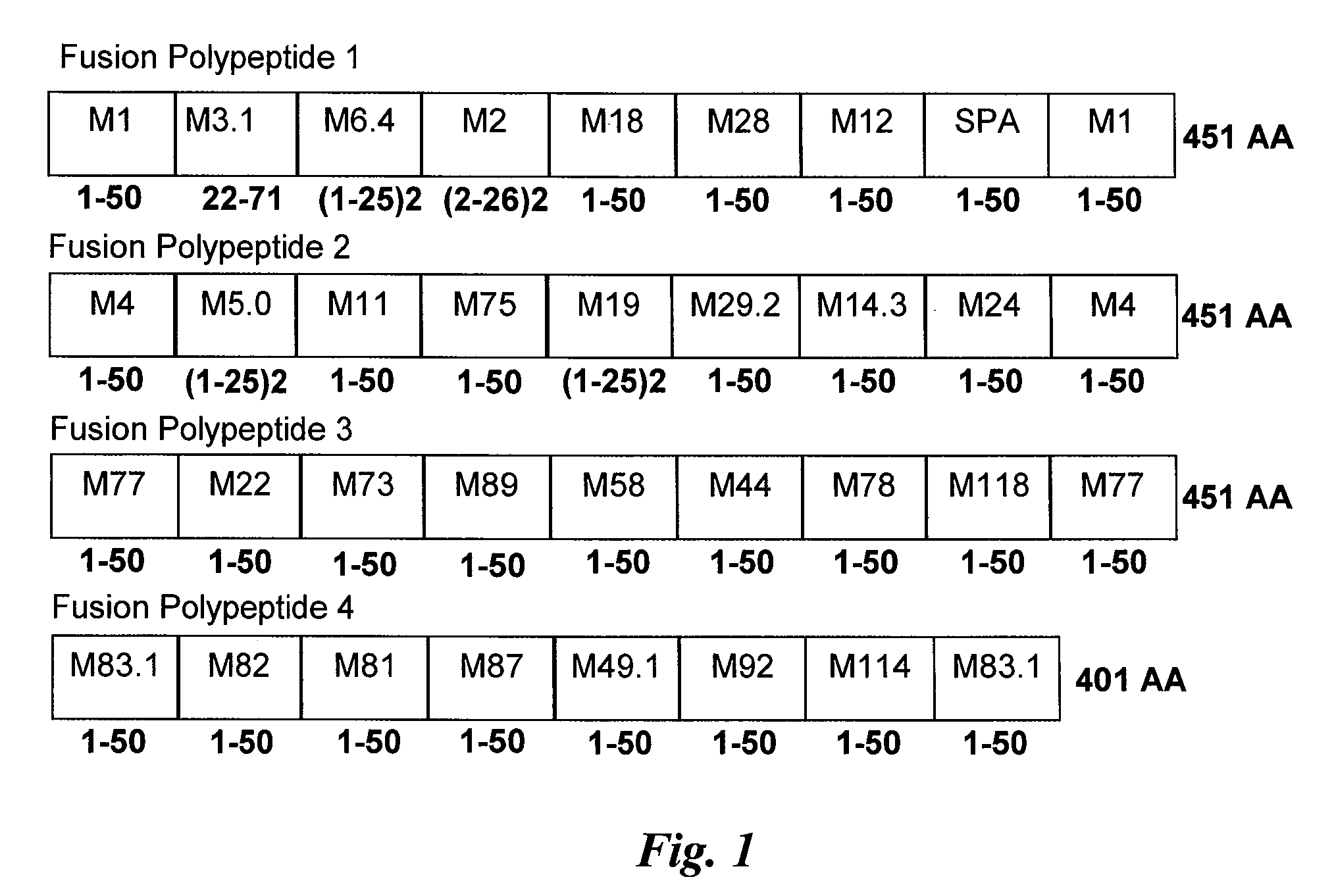
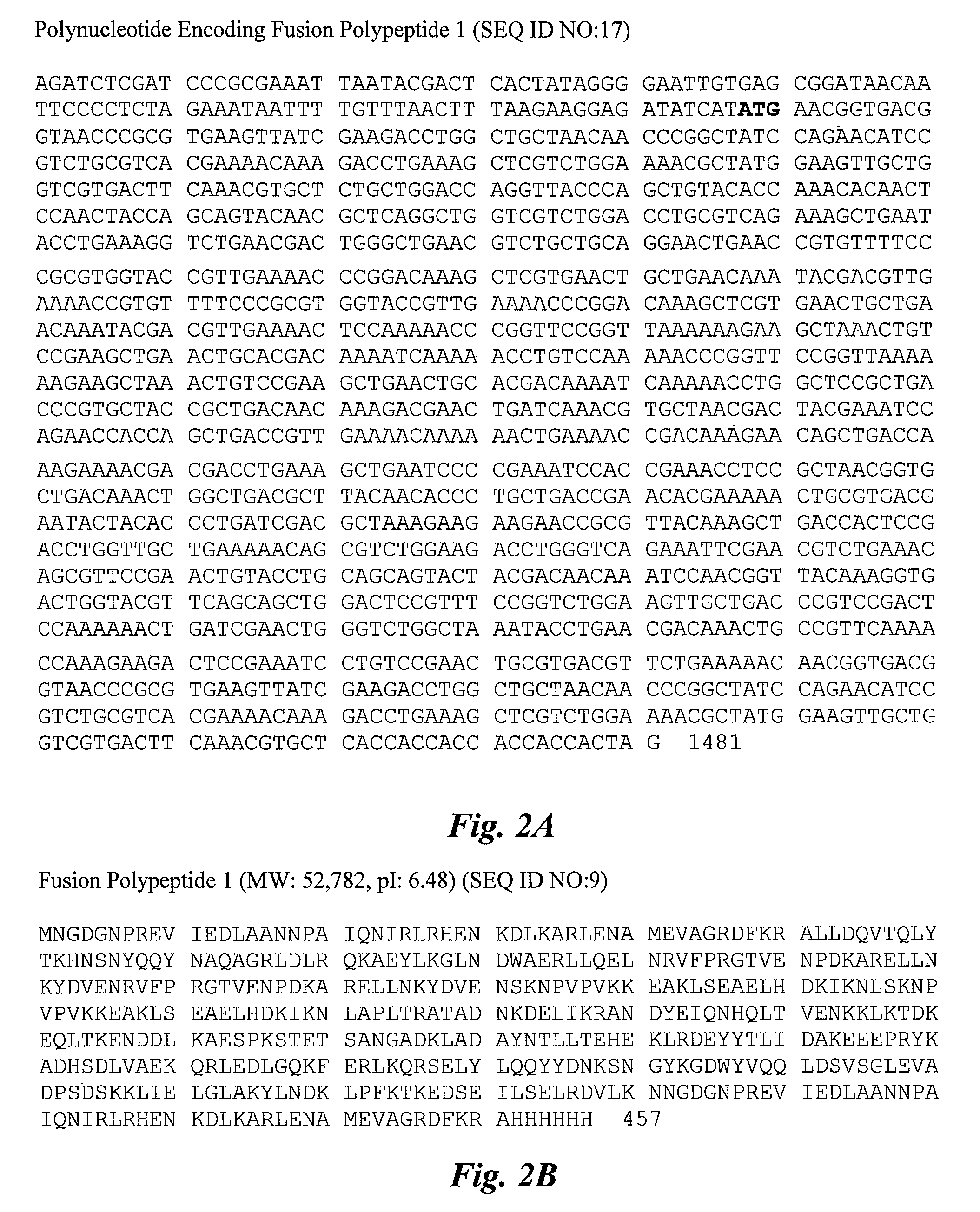
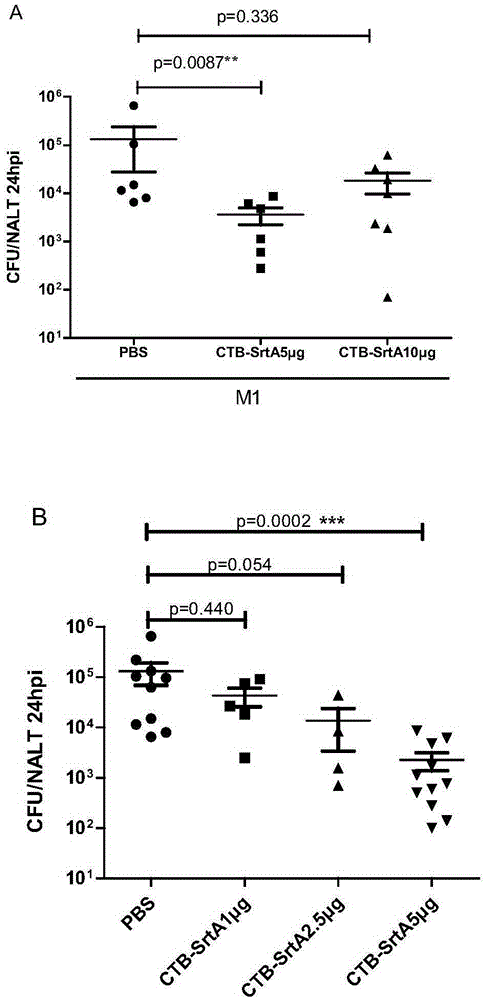
Immunogenic compositions are provided herein that are useful for inducing an immune response specific against group A streptococcus (GAS). Immunogenic compositions provided herein are multivalent and comprise a plurality of immunogenic peptides or fusion polypeptides comprising the immunogenic peptides that induce an immune response against GAS. The immunogenic compositions provided herein induce an immune response against the GAS serotypes represented by an immunogenic peptide (derived from an M protein or Spa protein) comprised within the immunogenic composition and also induce an immune response against serotypes that are unrepresented by any immunogenic peptide included in the immunogenic composition. Methods for using the compositions for inducing an immune response against GAS and for treating or reducing the likelihood of occurrence of a GAS infection are also provided.
Owner:UNIV OF TENNESSEE RES FOUND
Fusion protein vaccine capable of inhibiting Streptococcus and/or preventing Streptococcus infection
ActiveCN106554421AEasy to removeReduce dosageAntibacterial agentsFungiSide effectStreptococcus infection
The invention discloses a fusion protein vaccine capable of inhibiting Streptococcus and / or preventing Streptococcus infection. With fusion protein obtained through linkage of sortase A and a cholera toxin B subunit through connecting peptide, the Th17 cell activation level is remarkably increased, the effects of preventing pathogenic bacteria from settling and quickly removing the pathogenic bacteria can be realized, and the fusion protein has protecting effects on Group A Streptococcus of different serotypes and has the superiority of high efficiency, broad spectrum and low cost. With the fusion protein, the use amount of immunogen is reduced, the production technology is simplified, and the production cost is reduced. Meanwhile, the vaccine adopts the way of mucosal immunity, has the characteristics of being free of tissue damage, free of local side effects and convenient to use, and is easy to popularize and use.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
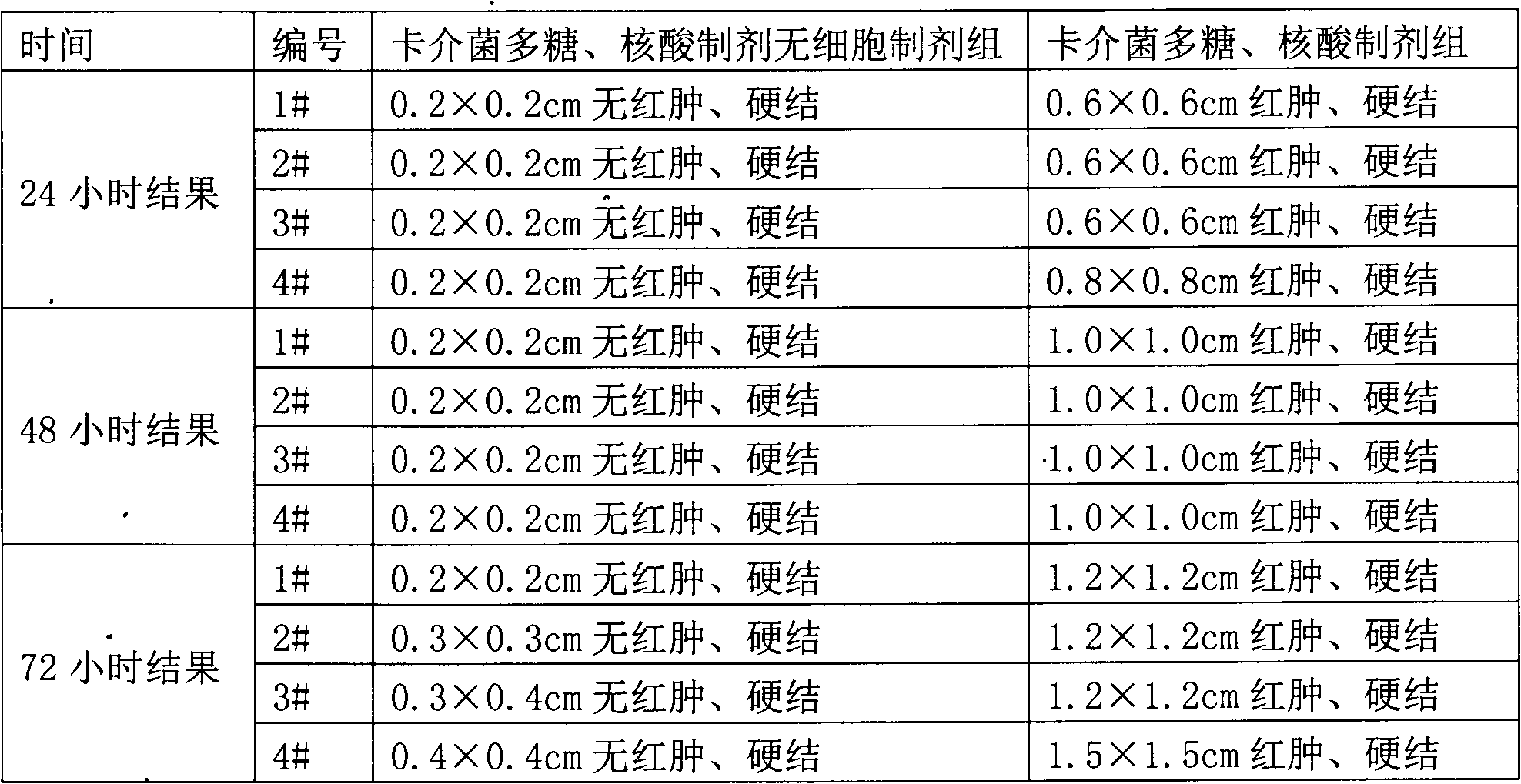
Cell-free preparations of immunopotentiators, and preparation methods and uses thereof
InactiveCN103505476AUniform particle sizePromote absorptionAntibacterial agentsOrganic active ingredientsGranularityBrucella
The invention relates to a cell-free preparation of a pseudomonas preparation, a cell-free preparation of a Bacillus Calmette-Guerin polysaccharide and nucleic acid preparation, a cell-free preparation of a Nocardia rubra cell wall skeleton preparation, a cell-free preparation of a Group A Streptococcus preparation, a cell-free preparation of a Pseudomonas aeruginosa preparation, and a cell-free preparation of a Brucella preparation. The above cell-free preparations have a granularity of 10-1000nm, preferably 10-800nm, and more preferably 10-500nm. The pyrogens of Gram-positive bacteria are below 320EU / ml, and preferably below 120EU / ml. The preparation methods of the cell-free preparations comprise the following steps: heating the preparations for boiling for 15-60min to obtain inactivated bacterial liquids; washing aseptic-test-qualified bacterial liquids, and breaking thalli under an aseptic condition by using a breaker; centrifuging a suspension obtained after breaking the thalli, collecting the above obtained precipitate, and washing the precipitate to prepare a suspension; and packaging the suspension, and carrying out heating disinfection to obtain the cell-free preparations of the preparations. The invention also relates to applications of the cell-free preparations.
Owner:熊慧
Method for preparing A-group streptococcus agent by bloodless medium and bloodless medium
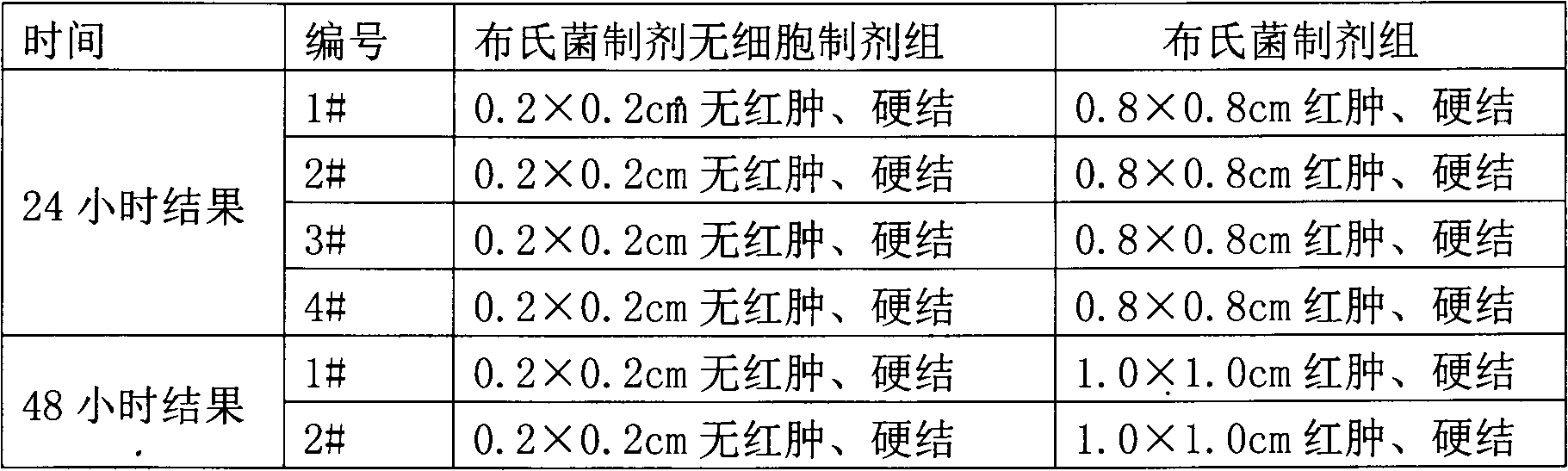
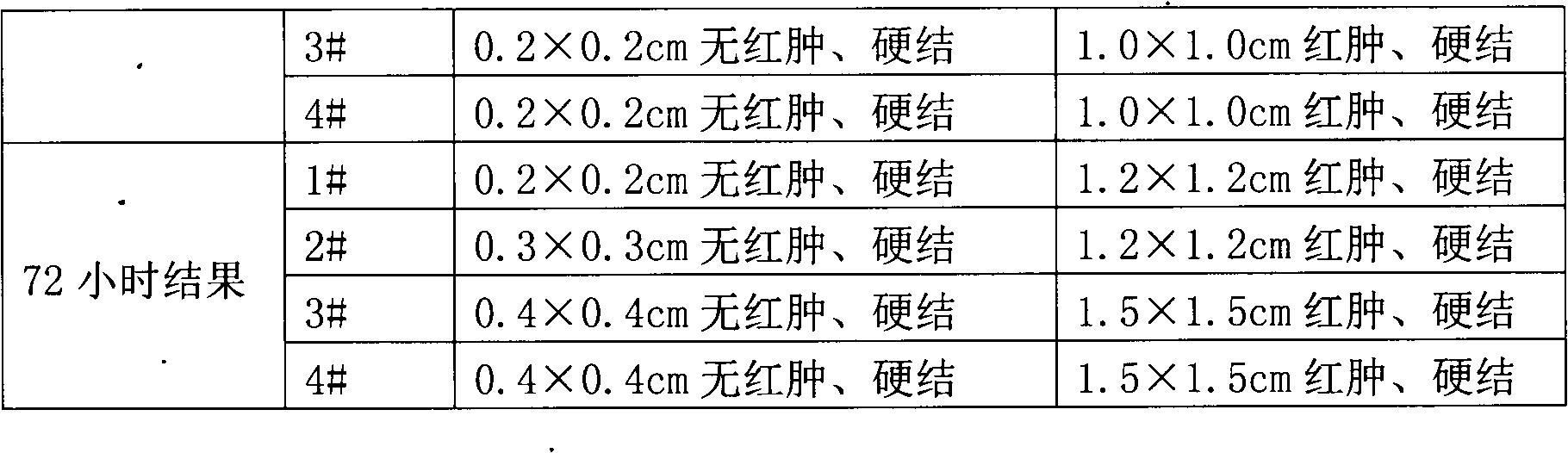
The invention discloses a preparing method of A-group streptococcus agent and avascular culture medium, which comprises the following steps: generating A-group streptococcus weak-toxic strain working seed; preparing seed liquid in the avascular culture medium; inactivating the cultured material; freezing and drying; obtaining the product. The avascular culture medium contains 1.2% tryptone, 0.9% glucose, 0.4% yeast powder, 0.5% sodium chloride, 0.5% potassium dihydrogen phosphate and water, wherein the pH value is 7.4-7.6.
Owner:CHANGCHUN UNIV OF TECH
Assay for diagnosing Streptococcus pneumoniae
InactiveUS8404457B2High detection sensitivityIncrease valuePeptide librariesMicrobiological testing/measurementAntigenStreptococcus pneumoniae
Owner:SANOFI PASTEUR LTD
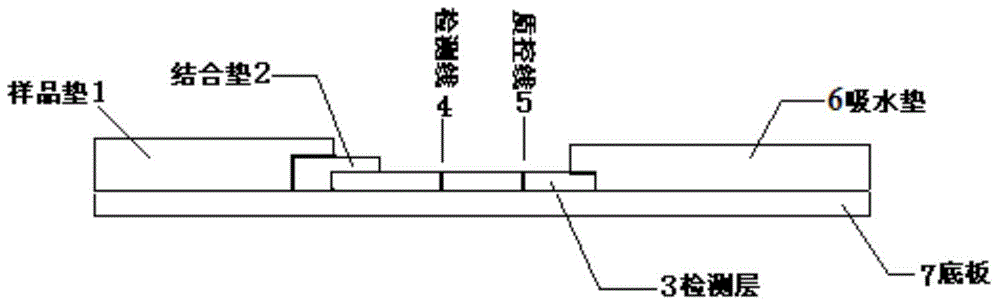
Human group A streptococci quantum dot immunochromatography detection card, preparation method and applications
The invention discloses a human group A streptococci quantum dot immunochromatography detection card, a preparation method and applications. The detection card comprises a base plate, a sample pad, a combination pad, a detection layer and a water absorption pad. The combination pad is coated with anti-human group A streptococci nano probes labeled with quantum dots. The detection layer is composed of a solid phase nitrocellulose membrane with a detection line and a quality control line. The detection line is coated with mouse-anti-human group A streptococci M protein polyclonal antibodies. The quality control line is coated with anti-rabit IgG. The detection layer is pasted on the base plate. The combination pad and the water absorption pad are pasted with the detection layer and the base plate respectively. The sample pad is arranged on the combination pad, overlaps with part of the combination pad and is pasted with the combination pad and the base plate. The human group A streptococci (M1, 3, 5, 6, 24 serotype) quantum dot immunochromatography detection card with advantages of simple operation, rapid detection, quantification, high sensitivity and the like, a preparation method and applications are provided.
Owner:湖北诺美华抗体药物技术有限公司
Composition and method for detecting group A streptococcus,
ActiveCN111020042AAccurate detectionUndisturbedMicrobiological testing/measurementMicroorganism based processesMicrobiologyBiochemistry
The invention relates to a composition for detecting group A streptococcus. The composition comprises a first primer with a sequence of 5'-TTAGCATTAGGTGGATTTGTTCTT-3', a second primer with a sequenceof 5'-GCTATCTTTTGCTTCTTTTTCGTTA-3'and a probe with a sequence of 5'-TAACCCAGTATTTGCCGATCAAAAC TTTGC-3'. Therefore, the group A streptococcus can be accurately detected. In addition, the invention alsoprovides a method for detecting the group A streptococcus and application thereof.
Owner:SANSURE BIOTECH INC
Vaccine
Effective stimulation of immune responses is achieved through the use of a group A streptococcal antigen combined with proteosome adjuvant. The compositions are provided in particular for intranasal administration. The vaccine compositions are provided for use in inducing an immune response in an individual for the treatment or prophylaxis of group A streptococcal infection in an individual, preferably via prevention or reduction of colonisation of the throat following intranasal administration.
Owner:ID BIOMEDICAL CORP LAVAL +1
Antibacterial cleaning composition and wound plaster for wounds
PendingCN110787293ASafe and effective killPlay a killing roleAntibacterial agentsAntimycoticsBiotechnologyYolk
The invention provides an antibacterial cleaning composition and wound plaster for wounds, and relates to the field of medicines. Active components of the antibacterial cleaning composition comprise acomposite yolk antibody, wherein the composite yolk antibody is prepared by the following method of mixing staphylococcus aureus with escherichia coli, baumanii, pseudomonas, group A streptococcus and candida albicans, which are inactivated to obtain a bacterial strain mixture, and enabling the bacterial strain mixture to dissolve to prepare composite antigen; and injecting the composite antigeninto a chicken body, performing immunologic facilitation, collecting eggs laid by chickens subjected to supplementary immunization, performing separation from the eggs, and performing refining, so that 6 pathogenic bacteria can be effectively restrained. Besides, the preparation method is simple, and antibodies can effectively exist, are in mutual coordination, are good in activity and good in specific disinfectant effects, and do not influence microecology balance. The wound plaster containing the antibacterial cleaning composition can effectively sterilize and clean the wounds, can prevent the wounds from being infected, and can promote the healing of the wounds.
Owner:GUANGZHOU PHGO BIOTECH
Liposomal group A streptococcus vaccine
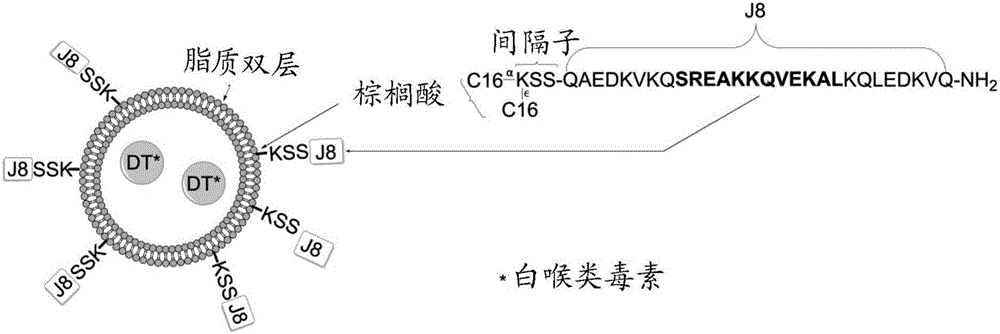
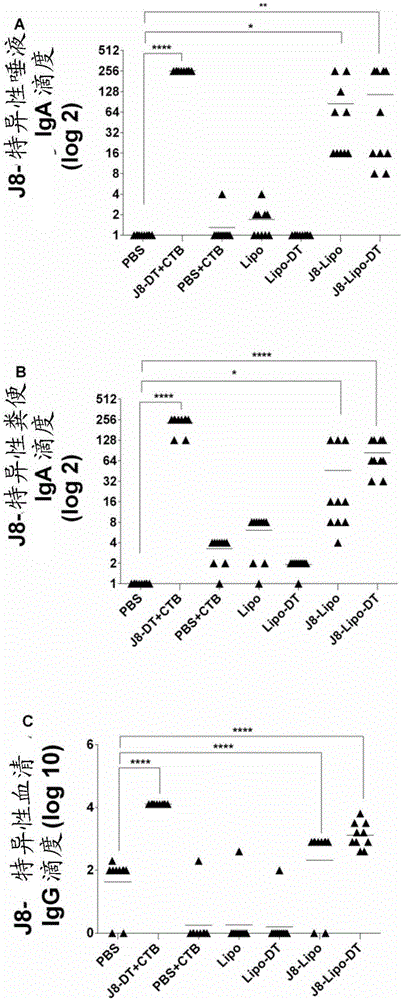
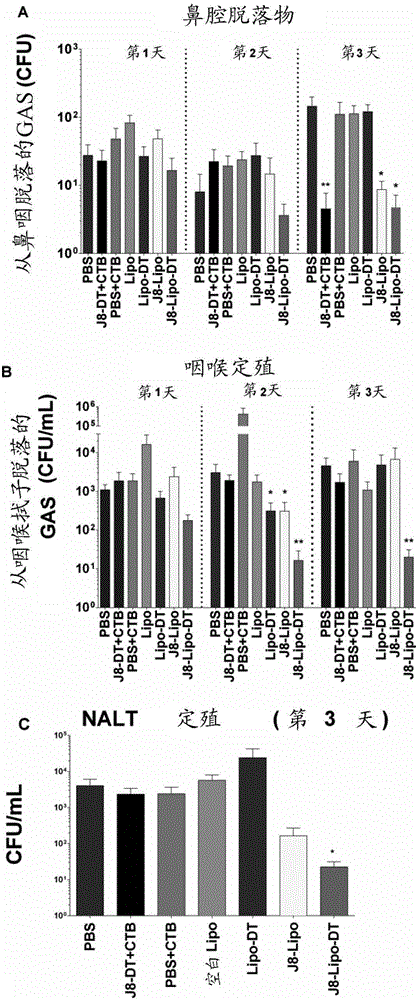
PendingCN106606775AAntibacterial agentsSsRNA viruses negative-senseMucosal Immune ResponsesCarrier protein
An immunogenic agent suitable for preventing, treating or immunizing against one or a plurality of different pathogen comprises an immunogenic agent which comprises one or a plurality of pathogen-derived proteins, fragments, variants or derivatives thereof displayed on a lipid vesicle and a carrier protein such as diptheria toxoid located in an intravesicular space. The immunogenic agent may be suitable for intranasal administration and may be capable of eliciting a mucosal immune response. The immunogenic agent may further comprise an activator of innate immunity such as trehalose-6,6'-dibehenate and / or a bile salt such as sodium deoxycholate. The one or plurality of pathogens may be group A streptococcus, viruses or hookworms.
Owner:GRIFFITH UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com