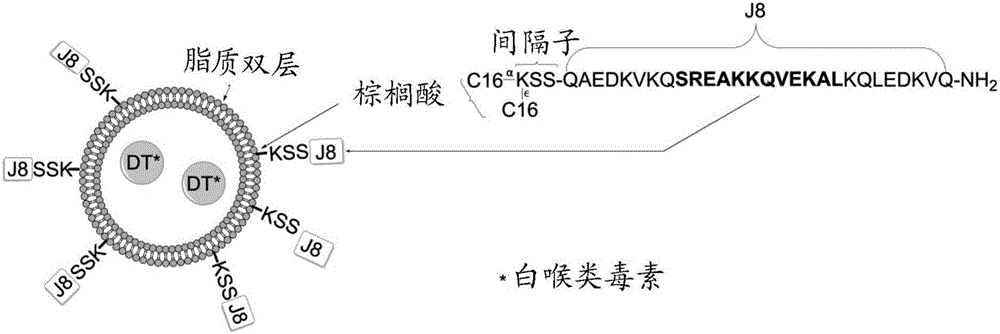
Liposomal group A streptococcus vaccine
A group A streptococcus and lipid vesicle technology, applied in the field of liposome group A streptococcus vaccine, can solve the problem that immunity is not optimal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] foreword
[0133]Group A Streptococcus (GAS) mainly infects the human upper respiratory tract (URT) mucous membrane and skin, causing many diseases. Infection can lead to toxic shock syndrome, necrotizing fasciitis, and myositis. The incidence of necrotizing fasciitis is 1 in 100,000, and the mortality rate is as high as 70% (1). Post-streptococcal diseases—rheumatic fever (RF) and rheumatic heart disease (RHD)—are also of concern. There are approximately 15.6 million active cases of RHD and almost 400,000 deaths each year (2). Pharyngitis was the most common disease following bacterial colonization of URT, whereas RF and RHD were strongly associated with untreated major infections of the pharynx (3). GAS infection and its associated diseases are prevalent in native populations in tropical regions, developing and developed countries, causing 500,000 deaths per year (4), underscoring the urgent need for a vaccine.
[0134] GAS vaccine candidates can be divided into M...
Embodiment 2
[0185] The following experiments were performed to investigate the effect of liposome size on immunogenicity.
[0186] Liposome extrusion was accomplished with a thermal block using a 1 mL syringe mini-extruder (Avanti Polar Lipids). The rehydrated solution was passed through 50 nm, 400 nm, 1000 mm filters (Avanti Polar Lipids) 11 times with the thermal block set at ~40°C. Liposome size measurements were performed by Nanosizer (Dynamic Light Scattering or DLS).
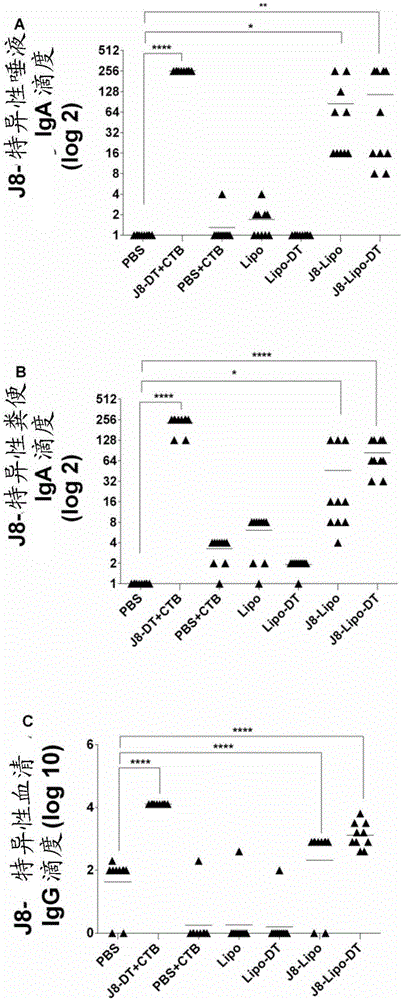
[0187] Such as Figure 12 As shown, J8-Lipo-DT can be extruded to form nano- or micron-sized particles. The majority of particle sizes have a narrow molecular weight distribution (low polydispersity index Figure 13The data showed that J8-Lipo-DT size did not affect the systemic IgG response. However, if Figure 14 As shown, liposomes of larger size induced J8-specific mucosal responses.
Embodiment 3
[0189] The following experiments were performed to investigate the effect of freeze-drying of liposomes on immunogenicity. Liposome films were rehydrated with milliQ water containing 10% trehalose and then lyophilized. 1, 4 and 7 weeks after lyophilization, J8-Lipo-DT powder was reconstituted with PBS.
[0190] Figure 15 Size results of liposome size measurements by Nanosizer (Dynamic Light Scattering or DLS) are shown. The majority of particle sizes have a narrow molecular weight distribution (low polydispersity index Figure 16 It was shown that reconstituted lyophilized J8-Lipo-DT liposomes induced J8-specific systemic responses without the need for additional adjuvants. This is a comparable immune response to freshly prepared J8-Lipo-DT. Trehalose is important for the immunogenicity of lyophilized J8-Lipo-DT. Figure 17 showed that reconstituted lyophilized J8-Lipo-DT liposomes induced J8-specific mucosal responses.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com