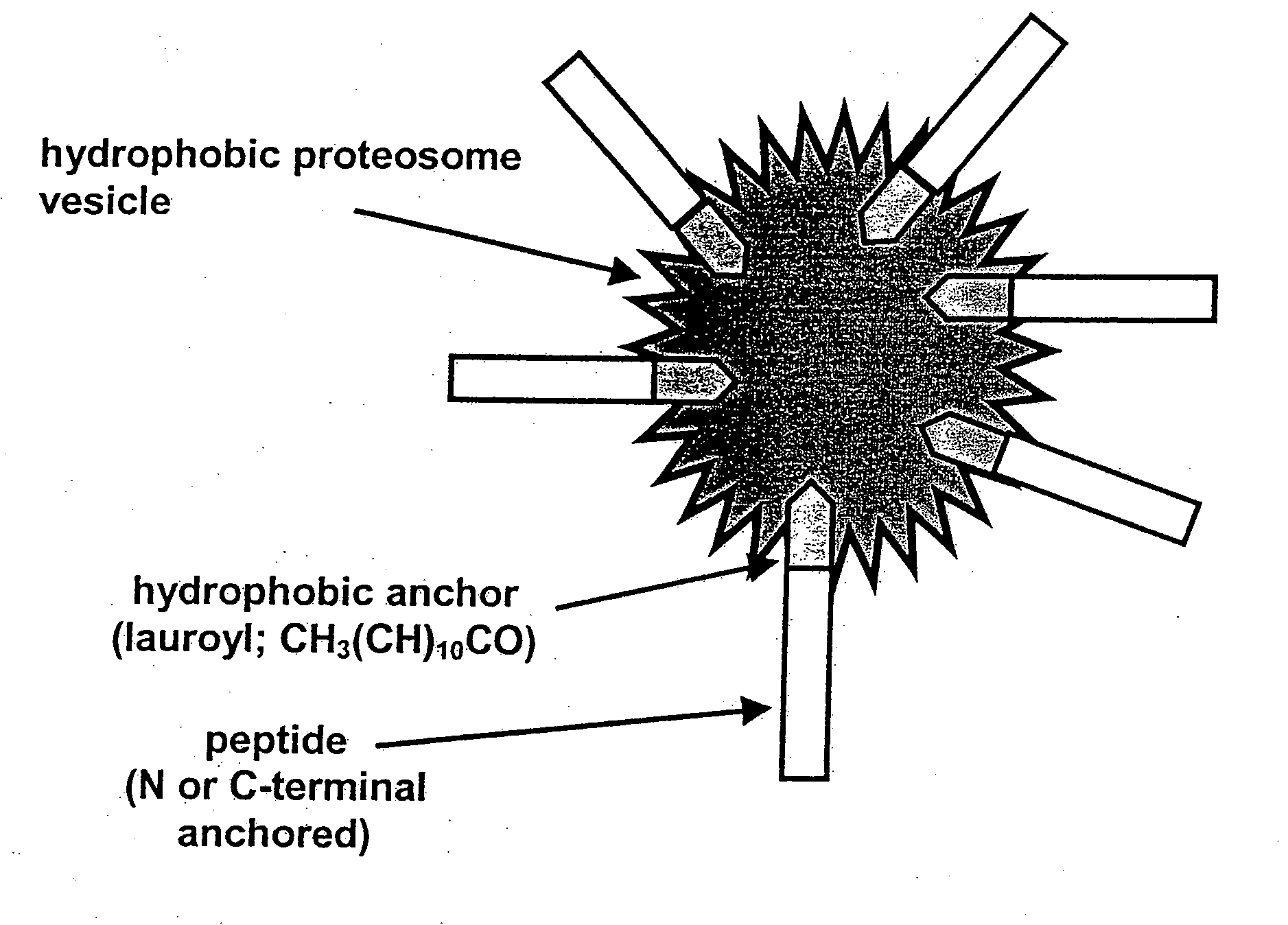
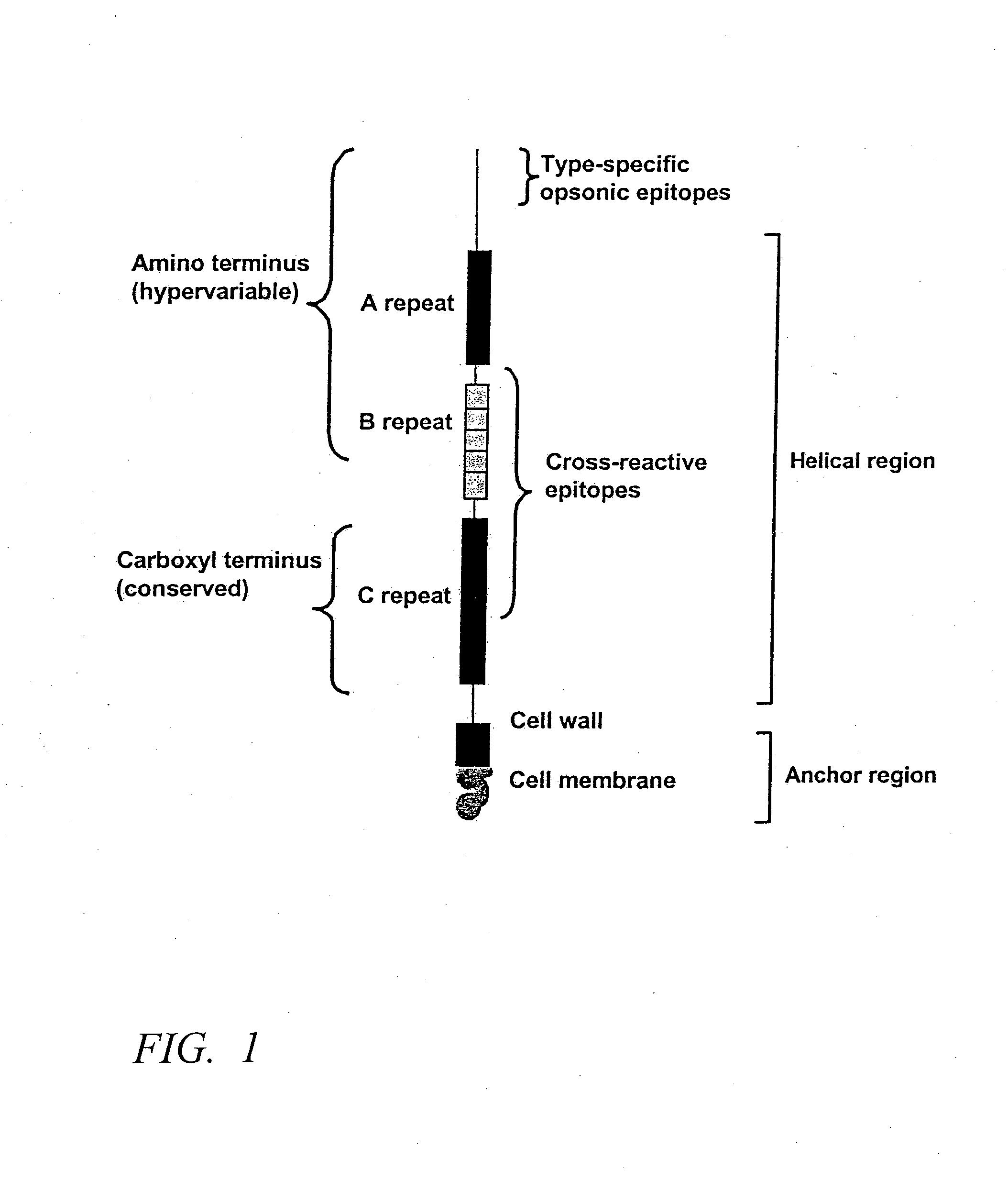
Vaccine
a technology of vaccines and antibodies, applied in the field of vaccines, can solve the problems of m proteins causing harmful host immune responses, affecting the immune response of the host, so as to reduce the colonisation of the throat, reduce the burden on healthcare resources, and effectively stimulate the immune response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Subcutaneous Immunisation
[0232]The integrity of the conformational structure and immunogenicity of the J14 and J14 anchor candidates were determined.
[0233]Peptides, with or without anchors, as described in table 3.2, were emulsified in Complete Freund's adjuvant CFA and administered subcutaneously at the tail base of B10.BR inbred mice. After the primary immunisation, boosts of 30 μg antigen in PBS were given on days 21 and 28. Sera were collected 1 day prior to boosts and 15 days after.
example 1a
Immunogenicity of the Peptide / Proteosomes Delivered Subcutaneously
[0234]Antibody titres, specific for the J14 peptide, are shown in FIG. 4. The antibody titres, after a primary immunisation in CFA and two boosts in PBS (groups 1-3), indicate that the J14 peptide with the addition of either the amino terminal (group 2) or carboxyl terminal (group 3) anchors, have resulted in sera that is able to recognise the standard chimeric J14 peptide. This infers that the addition of either the amino terminal or carboxyl terminal anchor did not disrupt the conformational structure of the J14 chimeric peptide.
TABLE 1Murine Groups for Example 1.GroupImmunisationNumberNumberImmunogenRouteof Mice1J14 (CFA)S / C52J14 amino terminal anchor (CFA)S / C53J14 carboxyl terminal anchor (CFA)S / C54PBS (CFA)S / C55J14 amino terminal anchor / S / C5proteosome adjuvant (ratio A)6J14 carboxyl terminal anchor / S / C5proteosome adjuvant (ratio A)7Proteosome adjuvant onlyS / C
[0235]The subcutaneous immunisation of the peptide-prot...
example 1b
Opsonic Potential of Sera from Immunised Mice
[0237]Sera were tested for their ability to opsonise or kill the M1 GAS reference strain (FIG. 5). All of the mice in groups one and three opsonised the GAS strain, with killing ranging from 18 to 43% and 22 to 62%, respectively. For group two only 3 of 5 mice opsonised the M1 GAS.
[0238]Both of the J14 / proteosome adjuvant immunised groups (five and six) opsonised the GAS strain. However, the opsonic potential of the individual mice varied with only 2 out of 4 mice from group five (mouse number 4 died before the opsonisation assay) and 3 out of 5 mice opsonising the bacteria from group six. Both of the control groups (four and seven) did not kill the bacteria in the in vitro assay.
Discussion and Conclusions
[0239]The experiment showed that sera raised to the peptides containing the anchors were able to recognise the standard J14 chimeric peptide and therefore the anchors did not appear to disrupt the conformational structure of the peptide....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com