Fusion protein vaccine capable of inhibiting Streptococcus and/or preventing Streptococcus infection
A fusion protein and protein technology, applied in the direction of bacteria, hybrid peptides, fungi, etc., can solve problems that hinder the development of type A streptococcal vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
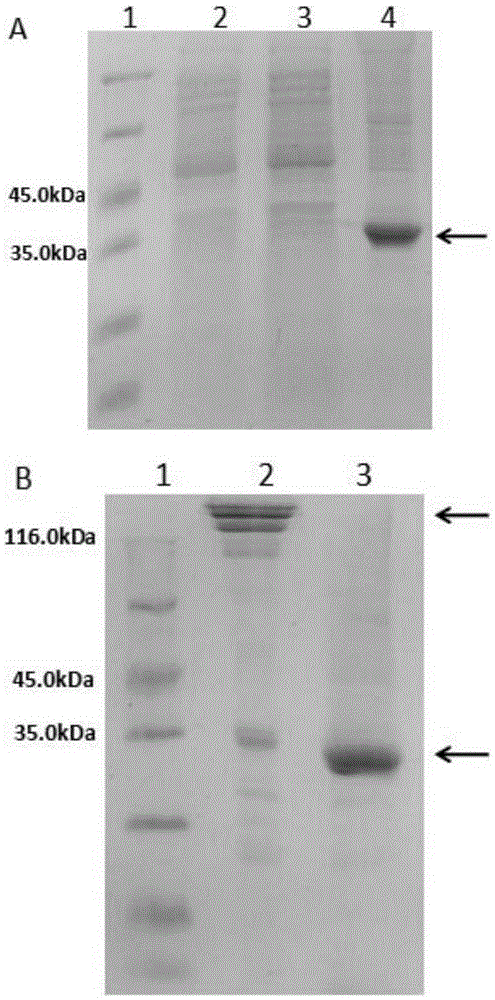
[0114] Example 1. Construction of CTB-Sortase A protein gene and preparation of CTB-Sortase A protein
[0115]1. Replace the DNA fragment between the NcoI and BamHI recognition sequences of the vector pET26b(+) with the DNA sequence shown in positions 112-996 of SEQ ID No.2, keep other DNA sequences unchanged, and obtain the recombinant expression vector pET26b- CTB-Sortase A. Sequencing results showed that the DNA sequence shown in positions 112-996 of SEQ ID No.2 was inserted into the recombinant expression vector pET26b-CTB-Sortase A, and the recombinant expression vector pET26b-CTB-Sortase A could express the first sequence of SEQ ID No.1. The CTB-Sortase A protein shown at positions 23-314, the DNA sequence shown at positions 115-996 of SEQ ID No.2 is the CTB-Sortase A protein shown at positions 23-314 of SEQ ID No.1. coding sequence.
[0116] 2. Transform the recombinant vector pET26b-CTB-Sortase A into BL21(DE3)plysS competent cells, and obtain the 23rd code containin...
Embodiment 2
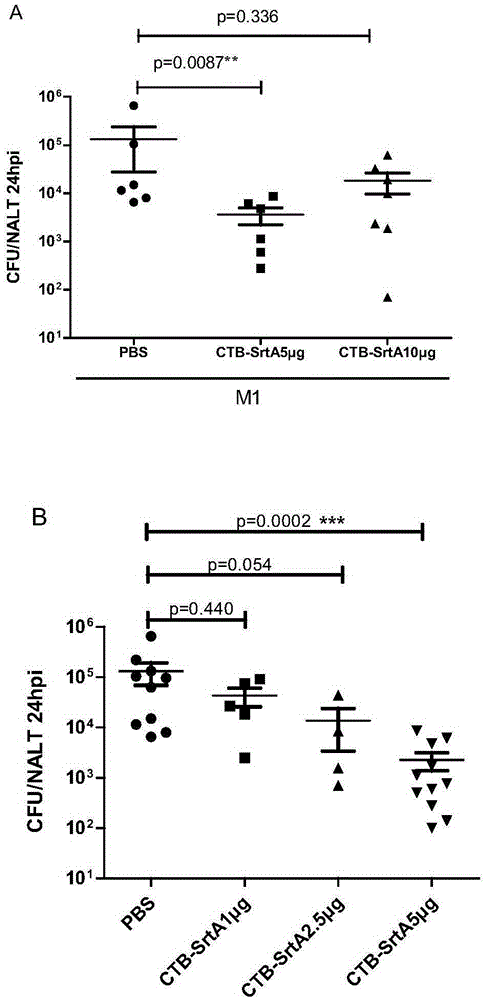
[0124] Example 2. Immune protection against type A streptococcus infection after nasal inhalation of CTB-Sortase A protein
[0125] Female BALB / c mice aged 6-8 weeks were randomly divided into three groups, 6 mice in each group, and the grouping treatment was as follows:
[0126] PBS group: 10 μL of PBS buffer was instilled through the nasal cavity on the first day, the fifth day, the 10th day and the 14th day of the experiment respectively;
[0127] CTB-Sortase A protein 5μg group: 10 μL CTB-Sortase A protein solution (vaccine liquid) was instilled through the nasal cavity on the 1st day, the 5th day, the 10th day and the 14th day of the experiment respectively. 5μg CTB-Sortase A protein;
[0128] CTB-Sortase A protein 10 μg group: 10 μL CTB-Sortase A protein solution (vaccine liquid) was instilled through the nasal cavity on the 1st day, the 5th day, the 10th day and the 14th day of the experiment respectively. is 10μg CTB-Sortase A protein;
[0129] On the 21st day of th...
Embodiment 3
[0131] Example 3. Immune protection against type A streptococcus infection after nasal inhalation of different doses of CTB-Sortase A protein
[0132] Female BALB / c mice aged 6-8 weeks were randomly divided into four groups, 6 mice in each group, and the grouping treatments were as follows:
[0133] PBS group: 10 μL of PBS buffer was instilled through the nasal cavity on the first day, the fifth day, the 10th day and the 14th day of the experiment respectively;
[0134] CTB-Sortase A protein 1 μg group: 10 μL of CTB-Sortase A protein solution (vaccine liquid) was instilled through the nasal cavity on the 1st day, the 5th day, the 10th day and the 14th day of the experiment respectively. is 1 μg CTB-Sortase A protein;
[0135] CTB-Sortase A protein 2.5 μg group: 10 μL CTB-Sortase A protein solution (vaccine liquid) was instilled through the nasal cavity on the 1st day, the 5th day, the 10th day and the 14th day of the experiment respectively. Both were 2.5μg CTB-Sortase A pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com